Abstract
Rationale
Ventricular enlargement is a robust phenotype of the chronically dependent alcoholic human brain, yet the mechanism of ventriculomegaly is unestablished. Heterogeneous stock Wistar rats administered binge EtOH (3 g/kg intragastrically every 8 h for 4 days to average blood alcohol levels (BALs) of 250 mg/dL) demonstrate profound but reversible ventricular enlargement and changes in brain metabolites (e.g., N-acetylaspartate (NAA) and choline-containing compounds (Cho)).
Objectives
Here, alcohol-preferring (P) and alcohol-nonpreferring (NP) rats systematically bred from heterogeneous stock Wistar rats for differential alcohol drinking behavior were compared with Wistar rats to determine whether genetic divergence and consequent morphological and neurochemical variation affect the brain’s response to binge EtOH treatment.
Methods
The three rat lines were dosed equivalently and approached similar BALs. Magnetic resonance imaging and spectroscopy evaluated the effects of binge EtOH on brain.
Results
As observed in Wistar rats, P and NP rats showed decreases in NAA. Neither P nor NP rats, however, responded to EtOH intoxication with ventricular expansion or increases in Cho levels as previously noted in Wistar rats. Increases in ventricular volume correlated with increases in Cho in Wistar rats.
Conclusions
The latter finding suggests that ventricular volume expansion is related to adaptive changes in brain cell membranes in response to binge EtOH. That P and NP rats responded differently to EtOH argues for intrinsic differences in their brain cell membrane composition. Further, differential metabolite responses to EtOH administration by rat strain implicate selective genetic variation as underlying heterogeneous effects of chronic alcoholism in the human condition.
Keywords: Alcoholism, Genetics, Selective breeding, Ventriculomegaly, Choline, Magnetic resonance (MR) imaging, MR spectroscopy
Introduction
Magnetic resonance imaging (MRI) of the brains of chronically dependent alcoholic human adults commonly reveals a robust phenotype of ventriculomegaly (for review, Oscar-Berman and Marinkovic 2007; Sullivan and Pfefferbaum 2005). Abstinence is associated with at least partial reversibility of ventricular enlargement (Pfefferbaum et al. 1995; Zipursky et al. 1989). The mechanisms of alcohol-induced brain changes, including reversible ventriculomegaly, however, remain controversial. Early brain recovery through abstinence, once considered to reflect rehydration (e.g., Goldstein 1983; Mann et al. 1993), is now hypothesized to reflect white matter remylination (e.g., Ruiz et al. 2013). In vivo study of animal models, by permitting longitudinal quantification of brain changes, their development and progression, as well as their potential resolution, can help determine the mechanisms underlying acute versus chronic effects of ethanol (EtOH) with the potential of identifying targets for therapeutics.
Longitudinal MRI of heterogeneous stock Wistar rats before (baseline), during (binge), and after (recovery) exposure to binge EtOH (i.e., 3 g/kg every 8 h for 4 days) to blood alcohol levels (BALs) of 258 mg/dL showed ventricular enlargement at the binge time point to 115 % of baseline (Zahr et al. 2010b); BALs of 292 mg/dL resulted in ventricular enlargement to 122 % of baseline (Zahr et al. 2013). Ventricular volume returned to baseline with 1 week of recovery. By contrast, chronic EtOH treatment via vapor chambers to BALs of 444 mg/dL achieved over 24 weeks resulted in only marginal ventricular expansion to 30 % above baseline when evaluated at the 24-week time point (Pfefferbaum et al. 2008). Differences in the volume of ventricular expansion argue for adaptive mechanisms in chronic versus acute exposure to EtOH.
MR spectroscopy (MRS) of the same groups of binge EtOH exposed rats revealed significantly lower levels of N-acetylaspartate (NAA) and higher levels of choline-containing compounds (Cho) at the binge compared with the baseline time point that were completely reversed with 1 week of recovery (Zahr et al. 2010b, 2013). Total creatine (tCr) levels were also reversibly lower in both groups of EtOH-exposed rats at the binge time point, but reached significance only in one of the two studies (i.e., Zahr et al. 2010b). In the chronic exposure model, decreases in NAA and tCr in response to EtOH treatment were moderate and did not reach significance, while increases in Cho were significant (Zahr et al. 2009). Although widely used as in vivo markers in clinical and research settings, the functions of MRS-detectable NAA, tCr, and Cho remain largely speculative. There are few, if any, pharmacological treatments that can directly modulate the levels of these metabolically important neurochemicals.
Selective, systematic, bi-directional breeding of rats for EtOH preference over successive generations has resulted in divergent lines that exhibit the extremes of EtOH preference without environmental manipulations (cf., Bell et al. 2012). Alcohol-preferring (P) and alcohol-nonpreferring (NP) lines of rats were developed by selection from a Wistar foundational stock at Indiana University School of Medicine in Indianapolis, IN, USA (Lumeng et al. 1977). P rats drink greater than 5 g/kg/day, whereas NP rats drink less than 1 g/kg/day EtOH under similar conditions (Lankford et al. 1991; Li et al. 1987). P rats satisfy criteria for an animal model of alcoholism (Cicero et al. 1971; Lester and Freed 1973) because they (1) orally self-administer EtOH, (2) consume EtOH to pharmacologically relevant BALs, (3) consume EtOH for its post-ingestive pharmacological effects (not strictly for caloric value or taste), (4) work for EtOH (positive reinforcement), (5) express metabolic and functional tolerance, and (6) show dependence, as indicated by withdrawal symptoms after access to EtOH is terminated (e.g., Bell et al. 2001, 2006a, 2012; Kampov-Polevoy et al. 2000; McBride and Li 1998; Murphy et al. 2002; Rodd-Henricks et al. 2000).
Compared with NP rats, P rats have altered serotonergic (Murphy et al. 1987, 1988; Weiss et al. 1993; Zhou et al. 1991), dopaminergic (McBride et al. 1993; Murphy et al. 1987; Zhou et al. 1995), GABAergic (Hwang et al. 1990; Thielen et al. 1993, 1997), and opiate (McBride and Li 1998) neurotransmitter systems. Their neuromodulatory systems (e.g., neuropeptide Y and corticotropin-releasing factor, Ehlers et al. 1992, 1998, 1999; Hwang et al. 2004a, b) are also modified. Because multiple neurotransmitter and neuromodulatory systems have been changed in the process of breeding rats with disparate alcohol-drinking behaviors (cf., Bell et al. 2012), it was predicted that these three rat lines would also demonstrate intrinsic differences in levels of MRS-detectable neurochemicals and, as a consequence, show differential responsiveness to binge EtOH treatment. We proposed that divergent MR findings in P and NP rats compared with heterogeneous stock rats could provide insight into the mechanisms underlying binge EtOH-induced ventricular expansion.
Methods
Animals
This study included two separate experiments: One studied 19 male P rats (339.0±4.5 g), the other 21 male NP rats (383.9±4.3 g, baseline weights) (Indiana University). All animals were singly housed with free access to food and water with lights on for 12 h starting at 8:00 a.m. Results are compared with previously published data from 19 male heterogeneous stock Wistar (W) rats (Charles River Laboratories, 264.5±4.5 g) (Zahr et al. 2010b). Animals used in these experiments were maintained in facilities fully accredited by the Association of the Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committees (IACUC) at SRI International and Stanford University approved all research protocols in accordance with the guidelines of the IACUC of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (The Guide 1996).
Treatment
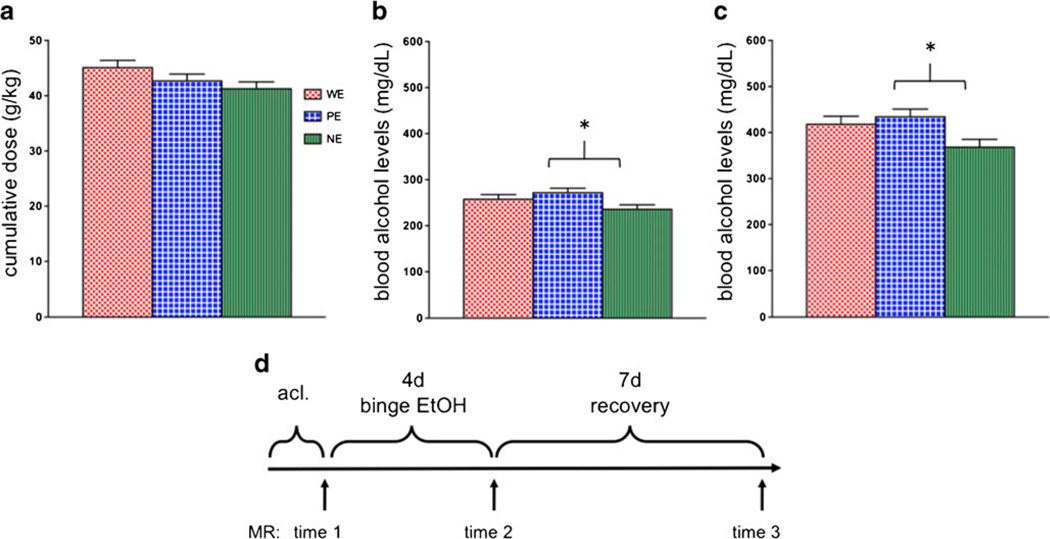
All 59 rats underwent baseline scanning after which 11 W, 12 P, and 12 NP rats were assigned to the EtOH-treatment group. These 35 rats received an initial “loading” dose of 5 g/kg 25% EtOH w/v via oral gavage, then a maximum of 3 g/kg every 8 h for 4 days (supplementary Figs. 1 and 3). On each of the 4 days, animals were weighed; tail vein blood samples were collected four times per day: ~90 min before each dose to determine the level of intoxication before further dosing or 90 min after the second dose of the day (i.e., 15:00) to determine peak BALs (supplementary Figs. 2 and 3). Plasma was assayed for alcohol content based on direct reaction with the enzyme alcohol oxidase (Analox Instruments Ltd., UK). Ethanol was administered according to body weight (supplementary Fig. 4), BALs, and behavioral intoxication state assessed using a modified Majchrowicz scale (range 0–5: 0—neutrality, 1—sedation, 2—mild ataxia, 3—moderate ataxia, 4—severe ataxia, 5—loss of righting reflex) (Majchrowicz 1975). There were no line differences (F(2, 34)=2.28, p =0.1182) in cumulative EtOH dose (i.e., 43.0± 0.76 g/kg/animal) across 4 days of treatment (Fig. 1a). However, a comparison of average BALs across the 4 binge days (i.e., 255.0±6.06 mg/dL) was significant (F(2,34)=3.5, p = 0.0424): P EtOH (PE) had significantly higher average BALs than NP EtOH (NE) rats (p =0.0131) (Fig. 1b). Peak BALs (i.e., 406.5± 10.78), defined as the highest measured BALs for each animal during the 4 days of treatment, were typically based on measurements taken 90 min after dosing. Peak BALs were significantly different across strains (F(2,34)= 4.1, p =0.0259) with PE>NE (p =0.0096) (Fig. 1c). The control (Ctrl) animals (W Ctrl (WC)=8, P Ctrl (PC)=7, N Ctrl (NC)=9) were treated and scanned in the same sessions as their respective EtOH rats and received volumes of 5 % dextrose equivalent to 3 g/kg EtOH at comparable times to the experimental animals, i.e., ~7:00, 15:00, and 23:00. In an attempt to maintain similar weights between animals in the EtOH and Ctrl groups, food was restricted to a maximum of two pellets (Certified Rodent Diet; LabDiet, Richmond, IN, USA) per animal per day. Experience from earlier experiments (e.g., Zahr et al. 2010b) indicated that binge EtOH-treated animals typically consume no more than two pellets per day and therefore lose weight. In an effort to weight-yoke the two groups of animals, all Ctrl animals were limited to two food pellets per day.
Fig. 1.
a Cumulative dose of ethanol (EtOH) across 4 days of treatment. b Average and c peak blood alcohol levels achieved in the 4 days of treatment. d Time course of experiment. Error bars in all figures represent standard error of the mean. *p ≤0.05, WE heterogeneous stock Wistar EtOH-treated rats, PE alcohol preferring EtOH rats, NE alcohol nonpreferring EtOH rats
MR scanning procedures and data analysis
Schedule
Animals were scanned at baseline (time 1), after 4 days of binge EtOH treatment (binge, time 2), and after 7 days of recovery (recovery, time 3; Fig. 1d).
Anesthesia and monitoring
Animals were held in an MR-invisible structure providing support for a radiofrequency (RF) coil and a nose cone for delivery of isoflurane anesthesia (1.5–3 %) and oxygen (1.5 L/min) (Adalsteinsson et al. 2004). For each rat, blood oxygen saturation, pulse rate, rectal temperature, and respiration were monitored throughout the ~2-h MR scan.
MRI acquisition
The scans were conducted on a clinical 3-T GE Signa MR scanner. A custom-made rat brain quadrature head coil (Ø=44 mm) was used for both RF excitation and signal reception. A gradient-recalled echo localizer scan was used to position the animals in the scanner and for graphical prescription of the subsequent scans. High resolution, dualecho, fast spin-echo (FSE) images were acquired in the rat axial plane, coronal to the magnet system bore (TE1/TE2/ TR=11.3/56.7/5,000 ms, field of view=64×48 mm2, 256× 192 matrix, echo train length=8, 50 slices, 0.3 mm thick, 0 mm separation, in-plane resolution=0.25×0.25mm2, four separate acquisitions each with two NEX).
Image post-processing
Motion-corrected FSE images were computed by aligning the second dual-echo acquisition for each animal with the first using rigid (translation and rotation) image-to-image registration of the early-echo channel. The aligned early- and late-echo images were then averaged. From each motion-corrected early-echo image, a second-order multiplicative intensity bias field was then estimated by entropy minimization (Likar et al. 2001). The same bias field was applied to the corresponding late-echo image to preserve quantities derived from the early-to-late echo ratio such as transverse relaxation time (T2).
A preliminary brain mask was computed for each FSE image pair by (1) thresholding the late-echo image at the 99th percentile, (2) eroding the resulting mask by four pixels, (3) computing connected components, (4) selecting the largest connected component, and (5) dilating the resulting region by seven pixels. The purpose of this coarse, slightly enlarged, approximate brain mask was to exclude the majority of non-brain tissue to facilitate alignment of the image to a whole-head image of a template animal, from which the final brain mask would then be derived by label propagation of the manually defined template brain mask.
To this end, baseline data from each animal were aligned with the template animal via a sequence of successively refined image transformations: (1) initial alignment based on principal axes of the whole-head late-echo FSE images, (2) rigid alignment of the whole-head late-echo images, (3) affine alignment of the brain-only late-echo template image and the animal late-echo image masked using the preliminary brain mask, and (4) full nonrigid alignment of the brain-only template image to the whole-head late-echo animal image. Steps 2 through 4 all used maximization of normalized cross correlation as the cost function for alignment, which was implemented to exclude non-brain pixels from computation where applicable, rather than set them to zero. This registration sequence resulted from balancing competing needs, such as the observation that brain alignment accuracy improves when brain-only images are coregistered, yet the quality of the initial animal brain masks is too poor for direct use and would interfere with the alignment.
For longitudinal alignment, each follow-up image was aligned with the baseline image for the same animal by coregistration of the whole-head, late-echo FSE images. Because the anatomy of the non-brain tissue is consistent over time in a single animal (as opposed to between different animals), use of the whole-head images for alignment yields better results compared with brain-only images because it avoids errors that would otherwise arise from longitudinally inconsistent brain masks. All software tools used to perform the processing outlined above were developed in house and are freely available, in source code, as part of the Computational Morphometry Toolkit (http://nitrc.org/projects/cmtk/).
For ventricular quantification, a rectangular template encompassing the majority of the lateral ventricles across seven contiguous slices was drawn on the template brain. This template was reformatted with rigid transformation onto each animal's native FSE images, and all pixels above a uniform threshold (CSF is much brighter than surrounding gray or white matter) were counted as ventricle.
MRS acquisition
FSE images were used to prescribe a voxel (10×4×4 mm3) in the dorsal hippocampus (coordinates according to the atlas of (Paxinos and Watson 2005): 2.0 mm anterior and posterior to −4.0 mm Bregma, 4.9 mm right and left of midline, and 4.0 mm inferior to −3.1 mm Bregma). Single-voxel spectroscopic data were acquired with constant time point-resolved spectroscopy (TE ranging from 36.6 to 241.4 ms, 1.6 ms increment, TR=2 s, six averages) (Dreher and Leibfritz 1999; Mayer and Spielman 2005) preceded by a three-pulse chemical shift selective sequence for water suppression. Additionally, data without water suppression from the same voxel were acquired at multiple TEs (same TE range, 12.8 ms increment, TR=2 s, two averages) to measure tissue water content for normalization of metabolite signal intensities to the amount of tissue water in the voxel (for details, see Zahr et al. 2009). The quality of the spectra allowed evaluation of signals of the major proton metabolites: NAA (2.01 ppm), tCr (3.03 ppm), Cho (3.20 ppm), Glu (2.36 ppm), and EtOH (1.18 ppm). The three singlet resonances (NAA, tCr, and Cho) were fit simultaneously, and the EtOH and Glu resonances fit independently, with a Gaussian function within a ±7.95-Hz window using a downhill simplex method (IDL AMOEBA). The integrated area under the fitted Gaussian was used for quantification.
Behavioral and liver analysis
Neurological examination (e.g., Becker 2000; Roberts et al. 1996; Yaksh et al. 1977) was performed on day 4 of binge and day 4 of recovery. Rats were rated (0 = absent, 1 = present) for the presence of neurological signs in the following categories: autonomic, sensory, anxiety-like, posture, motor, and central.
At euthanasia (2.5–3.5 % isoflurane, followed by decapitation), left lateral lobe liver specimens from all rats were collected and immersed in 10% buffered formalin solution. After fixation, the specimens were routinely processed for light microscopic examination of hematoxylin and eosin stain and Masson’s trichrome stain and evaluated by the veterinary pathologist (RL) for hepatic pathology on a 0 to 4 scale, where 0 = no pathology, 1 = minimal (affects <5% of tissue), 2 = mild (affects 5–20% of tissue), 3 = moderate (affects 20–50% of tissue), and 4 = severe (affects >50 % of tissue) (Zahr et al. 2009).
Statistical analysis
Differences between rat lines were tested with three-line (W, P, N) by two-treatment (Ctrl, EtOH) by three-time-point (baseline = time 1; binge = time 2; recovery = time 3) repeated-measures analyses of variance (ANOVA). Within rat line differences were assessed with two-treatment (Ctrl, EtOH) by three-time-point repeated-measures ANOVA. Rat line effects, treatment effects, and interactions were of interest to this analysis. Follow-up comparisons were conducted with a two-tailed t test. Simple regressions evaluated correlations.
Results
Binge ethanol affects weight
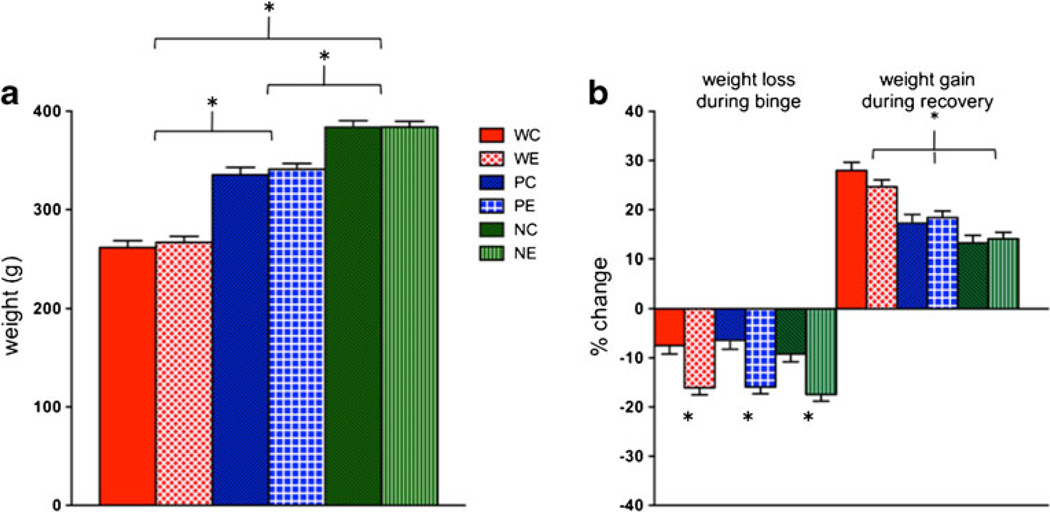
An ANOVA comparing baseline weight differences among the three lines (i.e., W, P, NP) was significant (F(2,56)=187.12, p ≤0.0001): NP>P>W rats (p ≤0.0001) (Fig. 2a). Baseline body weight and thus age differences were linked to logistic and quarantine constraints; however, all were young adults at the initiation of procedures. A two-treatment (EtOH, Ctrl) by three-rat line (W, P, NP) ANOVA comparing weight loss during binge treatment showed a treatment effect (F(1,2)=48.13, p ≤0.0001), but neither a line effect (F(2,2)=1.04, p =0.36) nor an interaction (F(2,2)=0.09, p =0.91). Regardless of line, all animals lost weight during treatment (Fig. 2b) and EtOH animals lost more weight than their Ctrl counterparts (W: p = 0.0003; P: p ≤0.0001; NP: p =0.0003). Weight gain during recovery showed a line effect (F(2,2)=36.98, p ≤0.0001), but neither a treatment effect (F(1,2)=0.14, p =0.71) nor an interaction (F(2,2)=1.31, p =0.28). All animals gained weight during recovery (Fig. 2b), but W rats gained more weight than P (t(36)= 4.82, p ≤0.0001) and NP (t(38)=7.89, p ≤0.0001) rats.
Fig. 2.
a Average baseline weights of the six groups of animals. b Weight loss and gain during binge ethanol (EtOH) exposure and recovery. *p≤0.05, WC heterogeneous stock Wistar control (Ctrl) rats, WE heterogeneous stock Wistar EtOH-treated rats, PC alcohol preferring P rat Ctrl, PE P EtOH rats, NC alcohol nonpreferring NP rat Ctrl, NE NP EtOH rats, Acl acclimation
Binge ethanol affects behavior
In each of these three experiments, EtOH rats exhibited similar behavioral changes during binge and recovery that differed from Ctrls (Table 1). On binge day 4, WE compared with WC rats showed dehydrated stool (t(17)=5.68, p ≤0.0001), sat motionless (t(17)=2.93, p = 0.0093), and had gait disturbances (t(17)=2.70, p =0.0151). Despite resolution of behavioral differences during recovery, WE on recovery day 4 were more irritable (t(17)=2.44, p =0.0258) and likely to vocalize when handled (t(17)=3.06, p =0.0071) than WC rats.
Table 1.
Behavioral signs
| WE | PE | NE | |
|---|---|---|---|
| Binge | |||
| Dehydrated stool | 0.0001 | 0.0005 | n.s. |
| Motionless | 0.0093 | 0.0025 | 0.0001 |
| Gait disturbances | 0.0151 | 0.0001 | 0.0001 |
| Chromodacryorrhea | n.s. | 0.0258 | 0.0032 |
| Heightened startle response | n.s. | 0.0228 | 0.0580 |
| Reduced trunk tone | n.s. | 0.0228 | 0.0662 |
| Loss of righting reflex | n.s. | 0.0001 | 0.0001 |
| Recovery | |||
| Irritable | 0.0258 | 0.0005 | n.s. |
| Likely to vocalize | 0.0071 | 0.0087 | n.s. |
| Chromodacryorrhea | n.s. | n.s. | 0.0112 |
t test results compared with corresponding Ctrl animals
WE heterogeneous stock Wistar rats exposed to EtOH, PE alcohol preferring rats exposed to EtOH, NE alcohol nonpreferring rats exposed to EtOH
Similarly to the WE rats, on binge day 4, PE rats showed dehydrated stool (t(17)=4.33, p =0.0005), sat motionless (t(17)=3.54, p =0.0025), and had gait disturbances (t(17)= 5.60, p ≤0.0001) compared with PC rats. Unlike WE rats, PE rats during binge EtOH treatment also showed chromodacryorrhea (t(17)=2.44, p =0.0258), heightened startle response (t(17)=2.50, p =0.0228), reduced trunk tone (t(17)= 2.50, p =0.0228), and loss of righting reflex (t(17)=5.5, p ≤ 0.0001). Again, by day 4 of recovery, these behaviors no longer distinguished PE and PC rats; instead, as observed for W rats, PE rats were more irritable (t(17)=4.33, p =0.0005) and likely to vocalize when handled (t(17)=2.96, p =0.0087) than PC rats.
The pattern of behavioral changes was similar for the comparison of NE and NC rats at binge day 4: NE rats sat motionless (t(19)=9.32, p ≤0.0001), had gait disturbances (t(19)=4.94, p ≤0.0001), showed chromodacryorrhea (t(19)= 3.38, p =0.0032), loss of righting reflex (t(19)=9.46, p ≤ 0.0001), and a trend toward differences in startle response (t(19)=2.02, p =0.0580) and trunk tone (t(19)=1.95, p = 0.0662). While chromodacryorrhea persisted in NE compared with NC rats (t(19)=3.42, p =0.0112), none of the other neurological signs distinguished NP rats at day 4 of recovery.
Binge ethanol does not affect the liver
Postmortem histopathology provided no evidence for alcohol-related hepatic steatosis, hepatitis, or cirrhosis. All EtOH-treated animals scored an average close to 0 (i.e., no pathology) on a number of hepatic pathology variables: hepatocyte swelling or necrosis, mallory bodies, inflammation (neutrophilic parenchymal), fibrosis (sinusoidal, centrilobular, portal–portal, or central–portal), and regeneration (microscopic or macroscopic). For the remaining variables, i.e., hepatocellular glycogenosis, microvesicular and macrovesicular lipidosis, inflammation (lympho-histiocytic portocentric), bile duct proliferation, and portocentric fibrosis, EtOH-treated animals scored averages close to 1 indicating minimal pathology, but there were no meaningful line or treatment effects.
Differential effects of binge ethanol on ventricular volume
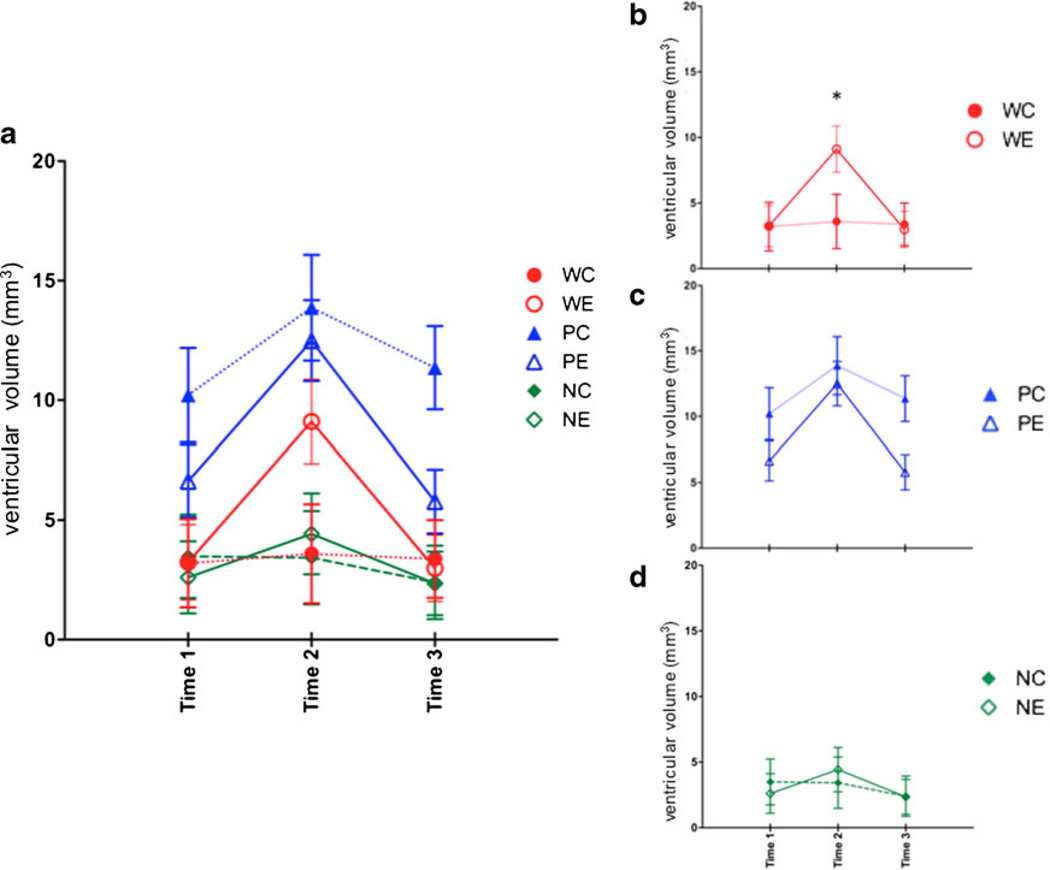
For ventricular volume, a two-treatment (EtOH, Ctrl) by three-line (W, P, NP) repeated measures (baseline = time 1; binge = time 2; recovery = time 3) ANOVA showed line (F(2, 53)=10.25, p =0.0002), time (F(2,106)=71.46, p ≤0.0001), and interaction effects (treatment by time: F(2,106)=20.92, p ≤0.0001; line by time: F(4,106)=8.82, p ≤0.0001; treatment by line by time: F(4,106)=3.80, p =0.0063). The simple treatment effect was not significant (F(1,53)=0.0048, p = 0.9448). At baseline, P rats had larger ventricles than either W (p =0.005) or NP (p =0.0025) rats (Fig. 3a). As previously reported (Zahr et al. 2010b) and replicated (Zahr et al. 2013), WE rats showed a pattern of profound but reversible ventricular enlargement (Fig. 3b), such that ventricular volume was larger in WE than WC rats at binge (t(17)=2.68, p = 0.0159) and larger in WE rats at binge than at baseline (t(10)=6.54, p ≤0.0001) and recovery (t(10)=−5.76, p = 0.0002). This pattern was not observed in P rats (Fig. 3c): There were no treatment differences at any time point, and ventricles were larger in both PE and PC rats at binge than at baseline (PE: t(11)=4.64, p =0.0007; PC: t(6)=4.49, p =0.0042) and recovery (PE: t(11)=−5.97, p ≤ 0.0001; PC: t(6)=−4.48, p =0.0042). Whereas NP rats also did not show treatment differences at any time point (Fig. 3d), ventricles of NE rats were larger at binge relative to baseline (t(11)=5.45, p =0.0002) and recovery (t(11)= −4.64, p ≤0.0001).
Fig. 3.
a Ventricular volume at the three time points in the six groups of animals. Time 1 baseline, time 2 binge, time 3 recovery. b Changes in ventricular volume for just heterogeneous stock Wistar rats, c for P rats, and d for NP rats. *p≤0.05, WC heterogeneous stock Wistar control (Ctrl) rats, WE heterogeneous stock Wistar EtOH-treated rats, PC alcohol preferring P rat Ctrl, PE P EtOH rats, NC alcohol nonpreferring NP rat Ctrl, NE NP EtOH rats
Differential effects of binge ethanol on proton metabolite levels
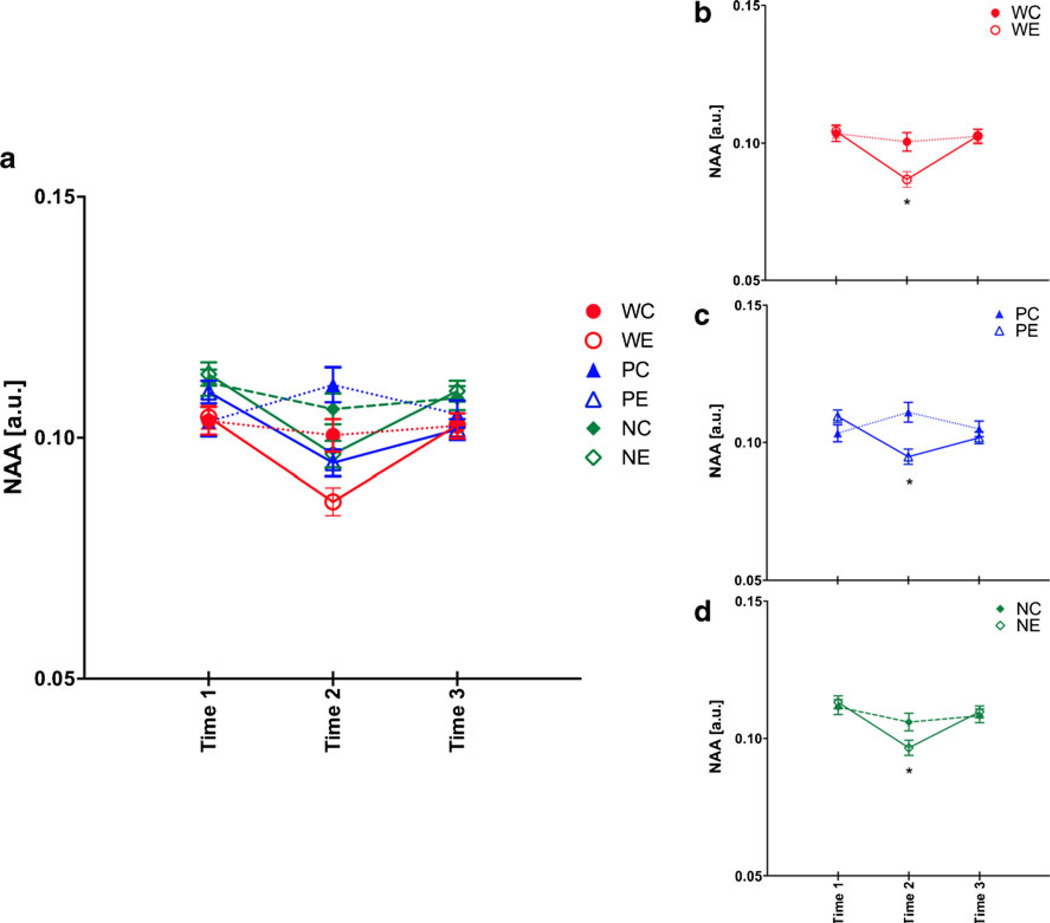
For NAA, a two-treatment by three-line repeated measures ANOVA showed treatment (F(1,53)=7.40, p =0.0088), line (F(2,53)=11.15, p ≤0.0001), time (F(2,106)=14.47, p ≤ 0.0001), and treatment-by-time interactions (F(2,106)=14.37, p ≤0.0001). At baseline, NP rats had higher levels of NAA than W (p =0.0017) and P (p =0.0474) rats (Fig. 4a). The pattern of NAA changes for W rats was as previously reported (Zahr et al. 2010b) and replicated (Zahr et al. 2013): WE rats showed reversible decreases in NAA (Fig. 4b), with NAA levels lower in WE than WC rats at binge (t(17)=5.23, p ≤0.0001) and lower in WE rats at binge than at baseline (t(10)=−4.38, p = 0.0014) and recovery (t(10)=6.83, p ≤0.0001). Both PE (Fig. 4c) and NE (Fig. 4d) rats demonstrated the same pattern of reversible NAA decreases. NAA levels at binge were lower in PE than PC rats (t(17)=3.33, p =0.004). NAA levels in PE rats at binge were lower than at baseline (t(11)=−5.01, p = 0.0004) but were not statistically lower than at recovery (t(11)= 1.59, p =0.1412). The lower NAA levels at binge in the NE than NC rats were not significant (t(19)=1.86, p =0.0785). Within NE rats, NAA levels at binge were lower than at baseline (t(11)=−5.13, p =0.0004) and recovery (t(11)=2.46, p =0.0336).
Fig. 4.
a N-Acetylaspartate (NAA) levels at the three time points in the six groups of animals. Time 1 baseline, time 2 binge, time 3 recovery. b Changes in NAA for just heterogeneous stock Wistar rats,c for P rats, and d for NP rats. *p ≤0.05, WC heterogeneous stock Wistar control (Ctrl) rats, WE heterogeneous stock Wistar EtOH-treated rats, PC alcohol preferring P rat Ctrl, PE P EtOH rats, NC alcohol nonpreferring NP rat Ctrl, NE NP EtOH rats
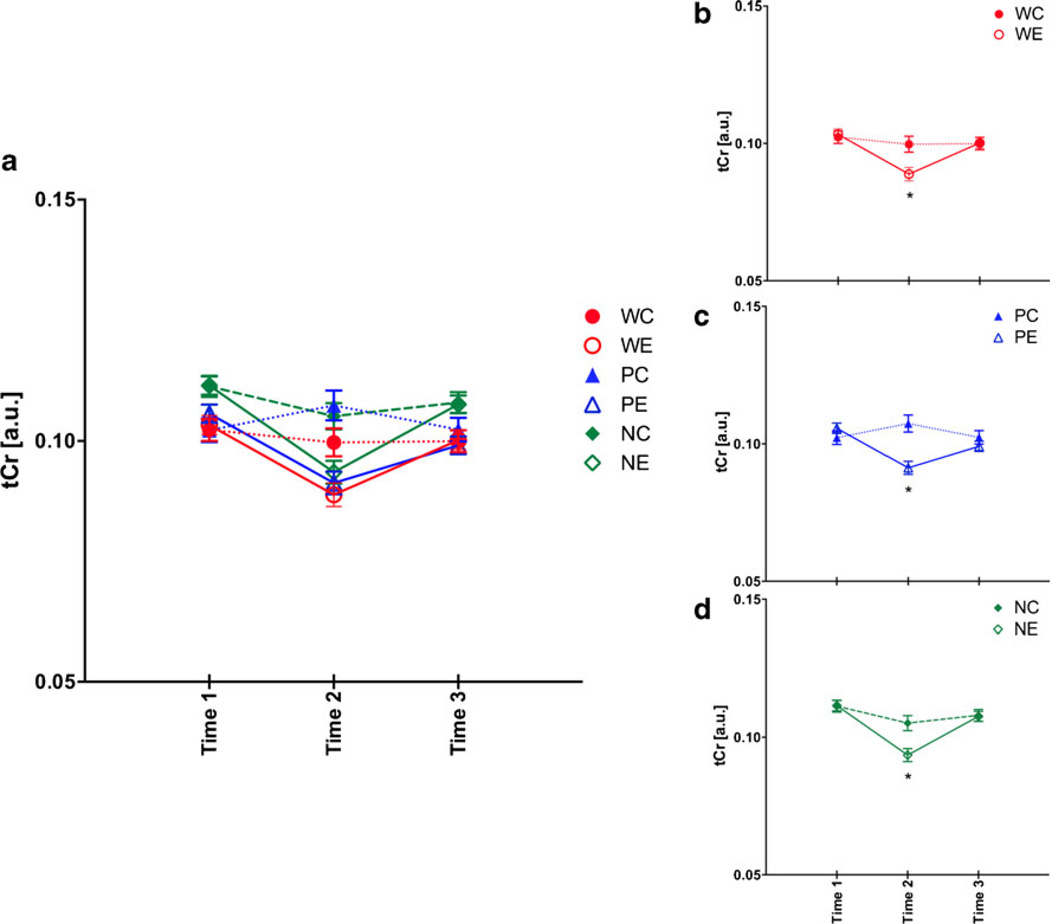
For tCr, a two-treatment by three-line repeated measures ANOVA showed treatment (F(1,53)=13.91, p =0.0005), line (F(2,53)=14.79, p ≤0.0001), time (F(2,106)=20.80, p ≤ 0.0001), and treatment-by-time interactions (F(2,106)= 16.90, p ≤0.0001). At baseline, NP rats had higher levels of tCr than W (p ≤0.0001) and P (p =0.0011) rats (Fig. 5a). The pattern of tCr changes for W rats was as previously reported (Zahr et al. 2010b): WE rats showed reversible decreases in tCr (Fig. 5b), with tCr levels lower in WE than WC rats at binge (t(17)=3.24, p =0.0048) and lower at binge than at baseline (t(10)=−3.84, p =0.0033) and recovery (t(10)= 4.81, p =0.0007). Both P (Fig. 5c) and NP (Fig. 5d) rats demonstrated the same pattern of reversible tCr decreases. tCr levels at binge were lower in PE compared with PC rats (t(17)=5.45, p ≤0.0001) and lower than at baseline (t(11)= −4.86, p =0.0005) and recovery (t(11)=2.63, p =0.0232). Similarly, NE rats at binge had lower tCr levels (t(19)=2.58, p =0.0184) than NC rats and lower levels at binge than at baseline (t(11)=−5.35, p =0.0002) and recovery (t(11)=2.95, p =0.0132).
Fig. 5.
a Total creatine (tCr ) levels at the three time points in the six groups of animals. Time 1 baseline, time 2 binge, time 3 recovery. b Changes in tCr for just heterogeneous stock Wistar rats, c for P rats, and d for NP rats. *p ≤0.05, WC heterogeneous stock Wistar control (Ctrl) rats, WE heterogeneous stock Wistar EtOH-treated rats, PC alcohol preferring P rat Ctrl, PE P EtOH rats, NC alcohol nonpreferring NP rat Ctrl, NE NP EtOH rats
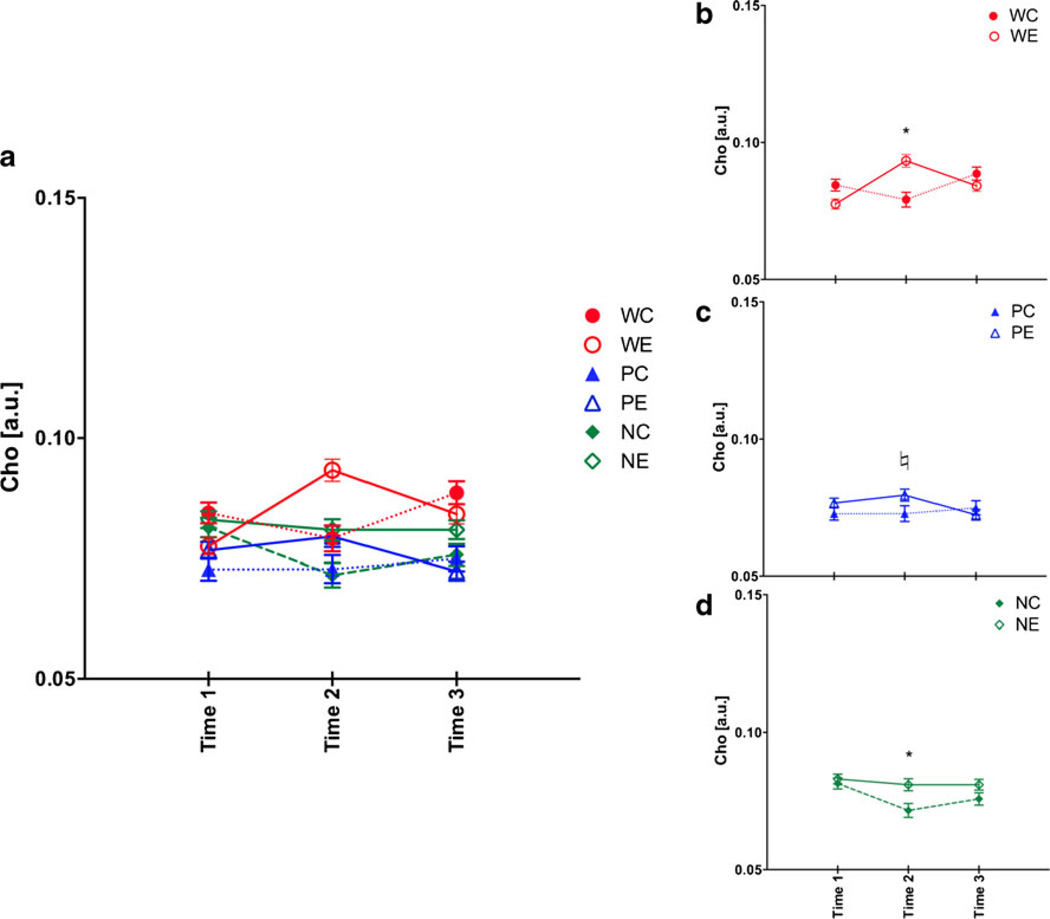
For Cho, a two-treatment by three-line repeated measures ANOVA showed treatment (F(1,53)=7.39, p =0.0088), line (F(2,53)=24.64, p ≤0.0001), treatment-by-time (F(2,106)= 12.12, p ≤0.0001), line-by-time (F(4,106)=4.48, p =0.0022), and treatment-by-line-by-time (F(4,106)=3.06, p =0.0198) interactions. At baseline, P rats had lower Cho levels than W (p =0.0145) and NP (p =0.0009) rats (Fig. 6a). The pattern of Cho changes for W rats was as previously reported (Zahr et al. 2010b) and replicated (Zahr et al. 2013): WE rats showed reversible increases in Cho (Fig. 6b), with Cho levels higher in WE than WC rats at binge (t(17)=3.48, p =0.0029) even though at baseline WE had significantly lower Cho than WC rats (t(17)=2.4, p =0.0275). WE rats at binge had higher Cho levels than at baseline (t(10)=6.78, p ≤0.0001) and recovery (t(10)=−2.64, p =0.0246). Both PE (Fig. 6c) and NE (Fig. 6d) rats appeared to have reversible Cho increases, but the pattern of changes were not as expected. Cho levels tended to differentiate PE and PC rats at binge (t(17)=1.97, p =0.0649). Although Cho levels in PE rats at binge were higher than at recovery (t(11)=−2.42, p =0.0340), they were not higher than at baseline (t(11)=0.82, p =0.4310). NE compared with NC rats had higher Cho levels at binge (t(19)=3.16, p =0.0052), but this was due to changes in the NC group: Cho levels were lower in the NC group at binge compared to baseline (t(8)= −3.91, p =0.0052) and recovery (t(8)=2.55, p =0.0340).
Fig. 6.
a Choline-containing compounds (Cho) levels at the three time points in the six groups of animals. Time 1 baseline, time 2 binge, time 3 recovery. b Changes in Cho for just heterogeneous stock Wistar rats, c for P rats, and d for NP rats. *p ≤0.05, ♮p =0.0649, WC heterogeneous stock Wistar control (Ctrl) rats, WE heterogeneous stock Wistar EtOH-treated rats, PC alcohol preferring P rat Ctrl, PE P EtOH rats, NC alcohol nonpreferring NP rat Ctrl, NE NP EtOH rats
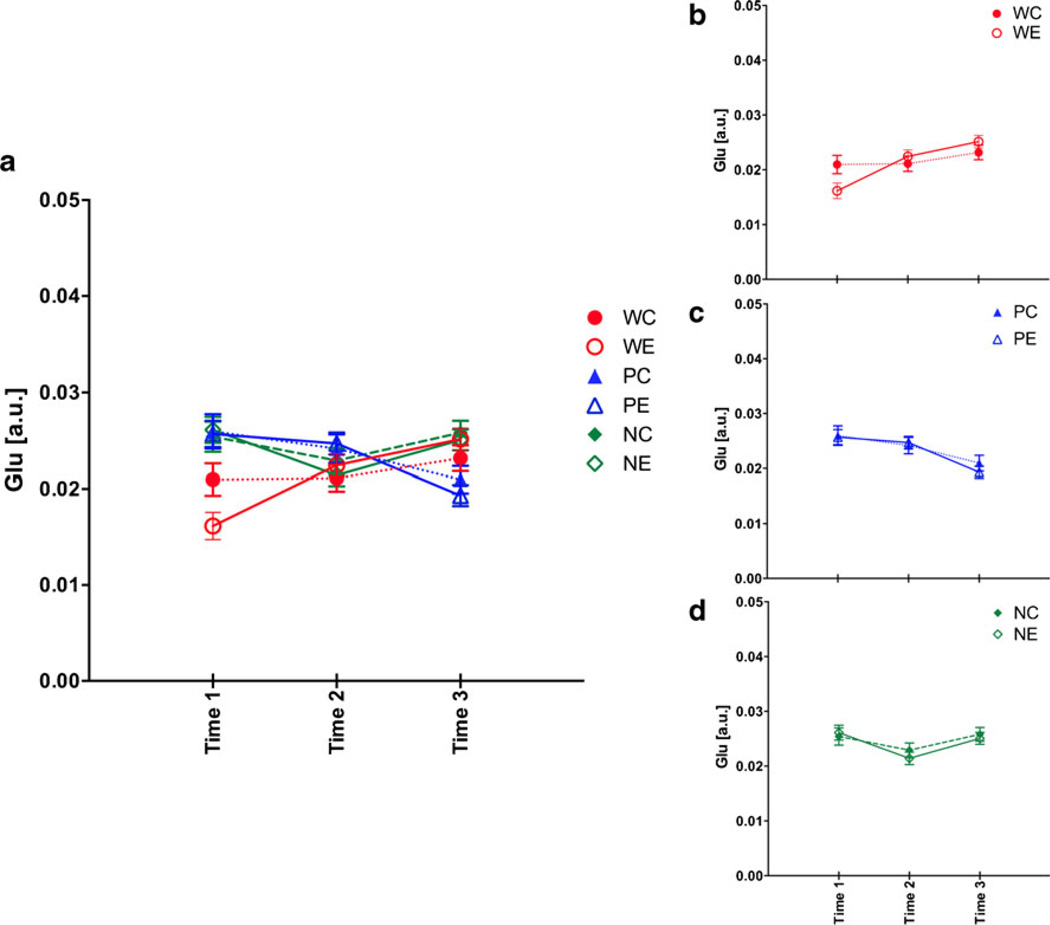
For Glu, a two-treatment by three-line repeated measures ANOVA showed only a line (F(2,53)=7.51, p =0.0013) and a line-by-time interaction (F(4,106)=10.88, p ≤0.0001). At baseline, W rats had lower Glu than P (p ≤0.0001) and NP (p ≤0.0001) rats (Fig. 7). Glu levels were not affected by binge EtOH treatment in W (Zahr et al. 2010b, 2013), P, or NP rats.
Fig. 7.
a Glutamate (Glu) levels at the three time points in the six groups of animals. Time 1 baseline, time 2 binge, time 3 recovery. b Changes in Glu for just heterogeneous stock Wistar rats, c for P rats, and d for NP rats. *p ≤0.05, WC heterogeneous stock Wistar control (Ctrl) rats, WE heterogeneous stock Wistar EtOH-treated rats, PC alcohol preferring P rat Ctrl, PE P EtOH rats, NC alcohol nonpreferring NP rat Ctrl, NE NP EtOH rats
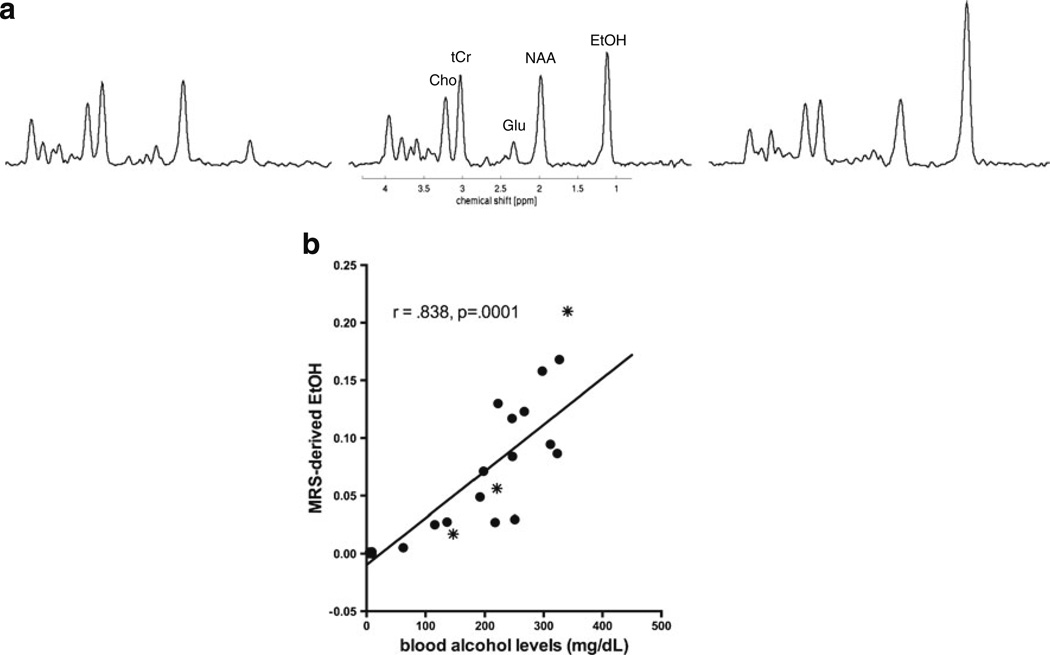
For MRS-derived brain EtOH levels (Fig. 8a), an ANOVA showed treatment (F(1,53)=27.38, p ≤0.0001), time (F(2, 106)=27.02, p ≤0.0001), and treatment-by-time interactions (F(2,106)=28.03, p ≤0.0001). In all three studies, EtOH levels were equivalent between groups at baseline and recovery, but differentiated EtOH from Ctrl animals at binge (W: t(17)=3.02, p =0.0077; P: t(17)=1.82, p =0.0857; NP: t(19)=4.43, p = 0.0003). In PE and NE animals, in which BALs were quantified at the time of the binge (time 2) scan, MRS-derived EtOH levels correlated with BALs (r =0.838, p =.0001) (Fig. 8b).
Fig. 8.
a MR spectra from three different NE animals showing varying MRS-detectable EtOH levels in brain. b Correlation between MRS-derived EtOH levels and BALs in PE and NE animals. The stars indicate the three animals whose spectra are displayed in a
Relationship between changes in ventricular volume and choline levels
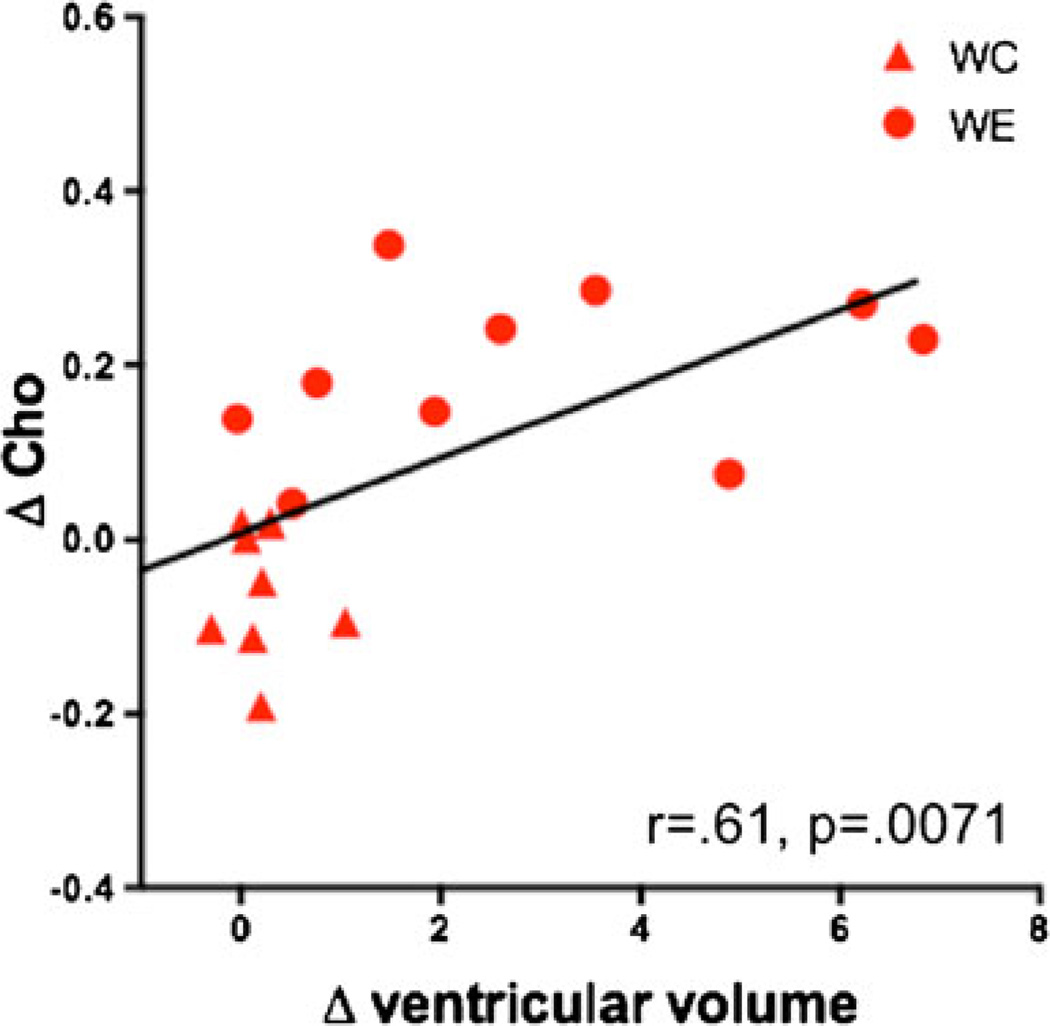
The results of binge EtOH treatment on changes in ventricular volume and in metabolite levels in the three rat lines are summarized in Table 2. Of note, ventricular volume expansion and increases in Cho levels were unique to W rats. Based on this observation, the relationship with regard to percent change between the baseline and binge time points in ventricular volume and Cho was evaluated in the three rat lines. Only W rats showed a relationship between these two variables (r = 0.61, p =0.0071; Fig. 9); P (r =0.10, p =0.6902) and NP (r = 0.05, p =0.8365) rats did not show this correlation. Further, in the W rats, a multiple regression to predict changes in ventricular volume from changes in the levels of the four metabolites (i.e., NAA, tCr, Cho, Glu) was significant (F(18)=3.09, p =0.05), with Cho as the only significant predictor of ventricular volume (t(18)=2.73, p =0.0162).
Table 2.
Summary of structural and neurochemical responses to binge EtOH
| WE | PE | NE | |
|---|---|---|---|
| Ventricular volume | Larger | n/e | n/e |
| NAA | Lower | Lower | Lower |
| tCr | Lower | Lower | Lower |
| Cho | Higher | n/e | n/e |
| Glu | n/e | n/e | n/e |
Compared with controls
n/e no effect
Fig. 9.
Relationship between change in ventricular volume and change in Cho levels between baseline and binge scans in WC and WE rats. WC heterogeneous stock Wistar control rats (triangles), WE heterogeneous stock Wistar EtOH-treated rats (circles)
Discussion
This study reports both baseline and EtOH-induced differences in brain structure and neurochemistry in rat lines differentially bred for EtOH preference. At baseline, P rats had larger ventricles and lower Cho levels than W or NP rats whereas NP rats had higher levels of NAA and tCr than W or P rats.
WE, PE, and NE rats received an equivalent cumulative dose (i.e., 42.9±0.76 g/kg) across four treatment days in resulting in average BALs of 255±6 mg/dL and peak BALs of 406.5±10.8mg/dL. Despite equivalent dosing, average and peak BALs were lower in NP than P rats (see Waller et al. 1983). In response to binge EtOH treatment, ventricular volume expanded substantially in W rats but was not a prominent feature in EtOH-exposed P and NP rats. NAA and tCr levels were lower and Glu levels unchanged in all three lines (i.e., W, P, NP) in the binge EtOH-treated animals compared with their controls. Similar to the selectivity of ventricular expansion, Cho was higher in WE than WC rats with EtOH, but not in P or NP rats exposed to EtOH compared with their controls.
Binge ethanol effects on weight and behavior
Treatment of animals with EtOH caused significant weight reductions in all three lines. WE, PE, and NE rats lost approximately the same amount of weight between baseline and binge scans (i.e., 16.5± 0.93 %). While Ctrl animals in all three lines also lost weight during that time (i.e., 7.8± 0.63 %), their weight loss was significantly less pronounced than that observed in the EtOH animals. EtOH-induced weight loss reported herein was more than previously reported in a study of P and NP rats in which weight loss across 4 days of binge EtOH was only ~2 % (Crews and Braun 2003). A likely explanation is that the previous study used a high caloric, nutritionally complete, liquid diet for gavage dosing animals. While Ctrl rats lost weight because of food restriction, EtOH animals lost weight because of EtOH exposure. EtOH-treated animals were observed to sit motionless, indicating that they were often too intoxicated to consume normal quantities of food. Moreover, previous studies have documented that BALs greater than 180 mg/dL can inhibit growth (Rivier and Vale 1983) and that isocaloric substitution of carbohydrates by EtOH results in weight loss (Lieber 1991).
The reported results, however, are unlikely related to nutritional deficiencies. First, acute weight loss does not necessarily result in malnutrition (e.g., Zawada Jr 1996). Additionally, a deficiency often associated with chronic alcoholism is for thiamine (vitamin B1). However, a combination of a thiamine deficient diet and daily administration of the thiamine antagonist, pyrithiamine, for a minimum of 2 weeks, is necessary to produce an animal model of thiamine deficiency (e.g., Ciccia and Langlais 2000; Langlais and Zhang 1997; Pitkin and Savage 2001; Pfefferbaum 2007 #16400). Given the excessive manipulations necessary to produce a thiamine-deficient animal model, binge EtOH treatment for 4 days is unlikely to result in thiamine deficiency. Indeed, the levels of thiamine and its phosphate derivatives were unaffected by the 4-day binge EtOH exposure paradigm (Zahr et al. 2010a). Further, MRS in the thiamine-deficient animal model shows reduced Cho (Lee et al. 2001), while heterogenous stock Wistar rats respond to binge EtOH treatment with elevated Cho (i.e., Fig. 6b).
In addition, malnutrition, as observed in anorexia nervosa (Enzmann and Lane 1977; Golden et al. 1996), kwashiorkor disease (Gunston et al. 1992), and vitamin D deficiency (Annweiler et al. 2013), results in ventriculomegaly. If binge EtOH treatment alone resulted in malnourishment, then all three strains of rat exposed to EtOH should have demonstrated ventricular enlargement.
A previous study comparing binge EtOH treatment using EtOH in a nutritionally complete liquid diet compared to EtOH mixed with water indicated that in fact, the nutritionally complete diet may have contributed to increased EtOH-induced brain damage (Crews et al. 2001). Final evidence against the possible role of nutritional deficits as contributing to the specific imaging and metabolite findings reported herein is provided by the current results: EtOH-exposed animals from all three strains demonstrated similar weight loss (i.e., weight loss between strains was not statistically different) but nevertheless showed differential binge EtOH effects in brain.
After 1 week of recovery, all rats gained weight (EtOH 18.9±1.1 %, Ctrl 19.3±1.5). W rats, however, gained more weight than either P or NP rats. The finding with W rats was probably due to the animal’s age, being slightly younger than the P and NP rats at the start of the experiment. Growth rate is sigmoidal across development with adolescence and young adulthood representing a growth spurt relative to younger and older animals (Kennedy 1967; Spear 2000). Nevertheless, the rat body weights indicate that the animals used in this study were tested during adulthood (cf., Bell et al. 2004, 2006b). Moreover, the present findings for the W rats parallel observations from larger (i.e., older) W rats (Zahr et al. 2013).
Two behavioral signs were observed in all three lines during intoxication: sitting motionless and gait disturbances. PE rats presented the most signs of altered “behavior” including chromodacryorrhea, heightened startle response, reduced trunk tone, and loss of righting reflex. This last observation is counter to a previous report that P rats are less affected by EtOH on a measure of motor impairment (Bell et al. 2001). However, this previous study was conducted with doses that resulted in much lower peak BALs (i.e., 100 to 150mg/dL) than those attained in the present study. Moreover, the present findings corroborate findings in Sardinian P and NP rats, also derived from a Wistar background (Colombo et al. 2006), in which Sardinian P rats took shorter times to lose the righting reflex and regained the reflex over longer periods of time and at lower blood ethanol levels than Sardinian NP rats (Colombo et al. 2000).
Despite resolution of EtOH-related signs during recovery, WE and PE but not NE rats were more irritable and likely to vocalize in the recovery week. There is some evidence that higher anxiety levels are associated with a greater propensity to abuse alcohol (e.g., Grant et al. 2004), and the latter finding would support previous work with P compared with NP rats (e.g., Jones et al. 2000). Thus, NP rats may have a neurobiological characteristic, associated with reduced anxiety or reactivity, which protects against alcohol abuse (cf., Bell et al. 2012).
Differential effects of binge ethanol on ventricular volume
Prior to EtOH treatment, P rats had significantly larger ventricular volume than either Wor NP rats. A potential interpretation of this finding is that the volumes of select brain structures are smaller in P than in W or NP rats. Indeed, relative to NP rats, P rats have been shown to have fewer serotonergic fibers in the hippocampus, nucleus accumbens, and cortex (Zhou et al. 1991), lower catecholaminergic innervation in the mesolimbic system (Zhou et al. 1995), and less densely packed astrocytes in the prelimbic cortex (Miguel-Hidalgo 2005). Such findings may implicate smaller volumes of these regions, which could contribute to the larger intrinsic ventricular volume in P rats. Relevant to this possibility, Marchigian-Sardinian P have smaller gray matter volumes in the thalamus, ventral tegmental area, insular, and cingulate cortices than outbred Wistar rats (Gozzi et al. 2013).
Binge EtOH treatment of W rats resulted in ventricular volume increases to 115 % of baseline with ventricular size returning to baseline with 7 days of recovery (Zahr et al. 2010b). This dynamic change was replicated in an independent group of older W rats (Zahr et al. 2013) but not observed in P rats. Rather, both PE and PC rats had larger ventricular volume at the binge time point compared with baseline and recovery, possibly indicating that P rats are especially susceptible to the stress of gavage or, alternatively, that P rats have greater difficulty maintaining fluid balance than W and NP rats. For instance, when experiencing an intermittent EtOH access protocol (i.e., four cycles of 4 days access to and 4 days of deprivation from EtOH), P rats have higher water intake during EtOH deprivation than during access by approximately 50 % (Bell et al. 2008).
Although NE rats did show a pattern of reversible ventricular enlargement, with ventricular volume expansion to 70 % greater than baseline values and values larger at the binge compared with the baseline and recovery time points, ventricular volume was not different from NC rats at any time point. NE rats displayed only negligible changes in ventricular size following binge EtOH compared with either WE or PE rats, indicating that the NP line may have a protective mechanism to avert the deleterious effect of high BALs. Thus, profound ventricular volume expansion in response to EtOH under the present experimental parameters appears to be selective to W rats.
Differential effects of binge ethanol on proton metabolite levels
In the dorsal hippocampus at baseline, NP rats had higher levels of NAA and tCr than W and P rats, P rats had lower levels of Cho than Wand NP rats, and W rats had lower levels of Glu than P and NP rats. Higher levels of NAA suggest that NP rats have a higher density of neurons in the hippocampus than W or P rats. P rats have been shown to have fewer serotonin fibers (Zhou et al. 1991) and fewer delta opioid receptors (Strother et al. 2001) than NP rats in the hippocampus. Further, proteomics demonstrate that the expression of some proteins is higher in the hippocampus of NP than P rats (Witzmann et al. 2003). Together, this type of data may imply strain differences in neuronal density, thereby accounting for the relatively higher levels of NAA in NP than in P rats.
A single study reported that local cerebral glucose utilization was significantly higher in several limbic, cortical, and subcortical regions in P compared with W and NP rats (Smith et al. 2001). It is possible that P rats have high local cerebral glucose utilization to compensate for low high-energy phosphate metabolism, i.e., low levels of tCr. Lower levels of Cho in P than W and NP rats may indicate differences in glial density, as demonstrated in the frontal cortex of P compared with W and NP rats (Miguel-Hidalgo 2005). Line differences in Glu levels may be accounted for by differential regulation of genes involved in glutamate neurotransmission, as noted in the ventral tegmental area of several rat lines (McBride et al. 2012)
In contrast to the differential effects of EtOH on ventricular volume in the three rat lines and despite differences in baseline metabolite levels, EtOH treatment resulted in similar effects on NAA, tCr, and Glu in WE, PE, and NE rats: NAA and tCr levels were lower at binge than at baseline and recovery while Glu levels were unaffected. That all three lines showed similar effects of binge EtOH treatment on NAA and tCr indicates a general response. Potential interpretations for lower NAA and tCr levels following binge EtOH treatment include effects on brain osmotic balance (Baslow et al. 1999, 2000; Ross and Bluml 2001), energy utilization (Bates et al. 1996; Moffett et al. 2007; Sartorius et al. 2008), or myelin homeostasis (Moffett et al. 2007). A more thorough discussion of these potential interpretations is presented in the context of findings in W rats (Zahr et al. 2010b). The absence of an effect on MRS-detectable Glu does not indicate that glutamate homeostasis is unaffected by binge EtOH treatment, but rather that gross Glu measures by in vivo MRS are perhaps insufficient to detect changes in response to EtOH at the molecular level as has previously been reported in P (Ding et al. 2013; Fitzgerald et al. 2012) and NP rats (McBride et al. 2012).
In contrast to the consistent effects of binge EtOH treatment on NAA, tCr, and Glu in the three rat lines, the current findings indicate that binge EtOH differentially affects Cho levels in W, P, and NP rats. Whereas binge EtOH exposure caused a clear increase in Cho levels in WE rats (Zahr et al. 2010b; Zahr et al. 2013), PE rats showed only a modest, non-significant increase. The effect in NE rats was significant only because Cho levels decreased in NC rats between baseline and binge (Fig. 6c).
MRS-visible Cho is composed of a number of metabolites including phosphocholine and glycerophosphocholine (Bluml et al. 1999; Boulanger et al. 2000), with residual contributions from other metabolites including free choline and acetylcholine (Lee et al. 2013). Because choline derivatives are precursors or degradation products of membrane phospholipids, variations in Cho levels are interpreted as representing changes in cell membrane synthesis and turnover (e.g., Boulanger et al. 2000; Sim and Pasternak 1976) and changes in response to EtOH may reflect adaptive changes in brain cell membranes (Lee et al. 2013). Indeed, Marchigian-Sardinian P rats with free access to EtOH for 10 weeks showed increased content of long-chain fatty acids and decreased content of short-chain fatty acids in the hippocampus (Berrettini et al. 2004), a finding which may underlie the consistent increase in Cho in W rats observed following binge EtOH treatment (i.e., Zahr et al. 2010b, 2013).
Although associated with changes to cell membranes, the functions and relative contributions of the various metabolites contributing to the MRS-detectable Cho signal may depend on the disease or animal model under investigation. For example, because Cho is elevated in gliomas (e.g., Gupta et al. 1999; Tedeschi et al. 1997), one interpretation for elevated Cho is infiltration of glia. Cho is also elevated in multiple sclerosis (Mader et al. 2008) and models of multiple sclerosis (Brenner et al. 1993) where interpretations include demyelination and inflammation. Elevations in Cho in renal failure (Sasaki et al. 2006), along with evidence that glycerophosphocholine is a cerebral osmolyte (e.g., Lien et al. 1990), suggest that elevations in Cho represent altered osmoregulation. Free choline levels increase during energy reduction (Djuricic et al. 1991), so another potential interpretation for elevated Cho is compromise in the brain’s normal energy utilization (Trovarelli et al. 1982).
With respect to EtOH effects on brain, treatment with EtOH (a single day of dosing or the 4-day binge model) results in upregulation of vimentin, a marker of reactive gliosis (Kelso et al. 2011). There is also evidence that alcoholism is associated with demyelination as inferred from findings in humans (e.g., Lewohl et al. 2005; Pfefferbaum et al. 2002, 2009) and animal models (Alfonso-Loeches et al. 2012). Alcoholism has also been associated with inflammation (e.g., He and Crews 2008), altered osmoregulation (e.g., Harding et al. 1996; Silva et al. 2002), and modified energy utilization (Altura and Altura 1999; Sonn and Mayevsky 2001). Thus, the elevations in Cho observed in W rats in response to binge EtOH treatment can be interpreted in various ways and additional research in necessary to determine precisely what the elevation in Cho in response to EtOH may indicate. The differential effects of EtOH on Cho in the three rat lines suggest that one or more of these variables may be intrinsically distinct.
Relationship between changes in ventricular volume and choline levels
Only W rats clearly showed both profound ventricular enlargement and increases in Cho in response to binge EtOH treatment. Evaluation of the relationship between changes in ventricular volume and Cho between the baseline and binge time points demonstrated that only W rats show a correlation between these two variables. Thus, EtOH-induced ventricular enlargement related to adaptive changes in brain cell membranes may be due to changes in glial, myelination, osmoregulatory, or energy utilization factors. Given the speculative nature of the current understanding of changes in Cho levels in the context of EtOH exposure, however, it is not clear which interpretation may account for EtOH-induced changes in ventricular volume.
Conclusion
This study evaluated the effects of binge EtOH treatment on three rat lines, two bred for differential alcohol preference. Key findings include intrinsic differences: P rats had larger ventricular volumes and lower levels of Cho at baseline than W or NP rats, whereas NP rats had higher levels of NAA and tCr at baseline than W or P rats. Additionally, differential responses to EtOH highlight unique responses in W rats regarding ventricular volume and Cho and suggest that changes in ventricular volume are related to adaptive changes in brain cell membranes, the precise mechanism of which remains to be determined.
Supplementary Material
Acknowledgments
This study was supported by NIH grant numbers AA013521-INIA, AA005965, and AA017168.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-013-3253-z) contains supplementary material, which is available to authorized users.
Conflict of interest All authors declare no competing financial interests.
Contributor Information
Natalie M Zahr, Email: nzahr@stanford.edu, Psychiatry & Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Rd, Stanford, CA 94305, USA; Neuroscience Program, SRI International, Menlo Park, CA 94025, USA.
Dirk Mayer, Neuroscience Program, SRI International, Menlo Park, CA 94025, USA; Radiology Department, Lucas MRS/I Center, Stanford University, Stanford, CA 94304, USA.
Torsten Rohlfing, Neuroscience Program, SRI International, Menlo Park, CA 94025, USA.
Oliver Hsu, Neuroscience Program, SRI International, Menlo Park, CA 94025, USA.
Shara Vinco, Neuroscience Program, SRI International, Menlo Park, CA 94025, USA.
Juan Orduna, Neuroscience Program, SRI International, Menlo Park, CA 94025, USA.
Richard Luong, Department of Comparative Medicine, Stanford University, Stanford, CA 94305, USA; Department of Comparative Medicine, Stanford University, Stanford, CA 94305, USA.
Richard L Bell, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Edith V Sullivan, Psychiatry & Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Rd, Stanford, CA 94305, USA.
Adolf Pfefferbaum, Psychiatry & Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Rd, Stanford, CA 94305, USA; Neuroscience Program, SRI International, Menlo Park, CA 94025, USA.
References
- Adalsteinsson E, Hurd RE, Mayer D, Sailasuta N, Sullivan EV, Pfefferbaum A. In vivo 2D J-resolved magnetic resonance spectroscopy of rat brain with a 3-T clinical human scanner. Neuroimage. 2004;22:381–386. doi: 10.1016/j.neuroimage.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Gomez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia. 2012;60:948–964. doi: 10.1002/glia.22327. [DOI] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Association of alcohol in brain injury, headaches, and stroke with brain-tissue and serum levels of ionized magnesium: a review of recent findings and mechanisms of action. Alcohol. 1999;19:119–130. doi: 10.1016/s0741-8329(99)00025-7. [DOI] [PubMed] [Google Scholar]
- Annweiler C, Montero-Odasso M, Hachinski V, Seshadri S, Bartha R, Beauchet O. Vitamin D concentration and lateral cerebral ventricle volume in older adults. Mol Nutr Food Res. 2013;57:267–276. doi: 10.1002/mnfr.201200418. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Suckow RF, Hungund BL. Effects of ethanol and of alcohol dehydrogenase inhibitors on the reduction of N-acetylaspartate levels of brain in mice in vivo: a search for substances that may have therapeutic value in the treatment of Canavan disease. J Inherit Metab Dis. 2000;23:684–692. doi: 10.1023/a:1005618526988. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Suckow RF, Sapirstein V, Hungund BL. Expression of aspartoacylase activity in cultured rat macroglial cells is limited to oligodendrocytes. J Mol Neurosci. 1999;13:47–53. doi: 10.1385/JMN:13:1-2:47. [DOI] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PMG, Clark JB. Inhibition of N-acetylaspartate production: Implications for H-1 MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li TK, McBride WJ. Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcohol Clin Exp Res. 2004;28:1867–1874. doi: 10.1097/01.alc.0000148101.20547.0a. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006a;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006b;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Schultz JA, Peper CL, Lumeng L, Murphy JM, McBride WJ. Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol. 2008;42:407–416. doi: 10.1016/j.alcohol.2008.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Stewart RB, Woods JE2nd, Lumeng L, Li TK, Murphy JM, McBride WJ. Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (P) and -nonpreferring (NP) rat lines. Alcohol Clin Exp Res. 2001;25:644–650. [PubMed] [Google Scholar]
- Berrettini M, Fedeli D, Falcioni G, Bevilacqua C, Massi M, Polidori C. Hippocampal and striated skeletal muscle changes in fatty acid composition induced by ethanol in alcohol-preferring rats. Toxicology. 2004;199:161–168. doi: 10.1016/j.tox.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Bluml S, Seymour KJ, Ross BD. Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31)P MRS in in vivo human brain. Magn Reson Med. 1999;42:643–654. doi: 10.1002/(sici)1522-2594(199910)42:4<643::aid-mrm5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Boulanger Y, Labelle M, Khiat A. Role of phospholipase A(2) on the variations of the choline signal intensity observed by 1H magnetic resonance spectroscopy in brain diseases. Brain Res Brain Res Rev. 2000;33:380–389. doi: 10.1016/s0165-0173(00)00037-0. [DOI] [PubMed] [Google Scholar]
- Brenner RE, Munro PM, Williams SC, Bell JD, Barker GJ, Hawkins CP, Landon DN, McDonald WI. The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med. 1993;29:737–745. doi: 10.1002/mrm.1910290605. [DOI] [PubMed] [Google Scholar]
- Ciccia RM, Langlais PJ. An examination of the synergistic interaction of ethanol and thiamine deficiency in the development of neurological signs and long-term cognitive and memory impairments. Alcohol Clin Exp Res. 2000;24:622–634. [PubMed] [Google Scholar]
- Cicero TJ, Snider SR, Perez VJ, Swanson LW. Physical dependence on and tolerance to alcohol in the rat. Physiol Behav. 1971;6:191–198. doi: 10.1016/0031-9384(71)90088-6. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL. Different sensitivity to ethanol in alcohol-preferring sP and -nonpreferring sNP rats. Alcohol Clin Exp Res. 2000;24:1603–1608. [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ. Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcohol Clin Exp Res. 2003;27:1075–1082. doi: 10.1097/01.ALC.0000075826.35688.0D. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Ali R, Knapp DJ. Interaction of nutrition and binge ethanol treatment on brain damage and withdrawal. J Biomed Sci. 2001;8:134–142. doi: 10.1007/BF02255982. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuricic B, Olson SR, Assaf HM, Whittingham TS, Lust WD, Drewes LR. Formation of free choline in brain tissue during in vitro energy deprivation. J Cereb Blood Flow Metab. 1991;11:308–313. doi: 10.1038/jcbfm.1991.63. [DOI] [PubMed] [Google Scholar]
- Dreher W, Leibfritz D. Detection of homonuclear decoupled in vivo proton NMR spectra using constant time chemical shift encoding: CT-PRESS. Magn Reson Imaging. 1999;17:141–150. doi: 10.1016/s0730-725x(98)00156-8. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI, Wall TL, Lumeng L, Li TK, Owens MJ, Nemeroff CB. Corticotropin releasing factor (CRF): studies in alcohol preferring and non-preferring rats. Psychopharmacology (Berl) 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P, Mathe AA. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–1782. [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Lumeng L, Li TK. Electrophysiological response to neuropeptide Y (NPY): in alcohol-naive preferring and non-preferring rats. Pharmacol Biochem Behav. 1999;63:291–299. doi: 10.1016/s0091-3057(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Enzmann DR, Lane B. Cranial computed tomography findings in anorexia nervosa. J Comput Assist Tomogr. 1977;1:410–414. doi: 10.1097/00004728-197710000-00005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GJ, Liu H, Morzorati SL. Decreased sensitivity of NMDA receptors on dopaminergic neurons from the posterior ventral tegmental area following chronic nondependent alcohol consumption. Alcohol Clin Exp Res. 2012;36:1710–1719. doi: 10.1111/j.1530-0277.2012.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden NH, Ashtari M, Kohn MR, Patel M, Jacobson MS, Fletcher A, Shenker IR. Reversibility of cerebral ventricular enlargement in anorexia nervosa, demonstrated by quantitative magnetic resonance imaging. J Pediatr. 1996;128:296–301. doi: 10.1016/s0022-3476(96)70414-6. [DOI] [PubMed] [Google Scholar]
- Goldstein D. Pharmacology of alcohol. Oxford: Oxford University Press; 1983. [Google Scholar]
- Gozzi A, Agosta F, Massi M, Ciccocioppo R, Bifone A. Reduced limbic metabolism and fronto-cortical volume in rats vulnerable to alcohol addiction. Neuroimage. 2013;69:112–119. doi: 10.1016/j.neuroimage.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gunston GD, Burkimsher D, Malan H, Sive AA. Reversible cerebral shrinkage in kwashiorkor: an MRI study. Arch Dis Child. 1992;67:1030–1032. doi: 10.1136/adc.67.8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Sinha U, Cloughesy TF, Alger JR. Inverse correlation between choline magnetic resonance spectroscopy signal intensity and the apparent diffusion coefficient in human glioma. Magn Reson Med. 1999;41:2–7. doi: 10.1002/(sici)1522-2594(199901)41:1<2::aid-mrm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Ng JL, Harper CG, Kril JJ. Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and time-dependent. Neuroscience. 1996;72:699–708. doi: 10.1016/0306-4522(95)00577-3. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BH, Lumeng L, Wu JY, Li TK. Increased number of GABAergic terminals in the nucleus accumbens is associated with alcohol preference in rats. Alcohol Clin Exp Res. 1990;14:503–507. doi: 10.1111/j.1530-0277.1990.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: a comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004a;1026:143–150. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Suzuki R, Lumeng L, Li TK, McBride WJ. Innate differences in neuropeptide Y (NPY) mRNA expression in discrete brain regions between alcohol-preferring (P) and -nonpreferring (NP) rats: a significantly low level of NPY mRNA in dentate gyrus of the hippocampus and absence of NPY mRNA in the medial habenular nucleus of P rats. Neuropeptides. 2004b;38:359–368. doi: 10.1016/j.npep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Jones AE, McBride WJ, Murphy JM, Lumeng L, Li T, Shekhar A, McKinzie DL. Effects of ethanol on startle responding in alcohol-preferring and -non-preferring rats. Pharmacol Biochem Behav. 2000;67:313–318. doi: 10.1016/s0091-3057(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience. 2011;197:381–393. doi: 10.1016/j.neuroscience.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GC. Ontogeny of mechanisms controlling food and water intake. In: Code CF, editor. Handbook of physiology:alimentary canal. Control of food and water intake. Washington, D.C.: American Physiological Society; 1967. pp. 337–352. [Google Scholar]
- Langlais PJ, Zhang SX. Cortical and subcortical white matter damage without Wernicke’s encephalopathy after recovery from thiamine deficiency in the rat. Alcohol Clin Exp Res. 1997;21:434–443. doi: 10.1111/j.1530-0277.1997.tb03788.x. [DOI] [PubMed] [Google Scholar]
- Lankford MF, Roscoe AK, Pennington SN, Myers RD. Drinking of high concentrations of ethanol versus palatable fluids in alcohol-preferring (P) rats: valid animal model of alcoholism. Alcohol. 1991;8:293–299. doi: 10.1016/0741-8329(91)90417-u. [DOI] [PubMed] [Google Scholar]
- Lee DW, Kim SY, Kim JH, Lee T, Yoo C, Nam YK, Jung JY, Shin HC, Kim HY, Kim DJ, Choe BY. Quantitative assessment of neurochemical changes in a rat model of long-term alcohol consumption as detected by in vivo and ex vivo proton nuclear magnetic resonance spectroscopy. Neurochem Int. 2013;62:502–509. doi: 10.1016/j.neuint.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lee H, Holburn GE, Price RR. In vivo and in vitro proton NMR spectroscopic studies of thiamine-deficient rat brains. J Magn Reson Imaging. 2001;13:163–166. doi: 10.1002/1522-2586(200102)13:2<163::aid-jmri1025>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lester D, Freed EX. Criteria for an animal model of alcoholism. Pharmacol Biochem Behav. 1973;1:103–107. doi: 10.1016/0091-3057(73)90062-2. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wixey J, Harper CG, Dodd PR. Expression of MBP, PLP, MAG, CNP, and GFAP in the human alcoholic brain. Alcohol Clin Exp Res. 2005;29:1698–1705. doi: 10.1097/01.alc.0000179406.98868.59. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–96. [PubMed] [Google Scholar]
- Lieber CS. Perspectives: do alcohol calories count? Am J Clin Nutr. 1991;54:976–982. doi: 10.1093/ajcn/54.6.976. [DOI] [PubMed] [Google Scholar]
- Lien YH, Shapiro JI, Chan L. Effects of hypernatremia on organic brain osmoles. J Clin Invest. 1990;85:1427–1435. doi: 10.1172/JCI114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likar B, Viergever MA, Pernus F. Retrospective correction of MR intensity inhomogeneity by information minimization. IEEE Trans Med Imaging. 2001;20:1398–1410. doi: 10.1109/42.974934. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Hawkins TD, Li TK. New strains of rats with alcohol preference and nonpreference. In: Thurman RG, Williamson JR, Drott HR, Chance B, editors. Alcohol and aldehyde metabolizing systems. New York: Academic; 1977. pp. 537–544. [Google Scholar]
- Mader I, Rauer S, Gall P, Klose U. (1)H MR spectroscopy of inflammation, infection and ischemia of the brain. Eur J Radiol. 2008;67:250–257. doi: 10.1016/j.ejrad.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mann K, Mundle G, Langle G, Petersen D. The reversibility of alcoholic brain damage is not due to rehydration: a CT study. Addiction. 1993;88:649–653. doi: 10.1111/j.1360-0443.1993.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Mayer D, Spielman DM. Detection of glutamate in the human brain at 3 T using optimized constant time point resolved spectroscopy. Magn Reson Med. 2005;54:439–442. doi: 10.1002/mrm.20571. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol. 1993;10:387–390. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Edenberg HJ, Lumeng L, Bell RL. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacol Biochem Behav. 2012;102:275–285. doi: 10.1016/j.pbb.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Lower packing density of glial fibrillary acidic protein-immunoreactive astrocytes in the prelimbic cortex of alcohol-naive and alcohol-drinking alcohol-preferring rats as compared with alcohol-nonpreferring and Wistar rats. Alcohol Clin Exp Res. 2005;29:766–772. doi: 10.1097/01.alc.0000164378.92680.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Contents of mono-amines in forebrain regions of alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 1987;26:389–392. doi: 10.1016/0091-3057(87)90134-1. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Waller MB, Gatto GJ, McBride WJ, Lumeng L, Li TK. Effects of fluoxetine on the intragastric self-administration of ethanol in the alcohol preferring P line of rats. Alcohol. 1988;5:283–286. doi: 10.1016/0741-8329(88)90066-3. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Elsevier Academic; 2005. [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Bell RL, Sullivan EV. Development and resolution of brain lesions caused by pyrithiamine- and dietary-Induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2007;32:1149–1177. doi: 10.1038/sj.npp.1301107. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Serventi KL, Sullivan EV. Corpus callosum, pons, and cortical white matter in alcoholic women. Alcohol Clin Exp Res. 2002;26:400–406. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Mayer D, Vinco S, Orduna J, Rohlfing T, Sullivan EV. Ventricular expansion in wild-type Wistar rats after alcohol exposure by vapor chamber. Alcohol Clin Exp Res. 2008;32:1459–1467. doi: 10.1111/j.1530-0277.2008.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkin SR, Savage LM. Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent. Behav Brain Res. 2001;119:167–177. doi: 10.1016/s0166-4328(00)00350-8. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Influence of ethanol on reproductive functions of the adult male rat as a function of body weight. Alcohol Clin Exp Res. 1983;7:210–212. doi: 10.1111/j.1530-0277.1983.tb05443.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000;24:747–753. [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcohol Clin Exp Res. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A, Lugenbiel P, Mahlstedt MM, Ende G, Schloss P, Vollmayr B. Proton magnetic resonance spectroscopic creatine correlates with creatine transporter protein density in rat brain. J Neurosci Methods. 2008;172:215–219. doi: 10.1016/j.jneumeth.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Sasaki O, Hattori N, Nakahama H, Inoue N, Nakamura S, Inenaga T, Kohno S, Sawada T, Kawano Y. Positive correlations between cerebral choline and renal dysfunction in chronic renal failure. Neuroradiology. 2006;48:300–306. doi: 10.1007/s00234-006-0063-6. [DOI] [PubMed] [Google Scholar]
- Silva SM, Madeira MD, Ruela C, Paula-Barbosa MM. Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Res. 2002;925:76–88. doi: 10.1016/s0006-8993(01)03261-9. [DOI] [PubMed] [Google Scholar]
- Sim E, Pasternak CA. The metabolism of the phosphonium analogue of choline in cultured cells. A useful nuclear-magnetic-resonance probe for membrane phosphatidylcholine. Biochem J. 1976;154:105–111. doi: 10.1042/bj1540105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Alcohol-naive alcohol-preferring (P) rats exhibit higher local cerebral glucose utilization than alcohol-nonpreferring (NP) and Wistar rats. Alcohol Clin Exp Res. 2001;25:1309–1316. [PubMed] [Google Scholar]
- Sonn J, Mayevsky A. The effect of ethanol on metabolic, hemodynamic and electrical responses to cortical spreading depression. Brain Res. 2001;908:174–186. doi: 10.1016/s0006-8993(01)02643-9. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Strother WN, Chernet EJ, Lumeng L, Li TK, McBride WJ. Regional central nervous system densities of delta-opioid receptors in alcohol-preferring P, alcohol-nonpreferring NP, and unselected Wistar rats. Alcohol. 2001;25:31–38. doi: 10.1016/s0741-8329(01)00162-8. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005 doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Lundbom N, Raman R, Bonavita S, Duyn J, Alger J, DiChiro G. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg. 1997;87:516–524. doi: 10.3171/jns.1997.87.4.0516. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, McBride WJ, Chernet E, Lumeng L, Li TK. Regional densities of benzodiazepine sites in the CNS of alcohol-naive P and NP rats. Pharmacol Biochem Behav. 1997;57:875–882. doi: 10.1016/s0091-3057(96)00464-9. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, McBride WJ, Lumeng L, Li TK. Housing conditions alter GABAA receptor of alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1993;46:723–727. doi: 10.1016/0091-3057(93)90568-e. [DOI] [PubMed] [Google Scholar]
- Trovarelli G, De Medio GE, Montanini I. The influence of CDP-choline on brain lipid metabolism during ischemia. Farmaco [Sci] 1982;37:663–668. [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Initial sensitivity and acute tolerance to ethanol in the P and NP lines of rats. Pharmacol Biochem Behav. 1983;19:683–686. doi: 10.1016/0091-3057(83)90345-3. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Witzmann FA, Li J, Strother WN, McBride WJ, Hunter L, Crabb DW, Lumeng L, Li TK. Innate differences in protein expression in the nucleus accumbens and hippocampus of inbred alcohol-preferring and -nonpreferring rats. Proteomics. 2003;3:1335–1344. doi: 10.1002/pmic.200300453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Kohl RL, Rudy TA. Induction of tolerance and withdrawal in rats receiving morphine in the spinal subarachnoid space. Eur J Pharmacol. 1977;42:275–284. doi: 10.1016/0014-2999(77)90294-1. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV, Pfefferbaum A. Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol Clin Exp Res. 2010a;34:1858–1870. doi: 10.1111/j.1530-0277.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hasak M, Hsu O, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol Psychiatry. 2010b;67:846–854. doi: 10.1016/j.biopsych.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology. 2013;38:1121–1129. doi: 10.1038/npp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology. 2009;34:1427–1442. doi: 10.1038/npp.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawada ET., Jr Malnutrition in the elderly Is it simply a matter of not eating enough? Postgrad Med. 1996;100:207–208. doi: 10.3810/pgm.1996.07.17. 211-4,220-2 passim. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Bledsoe S, Lumeng L, Li TK. Immunostained serotonergic fibers are decreased in selected brain regions of alcohol-preferring rats. Alcohol. 1991;8:425–431. doi: 10.1016/s0741-8329(91)90034-t. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Zhang JK, Lumeng L, Li TK. Mesolimbic dopamine system in alcohol-preferring rats. Alcohol. 1995;12:403–412. doi: 10.1016/0741-8329(95)00010-o. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Pfefferbaum A. MRI study of brain changes with short term abstinence from alcohol. Alcohol Clin Exp Res. 1989;13:664–666. doi: 10.1111/j.1530-0277.1989.tb00401.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.