Abstract
Renal proximal tubule epithelial cells express high levels of the hepatocyte growth factor receptor Met, and both the receptor and ligand are upregulated after ischemic injury. Activation of the Met receptor after hepatocyte growth factor stimulation in vitro promotes activities involved in kidney repair, including cell survival, migration, and proliferation. However, characterizing the in vivo role of these signaling events in proximal tubule responses to kidney injury has been difficult because global Met knockout results in embryonic lethality due to placental and liver abnormalities. Here, we used γGT-Cre to knockout Met receptor expression selectively in the proximal tubules of mice (γGT-Cre;Metfl/fl). The kidneys of these mice developed normally, but exhibited increased initial tubular injury, tubular cell apoptosis, and serum creatinine after ischemia/reperfusion compared with γGT-Cre;Met+/+ kidneys. These changes in γGT-Cre;Metfl/fl mice correlated with a selective reduction in PI3K/Akt activation in response to injury and subsequent decreases in inhibitory phosphorylation of the proapoptotic factor Bad and activating phosphorylation of the ribosomal regulatory protein p70-S6 kinase. Moreover, tubular cell proliferation after ischemia/reperfusion was delayed in γGT-Cre;Metfl/fl mice. In conclusion, this study identifies Met-dependent phosphoinositide 3-kinase activation in proximal tubules as a critical determinant of initial tubular cell survival and reparative proliferation after ischemic injury.
The cells of the renal proximal tubule have a large metabolic demand due to their role in bulk reabsorption of glomerular filtrate. The S3 portion of the proximal tubule lies in the outer medulla of the kidney, a region that normally receives proportionally less blood flow than the cortex, making epithelial cells lining this segment highly susceptible to injury during ischemia/reperfusion (I/R) of the kidney.1–3
Tubular epithelial cell responses to severe ischemia include sublethal injury with shedding of the brush border or cell death due to either necrosis or apoptosis.4,5 The endothelial injury that occurs in this setting initiates an innate inflammatory response of polymorphonuclear cells and macrophages that contributes to tubular cell death by promoting local reactive oxygen species generation and enhanced tubular cell apoptosis.6–9 Functional recovery of tubular architecture and glomerular filtration after such an event requires repopulation of the tubule with healthy segment-appropriate tubular cells, a process that is mediated by migration and proliferation of the surviving tubular cells.10
The hepatocyte growth factor (HGF) receptor Met is expressed by multiple cell types, including the renal proximal tubule. Binding of HGF to Met activates downstream signaling via multiple effectors, including the phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways, leading to diverse biologic events in culture systems including cell survival, differentiation, proliferation, and motility.11–14 These same phenotypic responses are predicted to be important for tubule repair, and previous studies have demonstrated upregulation of message levels for both Hgf and the Met receptor in rodent models of ischemic and nephrotoxic injury.15–18
Consistent with an important physiologic role for HGF-Met signaling in kidney repair, studies utilizing exogenously added Hgf, transgenically expressed Hgf, or neutralizing antibodies to Hgf in models of kidney injury all demonstrate a role for this pathway in the restoration of renal function.8,17,19–23 However, the cell type responsible and the mechanism by which Hgf promotes improved renal function remain unclear. To answer these specific questions, we mated Metfl/fl mice24–26 with γGT-Cre mice27 to generate γGT-Cre;Metfl/fl mice with conditional knockout of Met in the proximal tubule. These mice developed normally, but had a 2-fold greater rise in serum creatinine, increased initial tubular injury, and increased tubular cell apoptosis with diminished proliferation after I/R as compared with γGT-Cre;Met+/+ mice. These functional changes were found to directly correlate with a marked reduction in the initial activation of the PI3K/Akt signaling pathway with loss of downstream antiapoptotic and proproliferative signaling. Thus activation of the Met receptor on the proximal tubule cell appears to be critical for early Akt activation and cell survival after acute ischemic injury.
Results
Met Receptor, PI3K, and MAPK Are Rapidly Activated after I/R Injury
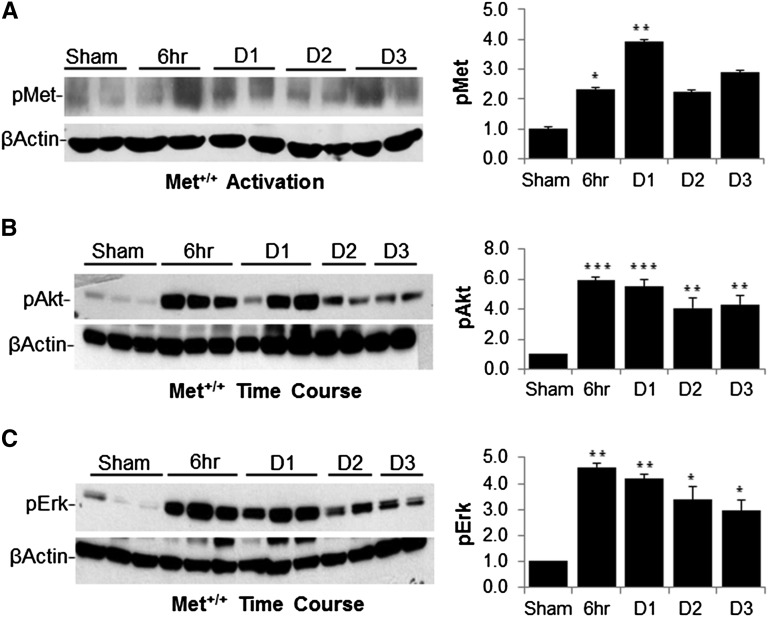
Activation of growth factor receptors such as Met results in intracellular signal transduction via the PI3K and MAPK pathways known to induce both antiapoptotic and proliferative responses in cell culture systems.12,19,28 To define the role of these pathways in vivo, wild-type mice were subjected to I/R injury followed by kidney harvest and Western blot analysis of Met receptor activation and the PI3K and MAPK effectors Akt and Erk. In this setting, Met activation was seen within 6 hours of reperfusion and persisted at days 1, 2, and 3 (Figure 1A). Similarly, Akt and Erk activation peaked between 6 and 24 hours after injury, and both remained elevated on days 2 and 3 compared with sham operation (Figure 1, B and C).
Figure 1.
Activation of Met, Akt, and Erk in wild-type mice after renal ischemia. (A) Western blot analysis of Met receptor phosphorylation at the activation site in wild-type mice at 6 hours, and 1, 2, and 3 days after I/R compared with sham operation. The graph shows quantification of pMet normalized to β-actin from four separate mice. (B) Kidney whole cell lysates of wild-type mice at the indicated times after I/R injury immunoblotted with pAkt and pErk (p42/44). The graph shows quantification from four separate mice as in A. (C) Lysates as in B immunoblotted and quantified for pErk. *P<0.05 versus sham; **P<0.01 versus sham; ***P<0.001 versus sham. n=4–6 per group.
Generation of Mice with Renal Proximal Tubule–Specific Deletion of the Met Receptor
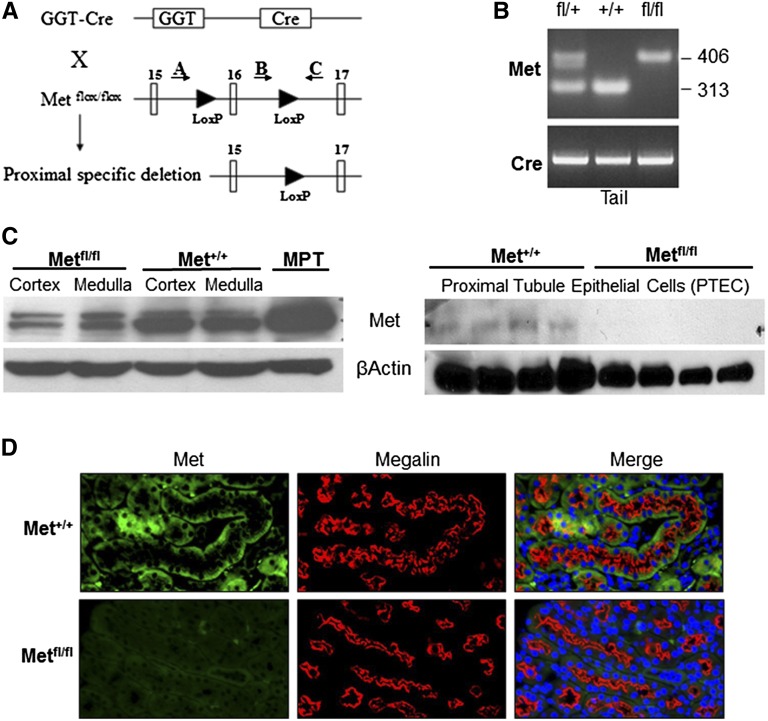
The predominant injury after I/R occurs in the S3 segment of the proximal tubule and thick ascending limb. To determine whether Met receptor activation is required for PI3K and MAPK activation after I/R, proximal tubule–specific deletion of the Met receptor was accomplished by mating Metfl/fl mice with γGT-Cre mice to produce γGT-Cre;Metfl/fl mice. Metfl/fl mice contained LoxP sites flanking exon 16 of the Met gene,25 the ATP-binding site required for activation of Met signaling (Figure 2A). Progeny heterozygous for the floxed Met allele (γGT-Cre;Metfl/+) were mated to produce γGT-Cre;Metfl/fl and γGT-Cre;Met+/+ mice used for these studies. Tail genotyping identified mice with the wild-type allele and mice heterozygous and homozygous for the floxed allele with all animals containing recombinase Cre (Figure 2B). Western blot analysis of lysates from renal cortex and medulla demonstrated a significant reduction in Met protein expression in the cortex of γGT-Cre;Metfl/fl mice, whereas renal proximal tubule epithelial cells (PTECs) isolated from these mice demonstrated absence of Met expression (Figure 2C). Finally, immunofluorescence staining of kidney sections revealed loss of basolateral Met expression in proximal tubular cells of γGT-Cre;Metfl/fl mice (Figure 2D). Renal histology revealed no apparent abnormalities in the cortical or medullary architecture in either group (Supplemental Figure 1A). There were no detectable differences in BUN, serum creatinine, or serum electrolytes in either group of mice at 8–10 weeks of age (Supplemental Figure 1B).
Figure 2.
Renal proximal tubule–specific deletion of the Met receptor. (A) Illustration of LoxP containing the Met allele with the location of genotyping primers. (B) PCR of genomic DNA using primers A–C. The 406 bp band occurs with inclusion of the LoxP sites, whereas the 313 bp band is the wild-type sequence. γGT-Cre recombinase is seen in all three groups. (C) Western blot of lysates from the renal cortex and medulla (left panel) in γGT-Cre;Metfl/fl and γGT-Cre;Met+/+ mice with mouse proximal tubule (MPT) cells as a positive control. Western blot analysis of primary tubular epithelial cells isolated from renal cortex (right panel). β-Actin is used as a loading control. (D) Immunofluorescence of the renal cortex with α-Met (green) and α-megalin (red, proximal tubule marker). Note the absence of basolateral staining for the Met receptor in the proximal tubules of γGT-Cre;Metfl/fl mice. DAPI (blue) is included in the merged image to identify nuclei.
Proximal Tubule–Specific Deletion of Met Results in Greater Functional Impairment and Structural Injury after I/R Surgery
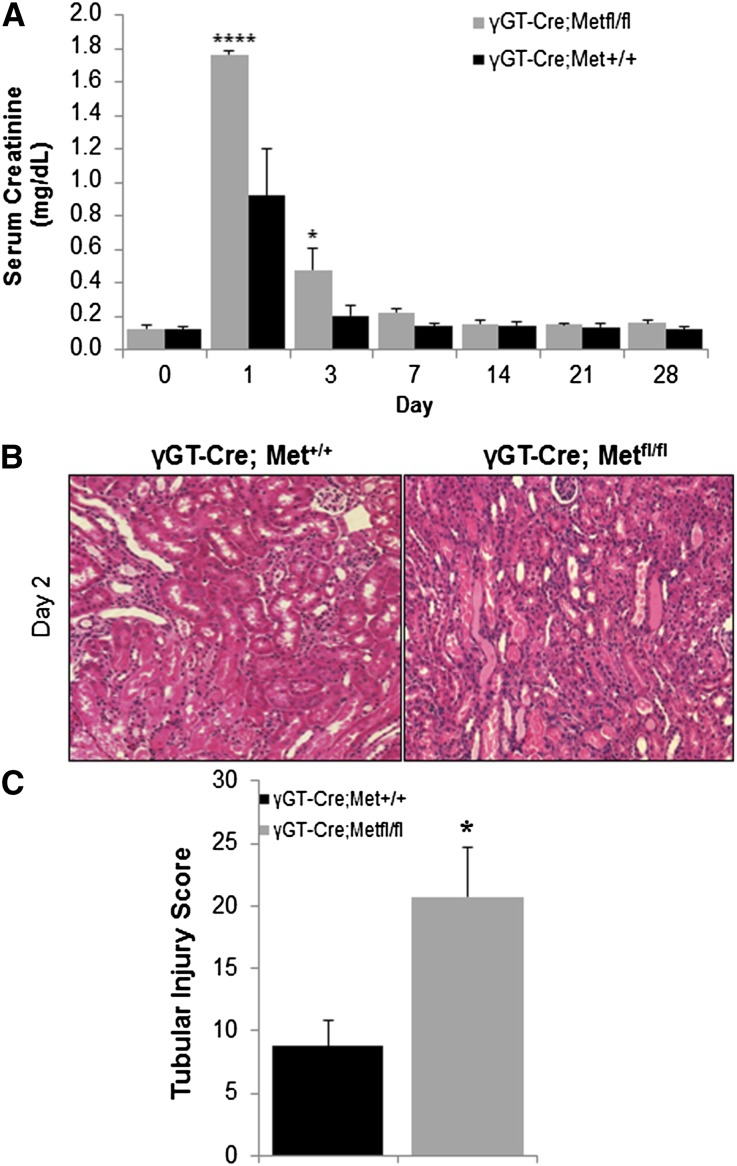
The importance of Met signaling in proximal tubule injury was assessed in γGT-Cre;Metfl/fl and γGT-Cre;Met+/+ mice exposed to I/R. On day 1 after I/R, serum creatinine was elevated 2-fold more in γGT-Cre;Metfl/fl mice than in γGT-Cre;Met+/+ mice (Figure 3A). Creatinine values remained significantly higher in γGT-Cre;Metfl/fl mice on day 3, but were indistinguishable at later time points. Initial BUN levels also tended to be higher in γGT-Cre;Metfl/fl mice, but the difference did not reach statistical significance (Supplemental Figure 1C). Consistent with the creatinine values, blinded tissue injury scoring on day 2 after I/R revealed increased tubular cell death and cast formation in γGT-Cre;Metfl/fl mice (Figure 3B, quantified in C). Of note, the mRNA for the Met ligand Hgf was equally upregulated after I/R in both groups of mice (Supplemental Figure 1D).
Figure 3.
γGT-Cre;Metfl/fl mice exhibit worse tubule injury after I/R. (A) Serum creatinine values are obtained on the indicated days in both groups. *P<0.05; ****P<0.0001 versus γGT-Cre;Met+/+ by two-way ANOVA. n=3–7 per group at each time point. (B) Renal histology 48 hours after injury reveals greater loss of brush border, nuclear drop out, and cast formation in γGT-Cre;Metfl/fl mice. (C) Tubular injury quantified in a single-blinded fashion. *P<0.05 versus γGT-Cre;Met+/+. n=5.
Increased Apoptosis with Marked Reduction in PI3K/Akt Signaling in γGT-Cre;Metfl/fl Mice after I/R
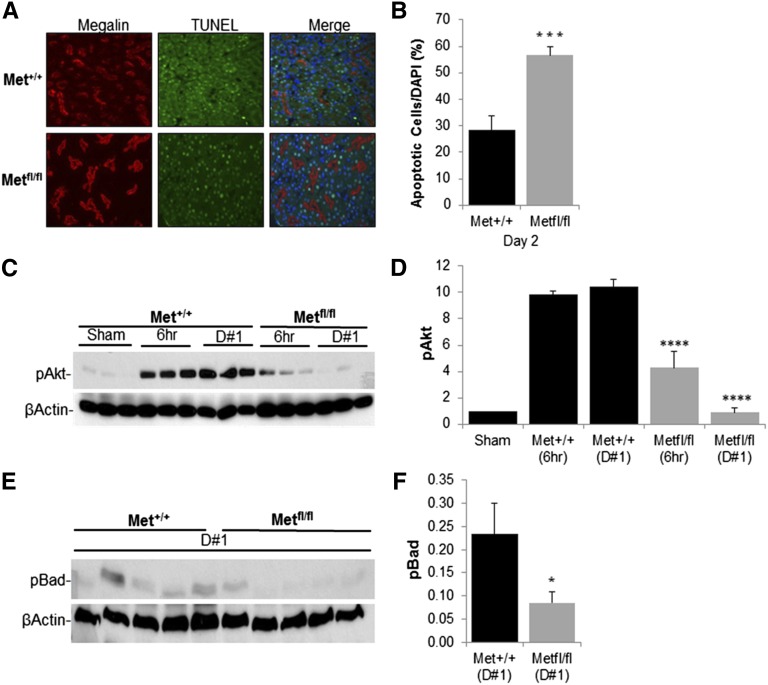
Tubular cell death seen after I/R occurs as a result of immediate necrosis and subsequent apoptosis.4 In light of the increased tubular cell death seen histologically in γGT-Cre;Metfl/fl mice, tubular cell apoptosis was quantified by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining. On day 2 after I/R, outer medullary proximal tubular cell apoptosis was significantly greater in γGT-Cre;Metfl/fl mice (Figure 4A, quantified in B).
Figure 4.
Decreased antiapoptotic PI3K/Akt signaling in γGT-Cre;Metfl/fl mice after I/R. (A) Representative outer medullary kidney sections from γGT-Cre;Metfl/fl and γGT-Cre;Met+/+ mice with TUNEL-positive staining for apoptotic cells (TUNEL-positive nuclei green stain) and α-megalin (red stain) for identification of proximal tubules. DAPI is used for counterstaining. (B) Percentage of apoptotic proximal tubular cells quantified as seen in A. ***P<0.001 versus γGT-Cre;Met+/+. n=4 per group. (C) Kidney lysates prepared from sham-operated mice, at indicated times after I/R surgery in γGT-Cre;Met+/+ and γGT-Cre;Metfl/fl mice and immunoblotted with pAkt antibody with β-actin as a loading control. (D) Quantification of pAkt as in C. ****P<0.0001 versus γGT-Cre;Met+/+ by two-way ANOVA. n=6 per group. (E) Western blot analysis of kidney lysates from γGT-Cre;Met+/+ and γGT-Cre;Metfl/fl mice obtained at day 1 after injury and immunoblotted with pBad. (F) Quantification of pBad as in E normalized to β-actin. *P<0.05 versus γGT-Cre;Met+/+. n=5 per group.
The observation that apoptosis was worse in those tubular cells lacking Met expression led us to investigate the possibility that antiapoptotic signaling downstream of Met might be diminished in the γGT-Cre;Metfl/fl mice after I/R. Of the known Met signaling pathways, PI3K/Akt activation has been the one that is most clearly associated with antiapoptotic responses.19,29 Similar to the observation in wild-type mice (Figure 1B), there was a robust activation of Akt in γGT-Cre;Met+/+ mice at both 6 and 24 hours after I/R (Figure 4C, quantified in D). In contrast, Akt activation in γGT-Cre;Metfl/fl mice subjected to I/R was <50% of that seen in γGT-Cre;Met+/+ mice at 6 hours after injury, and reduced to baseline by 24 hours.
Once phosphorylated and activated by phosphoinositide-dependent kinase 1, Akt can phosphorylate Bad, an apoptogenic member of the Bcl-2 family. Phosphorylation of Bad on serine 136 by pAkt promotes Bad:14-3-3 association and thus prevents Bad-dependent proapoptotic responses.30–32 Examination of Bad phosphorylation at serine 136 in whole kidney lysates 24 hours after I/R reveals a 64% reduction in Akt-mediated Bad inactivation in γGT-Cre;Met+/+ mice compared with γGT-Cre;Metfl/fl mice (Figure 4E, quantified in F).
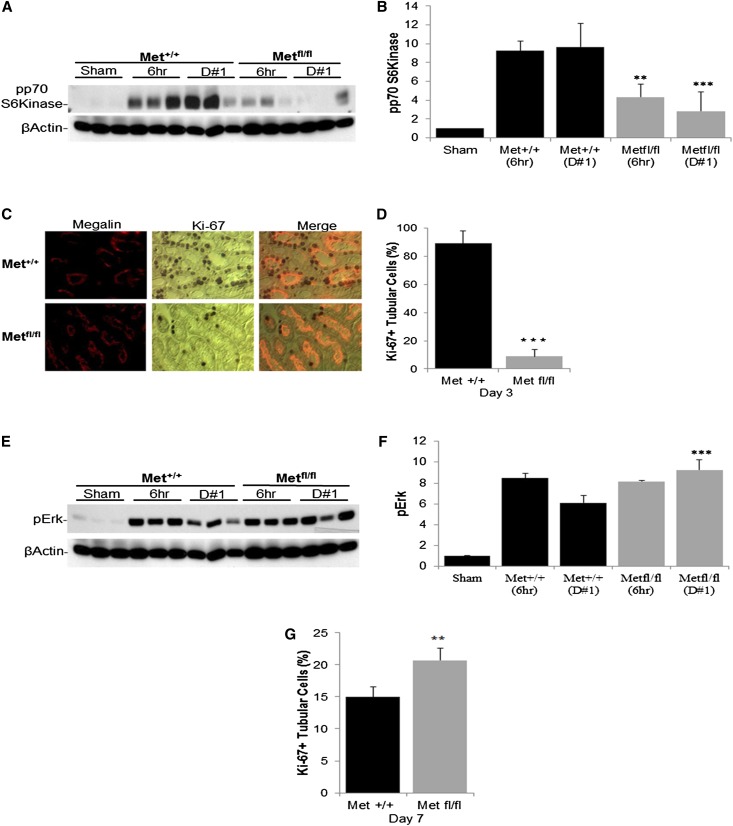
γGT-Cre;Metfl/fl Mice Exhibit a Reduction in p70-S6 Kinase Signaling and a Delayed Proliferative Response to Ischemic Injury
A separate target for activated Akt is p70-S6 kinase (p70S6K). This protein phosphorylates the S6 ribosomal protein and thus activates protein synthesis. Phosphorylation by pAkt activates p70S6K, providing the increased protein needed for cell division. Similar to the timing of Akt activation, p70S6K was phosphorylated at the activation site within 6 hours of I/R in γGT-Cre;Met+/+ mice, peaking at 24 hours (Figure 5A, quantified in B). In contrast, γGT-Cre;Metfl/fl mice exhibited a marked reduction of p70S6K activation at both time points. Analysis of tubular cell proliferation, assessed by Ki-67 staining, revealed the expected burst of proliferation on day 3 after I/R in γGT-Cre;Met+/+ mice (Figure 5C, quantified in D). However, this early reparative response was significantly reduced in the γGT-Cre;Metfl/fl mice. Interestingly, there was a significant increase in Erk activation (the prototypic signal for epithelial cell proliferation in response to growth factors) on day 1 after I/R in γGT-Cre;Metfl/fl mice (Figure 5E, quantified in F), suggesting that the reduction in Akt-dependent protein synthesis was sufficient to prevent successful Erk-dependent entry into the cell cycle.
Figure 5.
Met receptor inactivation leads to a reduction in early p70S6K phosphorylation and delayed tubular proliferation. (A) Kidney lysates prepared from sham-operated mice at 6 hours and 1 day after I/R surgery in γGT-Cre;Met+/+ and γGT-Cre;Metfl/fl mice. Lysates are immunoblotted with phosphorylated p70S6K antibody using β-actin as a loading control. (B) Quantification of phosphorylated p70S6K as in A. **P<0.01; ***P<0.001 versus γGT-Cre;Met+/+ by two-way ANOVA. n=5 per group. (C) Immunostaining of the renal outer medulla of γGT-Cre;Metfl/fl and γGT-Cre;Met+/+ mice with Ki-67 (nuclei stained brown) to assess tubular cell proliferation. (D) Quantification of proliferation as in C. ***P<0.001 versus γGT-Cre;Met+/+. n=4 per group. (E) Whole kidney lysates of γGT-Cre;Met+/+ and γGT-Cre;Metfl/fl mice probed with pErk (42/44) antibody after sham operation or I/R surgery at the indicated times with β-actin as the loading control. (F) Quantification of Erk activation as in E. ***P<0.001 versus γGT-Cre;Met+/+, by two-way ANOVA. n=6 in each group. (G) Quantification of tubular cell proliferation on day 7 after I/R injury. **P<0.01 versus γGT-Cre;Met+/+. n=4 per group.
Our observation that serum creatinine levels do improve in γGT-Cre;Metfl/fl mice, albeit more slowly than normal, suggested that Met-independent growth pathways might promote late tubular cell proliferation in these mice. Consistent with this, Ki-67 staining on day 7 after I/R revealed a significant increase in proximal tubular cell proliferation in γGT-Cre;Metfl/fl mice compared with the γGT-Cre;Met+/+ controls (Figure 5G).
Discussion
Signal transduction through the HGF/scatter factor receptor, Met, has been shown to activate a variety of in vitro physiologic responses essential to cell survival and repair after tissue injury. Most of these responses require activation of PI3K-Akt and MAPK-Erk signaling pathways when assessed in vitro.11–14 Our current analysis of these pathways in vivo confirmed that the Met receptor is phosphorylated and activated early after I/R coinciding with previous descriptions of a biphasic rise in plasma and renal HGF levels at 1 hour and 24 hours after similar injury in mice.8 This timing of Met receptor activation aligns well with effector signaling through pAkt and pErk, both of which peaked at 6 and 24 hours after I/R. It is also important to note that although these effects diminished on the second and third day after I/R surgery, they were not completely abolished, suggesting a role for sustained Met activation and Akt and Erk signaling during the reparative phase after injury to the renal epithelium.
To elucidate the role of Met receptor activation in regulating these signaling responses to injury, we performed selective knockout of Met expression in the renal proximal tubule. These mice were viable and demonstrated normal physiology and renal histology at baseline. Specifically, the kidney size of γGT-Cre;Metfl/fl mice was normal with normal tubule architecture and no evidence for glomerular hypertrophy or reduced numbers of nephrons. This is different than mice with Met knockout in the collecting duct, where we found small kidneys with reduced nephron numbers but normal architecture of the nephrons that did form.33 Cumulatively, these two studies conclusively demonstrate that Met is not required for the formation and elongation of the renal tubules during development, whereas it is required for maximal ureteric bud branching. Interestingly, the studies of Met function in the collecting duct demonstrated that Met normally acts in a cooperative manner with EGF-EGF receptor (EGFR) signaling to maximize ureteric bud branching.
Despite the finding that proximal tubules of γGT-Cre;Metfl/fl mice developed normally, we found marked differences in the response of these mice to ischemic injury of the proximal tubule. After I/R, γGT-Cre;Metfl/fl mice demonstrated a greater degree of renal dysfunction and tubular damage with elevated tubular injury scoring within the corticomedullary region of the kidney and increased rates of tubular cell apoptosis. Prior studies of renal I/R have revealed two waves of apoptosis present at 12–24 hours and then 2 days after reperfusion.34 Our study demonstrates that early activation of the Met receptor is critical in suppressing that second wave of apoptosis.
The PI3K/Akt pathway has been well characterized in vivo and in vitro for its ability to promote cell survival by suppressing apoptosis in various cell types, including renal tubular epithelial cells.35–37 In this study, we show that the PI3K/Akt pathway is markedly stimulated at 6–24 hours after reperfusion injury, and that Met receptor signaling is the dominant stimulus for this early response. Additional evidence in support of the role of Met-PI3K activation in normally suppressing apoptogenic responses after I/R is shown by the analysis of Bad inactivation.32 Bad is a proapoptotic member of the Bcl-2 family that is inactivated after Akt phosphorylation at serine 136. At 24 hours after reperfusion injury, the level of phosphorylation at this inhibitory site is significantly decreased in mice lacking the proximal tubule Met receptor. These results demonstrate that activation of the Met-PI3K-Akt signaling cascade is critical for promoting survival of sublethally injured proximal tubule cells after I/R injury, thus providing a sufficient reservoir of cells for subsequent repair.
In addition to the role of Met receptor signaling in suppressing tubular cell apoptosis, we also found that tubules lacking Met failed to proliferate normally in the early phase after reperfusion injury. One possible explanation for this is that the increase in apoptosis simply left fewer cells to proliferate. However, the reduction in tubular cell proliferation seen on day 3 is out of proportion to the increase in apoptosis seen on day 2, suggesting that entry into the cell cycle may be delayed in this model even in surviving tubular cells. In light of the clear decrease in Met-stimulated PI3K/Akt activation in the mice lacking tubular cell Met, we examined the possibility that loss of Akt signaling might impair the initial proliferative response. Once phosphorylated, Akt can induce activation of the mammalian target of rapamycin, leading to p70S6K phosphorylation/activation with subsequent phosphorylation of the S6 ribosomal protein and induction of protein synthesis.38–40 This pathway has been found to be required for proliferation due to both the general need for increased protein synthesis as well as the specific requirement for activated p70S6K in the expression of cyclin D proteins and entry into cell cycle.41–43 Consistent with the role of Met-induced activation of this pathway in the early proliferative response, γGT-Cre;Metfl/fl mice exhibited a >70% reduction in activated p70S6K expression 1 day after I/R. Therefore, we believe that the combination of greater initial tubular cell apoptosis and the concomitant reduction in p70S6K activation accounts for the marked reduction in the early proliferative phase of tubule repair seen in mice lacking the Met receptor in the proximal tubule.
Surprisingly, despite the dependence of the proximal tubule on Met receptor signaling to suppress early apoptosis and increase reparative proliferation, the GFRs of these young mice did improve at later time points to a level indistinguishable from wild-type controls. Analysis of tubular cell proliferation on day 7 after injury suggests that this recovery was due at least in part to sustained tubular cell proliferation at later time points. This is consistent with the large proliferative reserve seen in tubular cells isolated from young mice,44 and suggests that other growth factor pathways are likely to be important in the composite response to tubular injury, a conclusion that is supported by the finding that Erk was activated to a similar degree in both wild-type and knockout mice.
A plausible explanation for this can be found in the results of a recent study by Chen et al.45 that examined the effect of pharmacologic inhibition or selective deletion of the renal proximal tubule EGFR in mice subjected to renal ischemia. Renal dysfunction and structural injury were similar at early time points after I/R in all groups of mice but approached a statistical difference within the pharmacologic inhibition group and the proximal tubule–specific deletion group at 6 days postischemia. The authors demonstrated reductions in EGFR-Erk and EGFR-PI3K/Akt signaling beginning on day 2 after renal ischemic injury in mice with either pharmacologic inhibition or EGFR deletion in the proximal tubule. In contrast, we find that Met receptor activation alters initial PI3K/Akt signaling and promotes worse initial injury. Jointly, these data suggest that Met receptor activation and EGFR activation act in a complementary and sequential manner after I/R to promote tubular cell survival and proliferative repair, similar to our previous findings in ureteric bud branching.
In summary, we have described a mechanism by which HGF/Met receptor signaling through the PI3K/Akt pathway protects sublethally injured renal tubular cells against early apoptosis. Mice lacking the Met receptor in the proximal tubule have worsened renal function, structural injury, and apoptosis after ischemic injury. We conclude that Met-dependent PI3K activation after I/R is a nodal pathway for cytoprotection and early tubular repair, and thus might serve as a logical therapeutic target to promote recovery from AKI.
Concise Methods
Reagents and Antibodies
Antibodies against Met, phospho-Met, phospho-Erk, phospho-Akt, phospho-p70S6K, and phospho-Bad were purchased from Cell Signaling Technology (Beverly, MA). Met and β-actin antibodies were purchased from Santa Cruz Biotechnology (San Diego, CA). Anti-megalin antibody was obtained as a kind gift from Dr. Daniel C. Biemesderfer (Yale School of Medicine, New Haven, CT).
Generation of Conditional Met Knockout Mice
Metfl/fl mice on a mixed 129SV/C57Bl/6 background were developed as previously described.25 These mice were mated with transgenic mice containing γGT-Cre on the same 129SV/C57Bl/6 background. Tail genotyping was performed using primers for Met and Cre expression as previously described.33 Age-matched homozygous γGT-Cre;Metfl/fl and wild-type control mice γGT-Cre;Met+/+ were used in all experiments. All mouse experiments were conducted under a protocol approved by the Yale Institutional Animal Care and Use Committee.
I/R Surgery
γGT-Cre;Metfl/fl mice aged 8–10 weeks and their γGT-Cre;Met+/+ control were anesthetized using xylazine and ketamine. Exposure of bilateral renal pedicles was accomplished after using a midline ventral incision. The right renal pedicle was ligated with sutures and nephrectomized, whereas the left renal pedicle was clamped for 25 minutes with visually confirmed ischemia. Reperfusion of the left kidney was visualized after clamp release. Sham animals underwent midline ventral incision without clamping. To prevent dehydration, mice were administered 1 ml of prewarmed normal saline intraperitoneally before closure of muscle and skin layers.
Renal Functional Measurement
Mouse blood collection occurred at 3 days before and 1, 3, 7, 14, 21, and 28 days after I/R surgery. BUN levels and serum creatinine were analyzed using the Yale University School of Medicine Core Mouse Metabolic Phenotyping Center.
Isolation of PTECs
Mouse PTECs were isolated in a modified previously described protocol.9 In brief, uninjured mouse kidneys were harvested after cardiac perfusion with 0.125% collagenase type 1 (Worthington Biochemical Corporation, Lakewood, NJ) in M199 Hanks’ solution (Lonza Group, Basel, Switzerland). Isolated renal cortex was minced, placed in collagenase solution, and incubated in 37°C while 5% CO2 balanced with room air gas was infused into the solution for 40 minutes. Collagenase solution was removed and remnant tissues and cells were resuspended in renal epithelial growth medium (Lonza) containing penicillin/streptomycin and 2.5% FBS. Solution was passed through a 40-µm cell strainer, plated, and grown to confluence before protein isolation and immunoblotting.
Protein Isolation and Western Blot Analyses
Mice were anesthetized by intraperitoneal injection of xylazine and ketamine. Kidneys were harvested at 6 hours and 1, 2, and 3 days after surgery and immediately flash frozen in liquid nitrogen. Tissue was homogenized in radioimmunoprecipitation assay buffer (Teknova, Hollister, CA) containing EDTA-free protease and phosphatase inhibitors (Halt Inhibitor Cocktail; Thermo Scientific, Rockford, IL) using a Dounce homogenizer and centrifuged at 10,000×g for 15 minutes at 4°C. Equal amounts of protein lysate were separated by SDS-PAGE, transferred onto Immobilon-P membranes (Millipore, Bedford, MA), probed with the appropriate antibody, and visualized by enhanced chemiluminescence (Amersham Biosciences, Pittsburgh, PA) and SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). Quantification of signals was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Kidney Immunofluorescence, Immunohistochemistry, and Histology
Mice were anesthetized by intraperitoneal injection of xylazine and ketamine followed by in vivo perfusion fixation with 4% paraformaldehyde and processed for histology (hematoxylin and eosin, unstained sections from paraffin blocks). Sections were subjected to antigen retrieval (Retrievagen; BD, Franklin Lakes, NJ) and then blocked at room temperature using saline containing 0.1% BSA/10% goat serum for 1 hour. Immunostaining was performed with the appropriate primary antibody overnight at 4°C followed by Alexa Fluor goat anti-rabbit 594 or goat anti-mouse 488 secondary antibodies (Invitrogen, Carlsbad, CA) for visualization. Ki-67 staining was performed by Yale Mouse Research Pathology. The TUNEL assay (Roche, Indianapolis, IN) was completed for identification of apoptotic cells, as per manufacturer protocols. DAPI (4′,6,-diamidino-2-phenylindole) was used as a counterstain (Vector Laboratories, Burlingame, CA). Quantification of cells expressing the specified marker was conducted by manual count of positive cells/DAPI nuclei in outer medullary proximal tubule cells in 10 randomly chosen ×400 fields using a Nikon microscopy system (Nikon, Inc., Melville, NY).
Morphometric Evaluation of Kidney Injury
Kidney sections stained with hematoxylin and eosin were examined and quantified for histologic changes associated with tubular injury by a single-blinded renal pathologist (G.M.). Scoring was carried out by calculating the percentage of tubules in the corticomedullary junction that displayed cell necrosis, loss of brush border, cast formation, and tubule dilation as follows: 0, none; 1, 10%; 2, 11%–25%; 3, 26%–45%; 4, 46%–75%; and 5, ≥76%. Ten randomly chosen fields (×200) were examined for each slide.
Quantitative PCR
Kidneys were obtained at baseline, 1 day, and 7 days after surgery from γGT-Cre;Metfl/fl and γGT-Cre;Met+/+ mice with total RNA isolated using TRIzol reagent (Life Technologies, Carlsbad, CA). One microgram of RNA was reverse transcribed using random hexamer primers (SuperScript II; Invitrogen) and gene expression analysis was determined by quantitative PCR using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Berkeley, CA). Primers used for PCR were chosen for efficiency of 90%–100% (details available upon request). Normalization was to hypoxanthine guanine phosphoribosyl transferase expression from the same PCR reaction (ΔCt).
Statistical Analyses
All data were expressed as means ± SEMs for separate experiments. The two-tailed t test was used for statistical analysis, with a P value <0.05 compared with controls considered statistically significant. Between-groups comparison among postischemia time points was analyzed by two-way ANOVA for repeated measures and by multiple comparisons, with P<0.05 considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK65109, 5T32DK7276-33, and 5T32HD068201-02). Support for quantitative PCR and creatinine measurements was provided through the George M. O’Brien Kidney Center at Yale University.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050473/-/DCSupplemental.
References
- 1.Brezis M, Rosen S: Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332: 647–655, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Brezis M, Shanley P, Silva P, Spokes K, Lear S, Epstein FH, Rosen S: Disparate mechanisms for hypoxic cell injury in different nephron segments. Studies in the isolated perfused rat kidney. J Clin Invest 76: 1796–1806, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou SY, Porush JG, Faubert PF: Renal medullary circulation: Hormonal control. Kidney Int 37: 1–13, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV: Mechanisms of ischemic acute renal failure. Kidney Int 43: 1160–1178, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Havasi A, Borkan SC: Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Mizuno S, Nakamura T: Prevention of neutrophil extravasation by hepatocyte growth factor leads to attenuations of tubular apoptosis and renal dysfunction in mouse ischemic kidneys. Am J Pathol 166: 1895–1905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo JK, Cantley LG: Cellular maintenance and repair of the kidney. Annu Rev Physiol 72: 357–376, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Cantley LG, Barros EJ, Gandhi M, Rauchman M, Nigam SK: Regulation of mitogenesis, motogenesis, and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am J Physiol 267: F271–F280, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Cantley LG, Cantley LC: Signal transduction by the hepatocyte growth factor receptor, c-met. Activation of the phosphatidylinositol 3-kinase. J Am Soc Nephrol 5: 1872–1881, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Liu ZX, Nickel CH, Cantley LG: HGF promotes adhesion of ATP-depleted renal tubular epithelial cells in a MAPK-dependent manner. Am J Physiol Renal Physiol 281: F62–F70, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K, Nakamura T: Emerging multipotent aspects of hepatocyte growth factor. J Biochem 119: 591–600, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Joannidis M, Spokes K, Nakamura T, Faletto D, Cantley LG: Regional expression of hepatocyte growth factor/c-met in experimental renal hypertrophy and hyperplasia. Am J Physiol 267: F231–F236, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi K, Sasaki S, Sakamoto H, Hoshino Y, Nakamura T, Marumo F: Expressions of receptor gene for hepatocyte growth factor in kidney after unilateral nephrectomy and renal injury. Biochem Biophys Res Commun 187: 1454–1459, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Kawaida K, Matsumoto K, Shimazu H, Nakamura T: Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci U S A 91: 4357–4361, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabkin R, Fervenza F, Tsao T, Sibley R, Friedlaender M, Hsu F, Lassman C, Hausmann M, Huie P, Schwall RH: Hepatocyte growth factor receptor in acute tubular necrosis. J Am Soc Nephrol 12: 531–540, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Sun AM, Dworkin LD: Hepatocyte growth factor protects renal epithelial cells from apoptotic cell death. Biochem Biophys Res Commun 246: 821–826, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Mizuno S, Matsumoto K, Nakamura T: Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int 59: 1304–1314, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Yo Y, Morishita R, Nakamura S, Tomita N, Yamamoto K, Moriguchi A, Matsumoto K, Nakamura T, Higaki J, Ogihara T: Potential role of hepatocyte growth factor in the maintenance of renal structure: Anti-apoptotic action of HGF on epithelial cells. Kidney Int 54: 1128–1138, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Fiaschi-Taesch NM, Santos S, Reddy V, Van Why SK, Philbrick WF, Ortega A, Esbrit P, Orloff JJ, Garcia-Ocaña A: Prevention of acute ischemic renal failure by targeted delivery of growth factors to the proximal tubule in transgenic mice: The efficacy of parathyroid hormone-related protein and hepatocyte growth factor. J Am Soc Nephrol 15: 112–125, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Dai C, Yang J, Liu Y: Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol 13: 411–422, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C: Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A 101: 10608–10613, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS: Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A 101: 4477–4482, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, Saenko M, Opuko A, Togawa A, Soda K, Marlier A, Moeckel GW, Cantley LG, Ishibe S: Deletion of the Met receptor in the collecting duct decreases renal repair following ureteral obstruction. Kidney Int 76: 868–876, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W: Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol 149: 1419–1432, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantley LC: The phosphoinositide 3-kinase pathway. Science 296: 1655–1657, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kiley SC, Thornhill BA, Tang SS, Ingelfinger JR, Chevalier RL: Growth factor-mediated phosphorylation of proapoptotic BAD reduces tubule cell death in vitro and in vivo. Kidney Int 63: 33–42, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Korsmeyer SJ: BCL-2 gene family and the regulation of programmed cell death. Cancer Res 59[Suppl]: 1693s–1700s, 1999 [PubMed] [Google Scholar]
- 32.Liu Y: Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol 277: F624–F633, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Ishibe S, Karihaloo A, Ma H, Zhang J, Marlier A, Mitobe M, Togawa A, Schmitt R, Czyczk J, Kashgarian M, Geller DS, Thorgeirsson SS, Cantley LG: Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 136: 337–345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumer M, Colombel MC, Sawczuk IS, Gobé G, Connor J, O’Toole KM, Olsson CA, Wise GJ, Buttyan R: Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol 140: 831–838, 1992 [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwana H, Terada Y, Kobayashi T, Okado T, Penninger JM, Irie-Sasaki J, Sasaki T, Sasaki S: The phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int 73: 430–445, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Xie L, Zheng X, Qin J, Chen Z, Jin Y, Ding W: Role of PI3-kinase/Akt signalling pathway in renal function and cell proliferation after renal ischaemia/reperfusion injury in mice. Nephrology (Carlton) 11: 207–212, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR: Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A 98: 247–252, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J: PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature 370: 71–75, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Lieberthal W, Fuhro R, Andry CC, Rennke H, Abernathy VE, Koh JS, Valeri R, Levine JS: Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol 281: F693–F706, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Weng QP, Andrabi K, Klippel A, Kozlowski MT, Williams LT, Avruch J: Phosphatidylinositol 3-kinase signals activation of p70 S6 kinase in situ through site-specific p70 phosphorylation. Proc Natl Acad Sci U S A 92: 5744–5748, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH: G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol 287: C281–C291, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Pallet N, Thervet E, Le Corre D, Knebelmann B, Nusbaum P, Tomkiewicz C, Meria P, Flinois JP, Beaune P, Legendre C, Anglicheau D: Rapamycin inhibits human renal epithelial cell proliferation: Effect on cyclin D3 mRNA expression and stability. Kidney Int 67: 2422–2433, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Wullschleger S, Loewith R, Hall MN: TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Schmitt R, Marlier A, Cantley LG: Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol 19: 2375–2383, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Chen JK, Harris RC: Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.