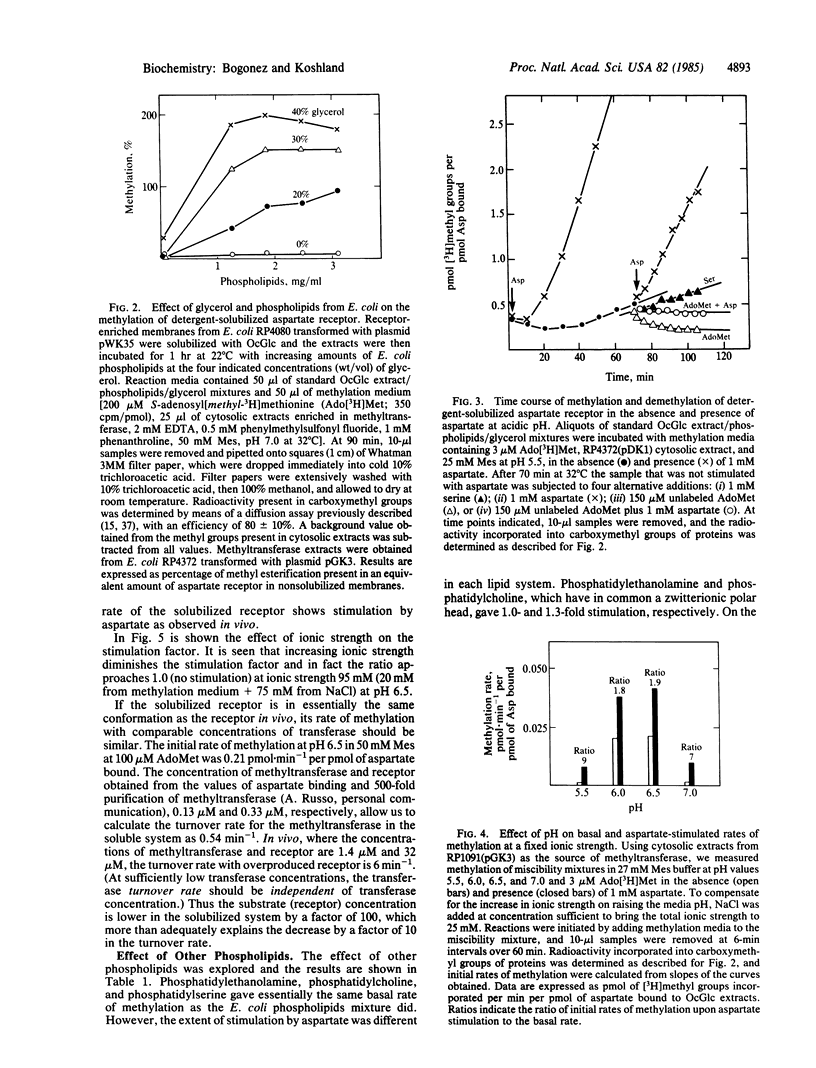
Abstract
The aspartate receptor, an integral membrane protein in the bacterial chemosensory system, has been solubilized in functional form by a combination of detergent, phospholipid, and glycerol. The conformation of the solubilized receptor is the same as that of the protein in vivo, as indicated by aspartate binding, rates of methyl esterification, and quantitative correlation of stimulus with this covalent modification. Studying the functional solubilized receptor in a homogeneous solution avoids many difficulties associated with an in vivo or a vesicle-reconstituted receptor. The technique of adding lipids, detergent, and glycerol to solubilize the protein in active form appears to be generally applicable.
Full text
PDF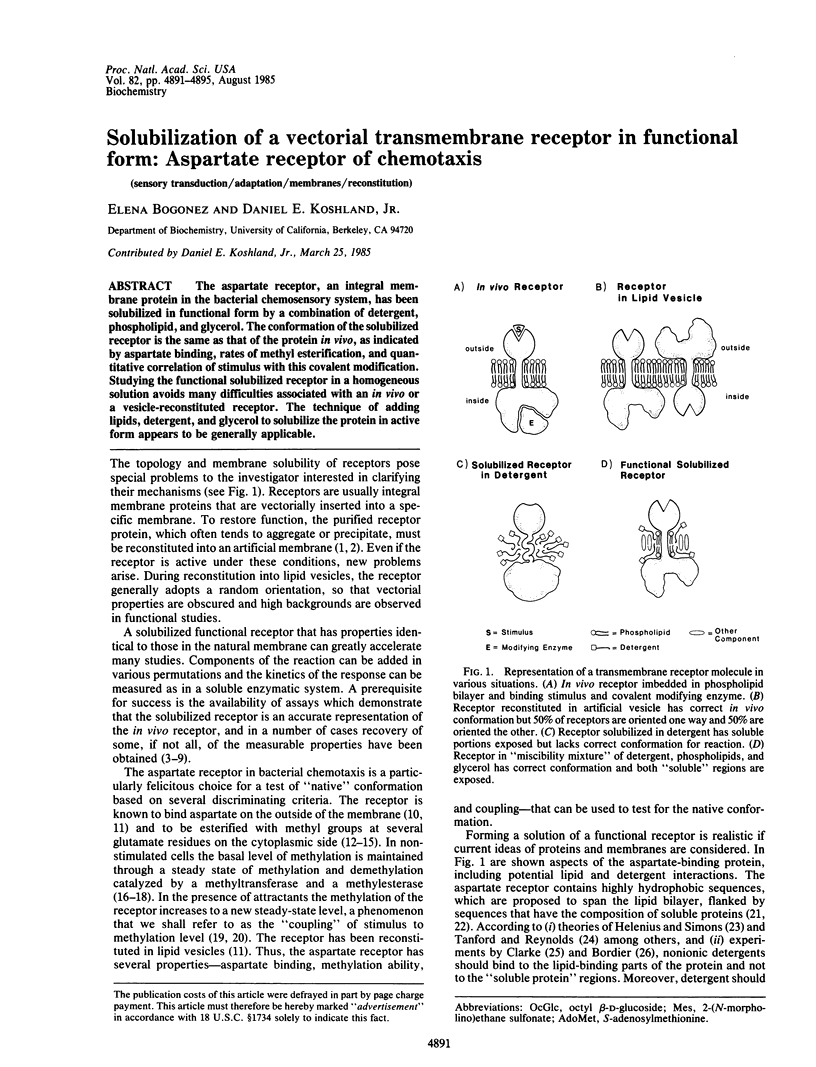



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R., Scarborough G. A. Solubilization and purification of the Neurospora plasma membrane H+-ATPase. J Biol Chem. 1981 Dec 25;256(24):13165–13171. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bowman B. J., Blasco F., Slayman C. W. Purification and characterization of the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12343–12349. [PubMed] [Google Scholar]
- Cerione R. A., Strulovici B., Benovic J. L., Lefkowitz R. J., Caron M. G. Pure beta-adrenergic receptor: the single polypeptide confers catecholamine responsiveness to adenylate cyclase. Nature. 1983 Dec 8;306(5943):562–566. doi: 10.1038/306562a0. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Gutterson N. I., Koshland D. E., Jr A diffusion assay for detection and quantitation of methyl-esterified proteins on polyacrylamide gels. Anal Biochem. 1984 Aug 15;141(1):143–148. doi: 10.1016/0003-2697(84)90437-8. [DOI] [PubMed] [Google Scholar]
- Clarke S., Koshland D. E., Jr Membrane receptors for aspartate and serine in bacterial chemotaxis. J Biol Chem. 1979 Oct 10;254(19):9695–9702. [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- DeFranco A. L., Koshland D. E., Jr Molecular cloning of chemotaxis genes and overproduction of gene products in the bacterial sensing system. J Bacteriol. 1981 Aug;147(2):390–400. doi: 10.1128/jb.147.2.390-400.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Formation and properties of bacteriorhodopsin monomers in the non-ionic detergents octyl-beta-D-glucoside and Triton X-100. FEBS Lett. 1978 Dec 15;96(2):322–326. doi: 10.1016/0014-5793(78)80427-x. [DOI] [PubMed] [Google Scholar]
- Epstein M., Racker E. Reconstitution of carbamylcholine-dependent sodium ion flux and desensitization of the acetylcholine receptor from Torpedo californica. J Biol Chem. 1978 Oct 10;253(19):6660–6662. [PubMed] [Google Scholar]
- Gal A., Braun S., Feder D., Levitzki A. Reconstitution of a functional beta-adrenergic receptor using cholate and a novel method for its functional assay. Eur J Biochem. 1983 Aug 1;134(2):391–396. doi: 10.1111/j.1432-1033.1983.tb07580.x. [DOI] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Sensory transduction in Escherichia coli: role of a protein methylation reaction in sensory adaptation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4964–4968. doi: 10.1073/pnas.74.11.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Kehry M. R., Bond M. W., Hunkapiller M. W., Dahlquist F. W. Enzymatic deamidation of methyl-accepting chemotaxis proteins in Escherichia coli catalyzed by the cheB gene product. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3599–3603. doi: 10.1073/pnas.80.12.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S. J., Toews M. L., Adler J. Isolation of glutamic acid methyl ester from an Escherichia coli membrane protein involved in chemotaxis. J Biol Chem. 1977 May 25;252(10):3214–3218. [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- Liscia D. S., Alhadi T., Vonderhaar B. K. Solubilization of active prolactin receptors by a nondenaturing zwitterionic detergent. J Biol Chem. 1982 Aug 25;257(16):9401–9405. [PubMed] [Google Scholar]
- London E., Khorana H. G. Denaturation and renaturation of bacteriorhodopsin in detergents and lipid-detergent mixtures. J Biol Chem. 1982 Jun 25;257(12):7003–7011. [PubMed] [Google Scholar]
- McIntosh D. B., Davidson G. A. Effects of nonsolubilizing and solubilizing concentrations of Triton X-100 on Ca2+ binding and Ca2+-ATPase activity of sarcoplasmic reticulum. Biochemistry. 1984 Apr 24;23(9):1959–1965. doi: 10.1021/bi00304a012. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Houts S. E. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982 Jul;151(1):106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue J. F., Chan J. K., Thibault C., Radaj P., Mills B., Daughaday W. H. The biochemical characterization of detergent-solubilized insulin-like growth factor II receptors from rat placenta. J Biol Chem. 1983 Jun 25;258(12):7800–7811. [PubMed] [Google Scholar]
- Russo A. F., Koshland D. E., Jr Separation of signal transduction and adaptation functions of the aspartate receptor in bacterial sensing. Science. 1983 Jun 3;220(4601):1016–1020. doi: 10.1126/science.6302843. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Protein methylation in behavioural control mechanisms and in signal transduction. Nature. 1979 Jul 26;280(5720):279–284. doi: 10.1038/280279a0. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Zanolari B., Pierzchala P. A. Ordered methylation of the methyl-accepting chemotaxis proteins of Escherichia coli. J Biol Chem. 1982 Jun 25;257(12):6861–6866. [PubMed] [Google Scholar]
- Steiner M., Oesterhelt D. Isolation and properties of the native chromoprotein halorhodopsin. EMBO J. 1983;2(8):1379–1385. doi: 10.1002/j.1460-2075.1983.tb01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Koshland D. E., Jr Changing reactivity of receptor carboxyl groups during bacterial sensing. J Biol Chem. 1981 Nov 10;256(21):10826–10833. [PubMed] [Google Scholar]
- Stock J. B., Maderis A. M., Koshland D. E., Jr Bacterial chemotaxis in the absence of receptor carboxylmethylation. Cell. 1981 Nov;27(1 Pt 2):37–44. doi: 10.1016/0092-8674(81)90358-5. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Bogonez E., Wang E. A., Koshland D. E., Jr Sites of methyl esterification on the aspartate receptor involved in bacterial chemotaxis. J Biol Chem. 1983 Aug 25;258(16):9608–9611. [PubMed] [Google Scholar]
- Terwilliger T. C., Koshland D. E., Jr Sites of methyl esterification and deamination on the aspartate receptor involved in chemotaxis. J Biol Chem. 1984 Jun 25;259(12):7719–7725. [PubMed] [Google Scholar]
- Van Der Werf P., Koshland D. E., Jr Identification of a gamma-glutamyl methyl ester in bacterial membrane protein involved in chemotaxis. J Biol Chem. 1977 Apr 25;252(8):2793–2795. [PubMed] [Google Scholar]
- Wang E. A., Koshland D. E., Jr Receptor structure in the bacterial sensing system. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7157–7161. doi: 10.1073/pnas.77.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]


