Abstract
Craving is a major motivator underlying drug use and relapse but the neural correlates of cannabis craving are not well understood. This study sought to determine whether visual cannabis cues increase cannabis craving and whether cue-induced craving is associated with regional brain activation in cannabis-dependent individuals. Cannabis craving was assessed in 16 cannabis-dependent adult volunteers while they viewed cannabis cues during a functional MRI (fMRI) scan. The Marijuana Craving Questionnaire was administered immediately before and after each of three cannabis cue-exposure fMRI runs. FMRI blood-oxygenation-level-dependent (BOLD) signal intensity was determined in regions activated by cannabis cues to examine the relationship of regional brain activation to cannabis craving. Craving scores increased significantly following exposure to visual cannabis cues. Visual cues activated multiple brain regions, including inferior orbital frontal cortex, posterior cingulate gyrus, parahippocampal gyrus, hippocampus, amygdala, superior temporal pole, and occipital cortex. Craving scores at baseline and at the end of all three runs were significantly correlated with brain activation during the first fMRI run only, in the limbic system (including amygdala and hippocampus) and paralimbic system (superior temporal pole), and visual regions (occipital cortex). Cannabis cues increased craving in cannabis-dependent individuals and this increase was associated with activation in the limbic, paralimbic, and visual systems during the first fMRI run, but not subsequent fMRI runs. These results suggest that these regions may mediate visually cued aspects of drug craving. This study provides preliminary evidence for the neural basis of cue-induced cannabis craving and suggests possible neural targets for interventions targeted at treating cannabis dependence.
Keywords: drug abuse, functional MRI, addiction
1. Introduction
Cannabis is the most commonly used illicit drug in the U.S. and an estimated 4% of the American population is cannabis dependent (Budney et al., 2007). Treatment for cannabis dependence is hindered because of limited knowledge of the neural underpinnings of cannabis dependence. Drug craving has been associated with continued drug use or returning to drug use after a period of sobriety (Preston et al., 2009) and cue-associated brain activation may predict relapse to alcohol (Braus et al., 2001;Grusser et al., 2004). Drug-related cues predispose drug users to drug use and drug cue-induced increases in drug craving likely account in part for the ability of cues to trigger drug use (Epstein et al., 2009). Understanding the neurobiology of drug craving is therefore critical to developing treatments to reduce craving and associated drug use behavior. For example, understanding the specific neural circuitry implicated in drug craving and relapse may lead to interventions targeted at brain activity in specific regions, e.g. psychotherapeutic approaches or psychopharmacological interventions. Although numerous studies have examined the neurobiology of drug-cue effects on brain activation and craving there has been remarkably little study of cannabis cue effects in cannabis users. Gray and colleagues (Gray et al., 2008) found that visual video clips of marijuana use cues, and manually handling marijuana and marijuana paraphernalia increased craving and some measures of autonomic reactivity in young users. Filbey and colleagues reported cannabis cue-induced brain activation to tactile cannabis cues in heavy cannabis users, but did not find a significant association of cannabis craving (measured as urge to use) and cueinduced brain activation (Filbey et al., 2009). In frequent cannabis users, visual cannabis images produced greater activation than control images in frontal, occipital, parietal, and cingulate cortices. When compared with sporadic users and control subjects, frequent users showed greater activation in the ventral tegmental area during cue viewing, but brain activation in these regions did not correlate with cannabis craving. Instead, portions of prefrontal cortex and putamen showed a negative association between visual cue-induced brain activation and craving (Cousijn et al., 2012). Goldman and colleagues recently reported an association of visual cannabis cue-induced brain activation and baseline craving in treatment seeking cannabis dependent subjects (Goldman et al., 2013). Determining which brain regions mediate cue-induced increases in cannabis craving is therefore a critical unresolved problem that has important implications for understanding the neurobiology of cannabis dependence. We used functional magnetic resonance imaging (fMRI) in conjunction with visual cannabis cue-exposure to test our hypotheses that visual cannabis cues increase craving in cannabis users and that cue-induced craving is associated with increased activation in regions most commonly implicated in visual drug cue-induced craving (orbitofrontal, anterior cingulate, visual cortices and striatum), reviewed in (Yalachkov et al., 2012).
2. Materials and Methods
2.1 Objectives
The present report is a component of a larger study designed to address the neural mechanisms of exercise effects on cannabis craving and cannabis use in cannabis-dependent participants. We have previously reported a subset of this data examining the effects of exercise on cannabis use, in which we demonstrated that exercise is associated with reductions in cannabis craving and use(Buchowski et al., 2011). The objectives of the present report were to examine the neural basis of cannabis cue-associated craving and all data were obtained in subjects at the initial imaging visit and prior to the exercise intervention.
2.2 Participants
Seventeen non-treatment seeking cannabis-dependent adults were recruited by advertisements and word of mouth as part of a larger study on the effects of exercise on the neurobiology of cannabis use and craving. Data derived from the current report occurred immediately after screening and prior to any other study interventions or tasks. All individuals were screened for psychiatric disorders and MRI safety and consented to participate. One subject was subsequently excluded for recent drug use, leaving a final sample size of 16 subjects. Inclusion criteria were: male or female; age 18–35; current cannabis dependence by MINI criteria; positive urine drug screen for cannabis on the study day; normal vision; if female, not pregnant or planning to become pregnant during the study period; right handed; no chronic medical conditions. Exclusion criteria were: current Axis I psychiatric disorder (other than substance abuse or dependence or substance-induced conditions); use of psychotropic or vasoactive medications within 6 weeks of the study day; use of other medications within 72 hours of the study day; contraindications to MRI study (implanted medical devices, non-secure metallic foreign bodies, claustrophobia); chronic medical conditions; history of head injury with loss of consciousness; regular exercise of more than 2 hour per week in the past month or participating in organized exercise; weight over 275 pounds (due to scanner size limits); and physical problems that might prevent participation in the exercise arm of the study. There was no minimum level of cannabis use required for study entry but all subjects had to meet criteria for current cannabis dependence.
Psychiatric and substance diagnoses as determined by the Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998) are listed in Table 1. Participants were asked to refrain from cannabis use for 8 h (overnight) before the imaging study and were required to have a positive urine drug test for cannabis (tetrahydrocannabinol) and to test negative for alcohol and other drugs on the study day. Urine testing was performed using a Triage Drugs of Abuse Panel (Biosite Diagnostics, San Diego, CA). Urine sensitivity to drugs of abuse varies with drug use; for cannabis, screens may remain positive for days to weeks after last use (reviewed in (Macdonald et al., 2010). Alcohol breath testing was performed using an alcohol breathalyzer (Intoximeters, St. Louis, MO). An 8-hour period of cannabis abstinence was chosen to balance the effects of recent/acute intoxication (where craving might be blunted or absent) and potential ceiling effects on craving that might be induced by more prolonged abstinence and subsequently high levels of craving. Abstinence was based on self-report and was not otherwise verified. This study was approved by the Institutional Review Board at Vanderbilt University and conformed to the Declaration of Helsinki. All participants were compensated $100 for their time.
Table 1.
Subject (N=16) characteristics
| Variable | |
|---|---|
| Age (years) (mean ± S.D.) | 23.7 ± 3.9 |
| Age (years) at first cannabis use (mean ± S.D.) | 15.1 ± 2.8 |
| Sex | 11 females/5 males |
| Number of uses of cannabis per week* (median, 25–75th IQR) | 15.5, 3.3–21 |
| Cannabis abstinence (hours) (mean ± S.D.) | 13.5 ± 2.1 |
| Other drug use | Number reporting use |
| Alcohol | 16 |
| Psilocybin | 12 |
| Cocaine | 10 |
| LSD | 10 |
| Non-heroin opiates | 7 |
| Sedative hypnotics | 6 |
| Methamphetamine | 5 |
| Heroin | 3 |
| Substance-related diagnoses | Number diagnosed |
| Cannabis dependence (current) | 16 |
| Alcohol abuse (lifetime) | 6 |
| Alcohol dependence (lifetime) | 1 |
| Benzodiazepine dependence (lifetime) | 1 |
| Hallucinogen dependence (lifetime) | 1 |
| Cocaine dependence (lifetime) | 1 |
| Substance-induced mood episode (lifetime) | 1 |
| Major depression (lifetime) | 1 |
N=15
2.3. Descriptions of procedures
2.3.1. Cannabis craving assessment
After abstaining from cannabis overnight, subjects reported to the imaging center and completed final questionnaires. We administered the Marijuana Craving Questionnaire - short form (MCQ-SF) (Heishman et al., 2009) as a baseline assessment (MCQ-baseline; approximately 15 minutes before the first cannabis cue-exposure fMRI run) and we reassessed craving with the MCQ-SF at the end of the first, second, and third (MCQ-post run 1; MCQ postrun 2; MCQ-post run 3) cannabis cue-exposure fMRI runs. Participants completed the paper version of the MCQ for the baseline assessment; after familiarizing themselves with the paper version of the questionnaire, participants were read the questions one by one at the end of each scanner run and provided verbal responses that were recorded by the experimenter on the paper version. The MCQ-SF is a validated (Heishman and Singleton, 2006) 12-item Likert response (where 1=strongly disagree and 7=strongly agree, with positive answers indicating greater craving) questionnaire that assesses situational cannabis craving using four subscales: compulsivity, emotionality, expectancy, and purposefulness (Heishman et al., 2009). The subscale scores are averaged to arrive at an overall index of craving ranging from a minimum value of 1.0 to a maximum of 7.0.The internal consistency values (Cronbach’s alphas) for the four times of assessment in this study ranged from 0.76 to 0.85.
2.3.2. Cannabis cue paradigm
Participants rested quietly in the scanner while viewing images of cannabis and control cues (nature scenes) during fMRI image acquisition. The fMRI cannabis-cue paradigm consisted of three functional runs. Each run was 3-minutes long and consisted of three, 30-second stimuli blocks of 10 cue images displayed for 3 seconds each and three 30-second baseline blocks. Each run contained one block of images from each of 3 cue categories: cannabis, nature scenes and food. We included nature scenes because they help control in part for: a) seeing a novel image, b) color, c) brightness, d) visual complexity, e) absence of content that might trigger or provoke craving. Food cues were included for exploratory examination of cannabis and feeding behavior. The baseline images we used were colorful blurry images. We expected that the nature scenes controlled (over the baseline images) in part for: a) attention because they have objects and complexity that can be visually explored, b) identifiable individual objects, such as trees, grass, mountains, sunsets, c) potential emotional responses, since nature scenes can be liked or admired, and possibly desired/craved. However, we did not specifically assess any parameters of the nature scenes. Nature images were generally matched for color, intensity, and brightness but not for additional visual parameters or complexity. Within each run, the order of cue blocks was random and was also random for each run. Participants saw each image only once across all three runs. Cannabis-related cues included close up pictures of cannabis in different forms (e.g. in bags, loose leaf, in joints), people using cannabis (e.g. smoking a joint or using a bong or pipe), and paraphernalia (e.g. bongs, pipes, papers). Images used for the baseline reference in the fMRI analysis (to account for non-specific visual activation) were Gaussian blurred images matched to the original cue image for color, intensity, and brightness. Nature scenes consisted of photographs of landscapes, animals, or insects that were a mixture of close up (e.g. a single blooming flower) and distant (e.g. a field of blooming flowers) images. The nature scenes did not include pictures of readily edible items, such as fruit or vegetables. Food images were included for a future exploratory analysis of the association of cannabis and feeding behavior and were not intended to serve as comparison images.
2.3.3. fMRI data preprocessing and analysis
All imaging was performed on a Philips Intera Achieva 3T MR scanner using a quadrature head coil. We analyzed fMRI data using Statistical Parametric Mapping (SPM5) (Wellcome Department of Cognitive Neuroscience, London, UK) utilizing the General Linear Model (GLM). Each individual’s functional data was spatially realigned to correct for motion. Next, the functional images were normalized into stereotactic space using SPM’s EPI.img template (Montreal Neurological Institute). The normalized functional images were then smoothed with a full-width half maximum (FWHM) 8 mm Gaussian kernel. Given that little published work has been conducted on cannabis cue activation, we conducted whole-brain exploratory analyses to precisely determine the pattern of regional brain activation produced by cannabis cues. To determine which regions of interest (ROIs) were activated or deactivated in response to cannabis cues, we analyzed the fMRI results of all 16 subjects using a 1-sample t-test (activation to cannabis cue>Gaussian baseline and activation to cannabis cue<Gaussian baseline) as our primary analysis within SPM5; a secondary analysis included an examination of activation to cannabis cue>nature and cannabis cue<nature, using the same methods. To reduce the number of multiple comparisons we applied a whole-brain gray matter mask to the data. We defined ROIs as those having significantly increased or decreased activation in response to cannabis-cues. Based on simulations performed with AlphaSim to control for Type I error http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf), an uncorrected voxel-level threshold of p<0.001 and an extent threshold of 30 contiguous voxels controlled for family-wise error at α = 0.05 for the gray matter mask search volume. For significant ROIs identified in the main analysis, we used MarsBar to calculate the BOLD response (as percentage signal change relative to Gaussian baseline) for each cue run for all subjects. The run-specific signal intensity data (i.e., BOLD response-run 1; BOLD response-run 2; BOLD response -run 3) was exported from SPM for assessing associations between craving scores and brain activation.
To determine if cue-induced activation in the cue-activated regions was relevant to craving, we analyzed the relationship of craving score to BOLD response during cue exposure in each of the activated or de-activated regions, starting with the cannabis versus baseline contrast. We chose to examine correlations between end of run MCQ and BOLD response in the cannabis versus baseline contrast as our primary analysis because we did not have a priori knowledge to predict that regions activated during liking or appreciation of nature cues were unique from those activated by cannabis cues. Additionally, we reasoned that regions irrelevant to cannabis craving would not show an association with craving score in subsequent analyses.
2.4. Ethics
All participants provided written informed consent for the study. This study was approved by the Institutional Review Board at Vanderbilt University and conformed to the Declaration of Helsinki. All participants were compensated for their time.
2.5. Statistical methods
The primary planned analysis was the correlation of end of run craving score with BOLD signal intensity in regions activated during the contrast of cannabis>Gaussian blurred baseline. Descriptive statistical summaries and associations of self-reported craving with cues and BOLD were conducted using SPSS (Windows version 17.0 software; SPSS Inc.). Descriptive statistical summaries used means and standard deviations unless otherwise specified (exceptions generally are due to the skewed nature of the drug use and brain activation data). In those cases, the median and 25th−75th interquartile range (IQR) representing the middle 50% of the observed values were used. Nonparametric statistical analytic methods were used to assess the statistical significance of changes in outcomes and associations with those variables because those data distributions were heavily skewed. Changes in self-reported craving (MCQ-SF scores) induced by exposure to cannabis cues were analyzed using a Freidman test (critical alpha=.05). Pairwise post-hoc comparisons were conducted using Wilcoxon Signed Ranks tests with a Bonferroni-corrected alpha of 0.008 (equivalent to p=0.05 divided by 6 to account for the number of possible pairs). Cohen’s d effect size estimate was also generated to examine the effect of cue exposure on changes in cannabis craving. Spearman correlations were used to measure the strength of the associations between regional BOLD signal intensity during cue-induced activation and self-reported craving scores. Because of the preliminary nature of this study, we did not correct for the number of correlation analyses conducted in this study. A Spearman correlation of 0.50 was sufficient to be statistically significant (p = 0.05) and was indicative of a strong effect size for correlations (25% shared variance between two variables).
3. Results
3.1. Cannabis use
The 16 subjects (11 females) had a mean age 23.7 (S.D. = 3.9 years) (Table 1). The mean age of onset for cannabis use was 15.06 (S.D. = 2.79); the median cannabis use was 15.5 occasions per week (25th−75th IQR=3.3–21.0; min = 1; max = 63). All subjects reported at least 8 hours cannabis abstinence; precise duration of cannabis abstinence was available for 15 of 16 subjects. For those 15 subjects, the minimum abstinence was 8 hours 15 minutes; the maximum was 19 hours and the mean duration of abstinence was 13.5 (S.D. = 2.1) hours.
3.2. Cue-induced Craving
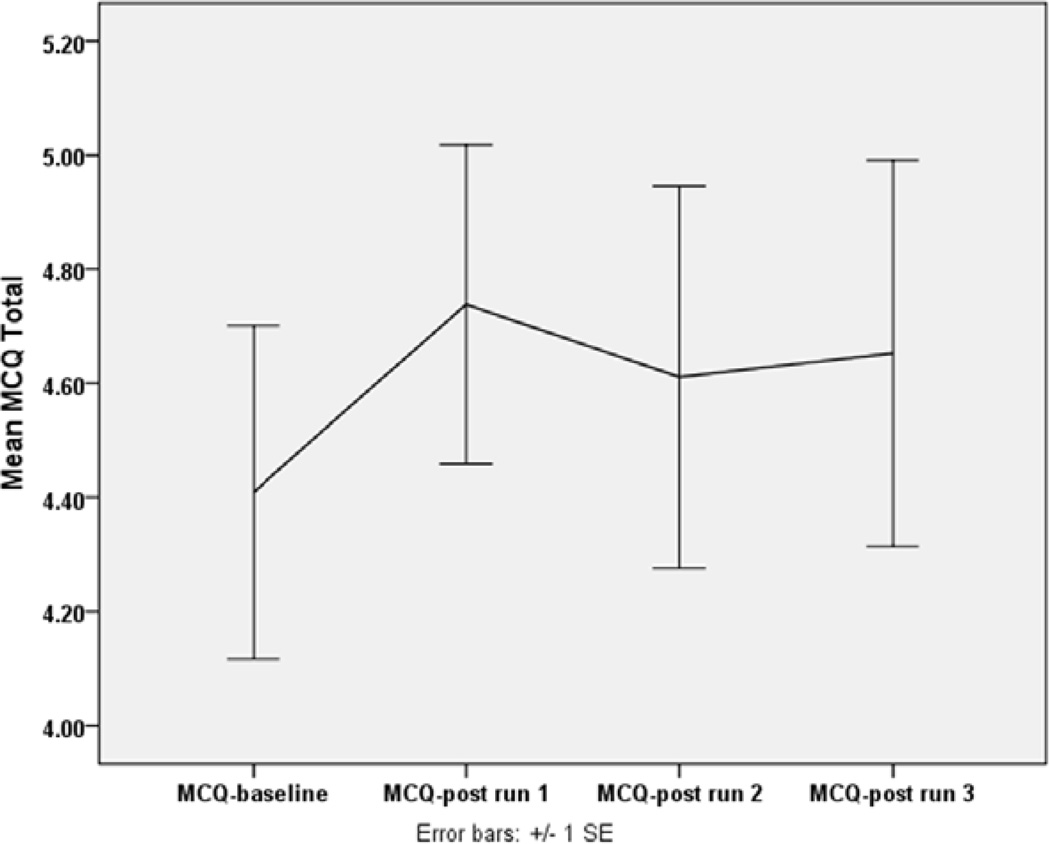
There was a statistically significant increase in self-reported craving due to exposure to cannabis cues (p=0.030, Friedman test) (Figure 1). Post-hoc comparisons revealed that the increase in reported craving from baseline (MCQ score at baseline to peak craving (MCQ score at post run 1) was statistically significant (p = 0.005, Wilcoxon Signed Ranks test) and the average of the three post exposure MCQ scores remained higher than baseline (p=0.004, Wilcoxin Signed Ranks test). This increase was 0.33 (on average) on a 7-point scale and indicated an effect size (Cohen’s d) of 0.29.
Figure 1. Craving score at baseline and after each cue exposure run.
Data are plotted as mean ± standard error of the mean for MCQ=Marijuana Craving Questionnaire at baseline (MCQ-baseline) and after each cue-exposure run (MCQ-post run 1, MCQ-post run 2, MCQ-post run 3).
3.3. Brain activation to visual cannabis cues
We examined brain activation to cannabis cues by three sets of contrasts: 1) activation to cannabis cues versus Gaussian baseline, 2) activation to cannabis cues versus nature cues, and 3) activation to cannabis cues versus food cues.
3.3.1. Brain activation to visual cannabis cues versus Gaussian baseline
3.3.1.1. Cannabis cues>Gaussian baseline
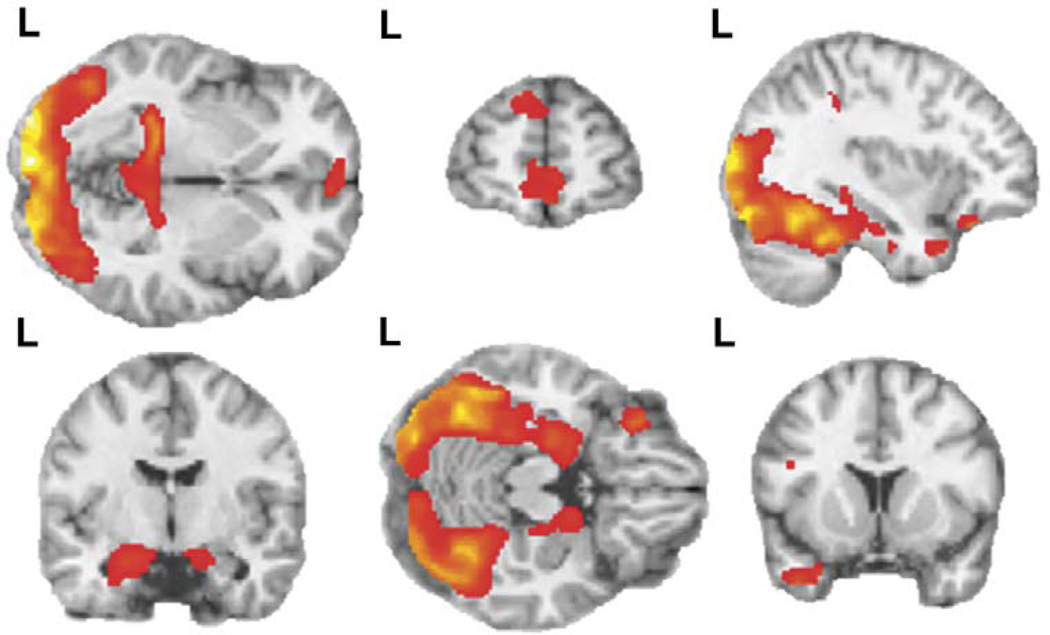
The contrast of cannabis cues (versus Gaussian baseline) across all cue-exposure runs produced 15 clusters in which activation was significantly greater during cannabis cue viewing than during Gaussian baseline viewing (Table 2, Cannabis greater than baseline—clusters 1–15; Figure 2). Cannabis cues produced increased activation in limbic regions (clusters 1, 2, 3,4, 5, and 6 in Table 2; parahippocampal gyrus, posterior cingulate gyrus, hippocampus, and amygdala), temporal regions (clusters 3, 7, 8, and 10 in Table 2; middle temporal gyrus, fusiform gyrus, and temporal pole), frontal regions (clusters 12, 13, 14, and 15 in Table 2; including the superior frontal, medial frontal, and inferior orbitofrontal, gyri), occipital regions (clusters 9, 10, and 11 in Table 2; calcarine, lingual, cuneus, and middle occipital gyrus), parietal cortex (cluster 2 in Table 2; precuneus), and thalamus (cluster 4 in Table 2).
Table 2.
Run 1 brain activation to cannabis cues>Gaussian baseline: correlation with cannabis craving
| MNI Coordinates |
Correlation Coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster # |
Side | Regions | Brodmann’s Area | x | y | z | ES | Pre- Run |
Run 1 |
Run 2 |
Run 3 |
| Cannabis greater than baseline | |||||||||||
| 1 | R | Posterior cingulate | 29 | 4 | 48 | 6 | 2.91 | 0.399 | 0.432 | 0.341 | 0.311 |
| 2 | L | Posterior cingulate, precuneus | 29, 30, 23 | −4 | −50 | 8 | 3.13 | 0.442 | 0.462 | 0.418 | 0.396 |
| 3 | R + L | Occipital cortex (including calcarine, lingual, cuneus), | 17–20, 23, 27–28, 34–37, 39 | −8 | −94 | −2 | 9.72 | 0.431 | 0.285 | 0.471 | 0.530 |
| fusiform gyrus, parahippocampal gyrus, hippocampus, amygdala | |||||||||||
| 4 | R | Parahippocampal gyrus; thalamus; hippocampus | 27, 35 | 18 | −32 | −4 | 2.75 | 0.487 | 0.494 | 0.456 | 0.511 |
| 5 | R | Parahippocampal gyrus, including amygdala; hippocampus | 34 | 16 | −2 | −18 | 2.87 | 0.346 | 0.321 | 0.368 | 0.427 |
| 6 | L | Hippocampus | −28 | −32 | −8 | 4.14 | 0.568 | 0.518 | 0.535 | 0.552 | |
| 7 | L | Superior temporal pole | 38, 28 | −28 | 12 | −30 | 3.00 | 0.512 | 0.529 | 0.509 | 0.572 |
| 8 | L | Middle temporal pole | 21, 38 | −40 | 12 | −34 | 3.51 | 0.046 | 0.065 | 0.006 | 0.082 |
| 9 | R | Middle occipital gyrus | 19 | 30 | −90 | 16 | 4.91 | 0.484 | 0.300 | 0.488 | 0.514 |
| 10 | R | Middle occipital gyrus; middle temporal gyrus | 19, 18, 39 | 28 | −94 | 4 | 5.80 | 0.305 | 0.144 | 0.365 | 0.418 |
| 11 | L | Middle occipital gyrus | 19 | −32 | −90 | 16 | 5.69 | 0.378 | 0.221 | 0.412 | 0.434 |
| 12 | R | Superior frontal gyrus | 9, 10 | 2 | 62 | 0 | 3.29 | 0.152 | 0.174 | 0.094 | 0.138 |
| 13 | L | Superior frontal gyrus | 9 | −10 | 58 | 34 | 2.78 | 0.231 | 0.153 | 0.203 | 0.202 |
| 14 | L | Medial frontal gyrus | 10 | −4 | 62 | 2 | 2.76 | 0.362 | 0.415 | 0.294 | 0.296 |
| 15 | L | Inferior orbitofrontal | 47, 11 | −30 | 30 | −20 | 4.32 | 0.369 | 0.368 | 0.379 | 0.368 |
| Cannabis less than baseline | |||||||||||
| 16 | R | Inferior parietal; supramarginal gyrus; angular gyrus | 40 | 56 | 46 | 46 | 3.57 | 0.081 | 0.176 | 0.091 | 0.202 |
| 17 | L | Insula; superior temporal gyrus | 22 | 48 | 16 | 6 | 2.89 | 0.015 | 0.041 | 0.029 | −0.025 |
| 18 | R | Superior temporal | 42, 41 | 64 | 22 | 10 | 2.34 | 0.353 | 0.321 | 0.368 | 0.402 |
| 19 | L | Superior temporal | 41 | 46 | 36 | 12 | 2.56 | −0.088 | 0.021 | 0.115 | −0.035 |
Statistical Parametric Analysis (SPM) was performed using a 1-sample t-test for all subjects across all runs with the contrast of activation during cannabis cues versus baseline activation (n=16). Voxel threshold p=0.001, extent threshold k=30, family-wise corrected p= 0.05. Montreal Neurological Institute (MNI) coordinates and effect size (ES; Cohen’s d) correspond to peak voxel in each region. Bold font indicates that BOLD signal intensity (as the measure of activation) in this region was found to be correlated with craving at the threshold of p≤0.05.
Figure 2. Brain activation during comparison of visual cannabis cues > Gaussian baseline.
Both rows depict activation in response to visual cannabis cues versus baseline (activation is overlaid on sagittal, coronal, and horizontal sections of MNI single subject template). There were four clusters bilaterally in the occipital cortex including portions of calcarine, lingual, cuneus, fusiform and middle occipital gyri (Table 2). Four clusters were activated in bilateral temporal lobe, including parahippocampal gyrus, hippocampus, and amygdala. An additional confluent cluster included regions in both the occipital cortex and parahippocampal gyrus. Further clusters were present in the temporal gyrus including the superior temporal pole (left) and middle temporal gyrus (bilaterally), and in the posterior cingulate (bilaterally). In frontal cortex, activated clusters included the inferior orbitofrontal cortex (left), superior frontal gyrus (bilaterally), and medial frontal gyrus (left). Voxel threshold p=0.001, extent threshold k=30; for display, gray matter mask removed to show regional continuity. t-score color bar corresponding to activated regions is shown in figure 2. L=left side of brain for each figure.
Correlation with craving
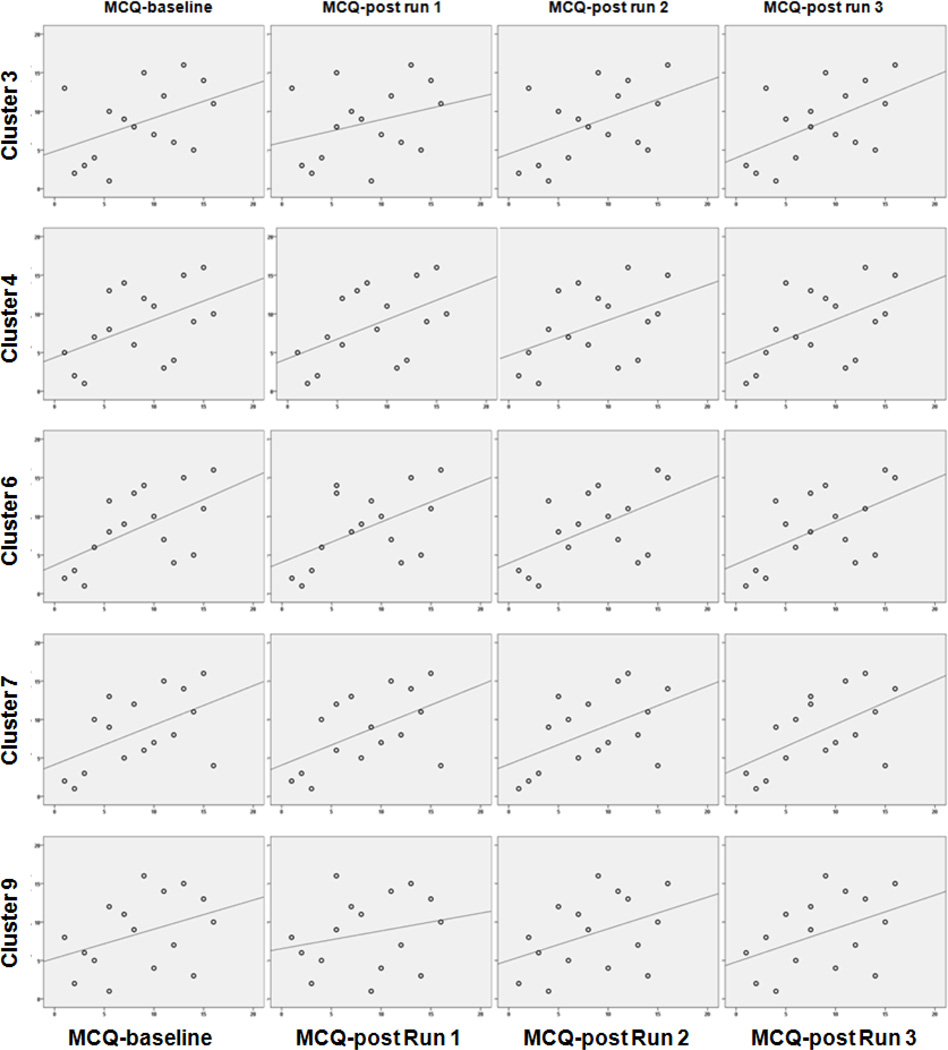
The strongest and most consistent patterns of associations of the self reports of craving with activation were found within five of the clusters activated more strongly by cannabis cues versus Gaussian baseline (clusters 3, 4, 6, 7 and 9; bolded correlations, rs >= 0.50, p < 0.05, Table 2). These patterns were particularly apparent in the occipital cortex (cluster 3), parahippocampal gyrus, thalamus, hippocampus regions (cluster 4), hippocampus (cluster 6), superior temporal pole (cluster 7), and middle occipital gyrus (cluster 9). These patterns are also depicted graphically in Figure 3. Statistically significant correlation coefficients ranged from 0.509 to 0.572; p values ranged from 0.044 to 0.020).
Figure 3. Correlation of MCQ craving score with BOLD signal intensity (cannabis>Gaussian baseline) during Run 1.
Scatterplots of craving score correlation with activation (BOLD signal intensity from the contrast of cannabis cues>Gaussian images) from Run 1. Rows correspond to the 5 clusters (highlighted in bold font in Table 2) that showed at least one significant correlation of marijuana craving questionnaire (MCQ) total score and BOLD signal intensity-run 1. X-axes indicate rank MCQ score from left to right as MCQ-baseline, MCQ-post run 1, MCQ-post run 2, MCQ-post run 3. Y-axes indicate rank BOLD signal intensity-run 1 extracted from each cluster. Correlations coefficients for the depicted data are shown in Table 2.
3.3.1.2. Cannabis cues<Gaussian baseline
The contrast of visual cannabis cues versus Gaussian baseline across all cue-exposure runs produced four clusters in which activation was significantly lower during visual cannabis cue viewing than during Gaussian baseline viewing (Table 2, Cannabis less than baseline—clusters 16–19; Figure 2). Visual cannabis cues produced decreased activation in parietal regions (cluster 16 in Table 2; inferior parietal cortex, supramarginal and angular gyri), insula (cluster 17 in Table 2), and temporal regions (clusters 17, 18 and 19 in Table 2; superior temporal gyrus).
Correlation with craving
No statistically significant correlations of Run 1 activation with craving scores were observed in these regions (Table 2).
3.3.2. Brain activation to visual cannabis cues versus nature cues
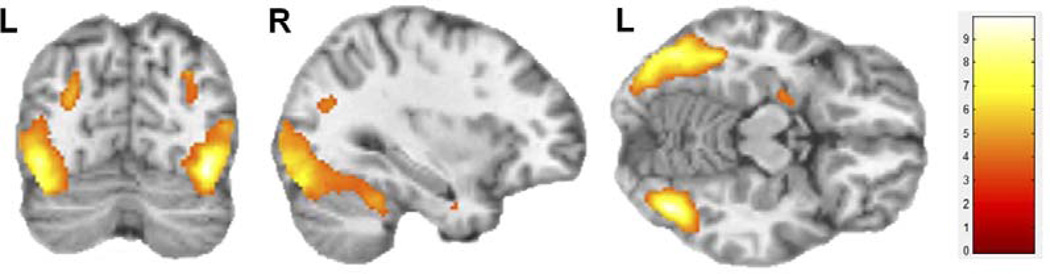
3.3.2.1. Cannabis cues>nature cues
The contrast of visual cannabis versus nature cues produced fewer clusters of activation than did the contrast of cannabis versus baseline but these clusters largely overlapped those found in the cannabis versus Gaussian baseline contrast (Table 3; Figure 4). Five regions demonstrated greater activation during cannabis cues than during nature images (Table 3; Figure 4). These activated clusters included occipital regions (clusters 1, 2, 3, and 4 in Table 3; inferior and middle occipital gyri), temporal regions (clusters 2, 3, 4 and 5 in Table 3; fusiform, middle, and inferior temporal gyri and uncus), limbic regions (cluster 5; hippocampus, amygdala, uncus), and cerebellum (cluster 2 in Table 3).
Table 3.
Run 1 brain activation to cannabis cues>nature cues: correlation with cannabis craving
| MNI Coordinates |
Correlation Coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clus ter # |
Side | Regions | Brodmann’s Area | x | y | z | ES | Pre- Run |
Run 1 | Run 2 | Run 3 |
| Cannabis greater than nature | |||||||||||
| 1 | R | Inferior occipital gyrus | 18, 19 | 42 | −74 | −8 | 4.64 | −0.196 | −0.262 | −0.209 | −0.275 |
| 2 | L | Fusiform gyrus (inferior occipital gyrus, middle occipital gyrus, middle temporal gyrus, cerebellum crus1, cerebellum) | 18, 19, 20, 37 | −40 | −42 | −22 | 4.21 | −0.177 | −0.218 | −0.133 | −0.146 |
| 3 | L | Inferior occipital gyrus (lingual gyrus, fusiform gyrus) | 18, 19 | −42 | −74 | −8 | 4.15 | −0.004 | −0.085 | −0.021 | −0.093 |
| 4 | R | Fusiform gyrus (middle temporal gyrus, inferior temporal gyrus, inferior occipital gyrus) | 18, 19, 37, 39 | 42 | −68 | −18 | 4.07 | −0.118 | −0.237 | −0.043 | −0.065 |
| 5 | L | Hippocampus (amygdala, uncus) | 34 | −20 | −6 | −20 | 2.63 | 0.001 | 0.071 | −0.015 | 0.085 |
Statistical Parametric Analysis (SPM) was performed using a 1-sample t-test for all subjects across all runs with the contrast of activation during cannabis cues > activation during nature images (n=16). Voxel threshold p=0.001, extent threshold k=30, family-wise corrected p= 0.05. Montreal Neurological Institute (MNI) coordinates and effect size (ES; Cohen’s d) correspond to peak voxel in each region. BOLD signal intensity (as the measure of activation) was extracted from activated regions for all runs. Only the correlation of Run 1 BOLD signal intensity with craving score is shown. There were no regions in which BOLD signal intensity was found to be correlated with craving at the threshold of p≤0.05.
Figure 4. Brain regions activated during comparison of visual cannabis cues > nature cues.
Statistical Parametric Analysis (SPM) was performed using a 1-sample t-test for all subjects across all runs with the contrast of activation during cannabis cues versus natures cues (n=16). This contrast revealed 5 clusters as shown in Table 3. Voxel threshold p=0.001, extent threshold k=30; for display, gray matter mask removed to show regional continuity. Montreal Neurological Institute (MNI) coordinates used for labeling. Color bar represents t-scores.
Correlation with craving
No statistically significant correlations of Run 1 activation with craving scores were observed in the regions showing greater activation during cannabis cue viewing versus nature cues. (All correlations were Spearman’s rho, using p≤0.05 as significance threshold; Table 3). Across other runs, the only significant relationship found was an inverse association of craving score and activation in the cluster encompassing right fusiform gyrus for activation during run 2 and craving during baseline (r= −0.539; p=0.031), run 2 (r= −0.568; p=0.022) and run 3 (r= −0.545; p=0.029). However, a similar but non-significant association of craving for run 1 was seen for the activation during run 2 (r= −0.42; p=0.105).
3.3.2.2. Cannabis cues<nature cues
No regions demonstrated greater activation to nature than to cannabis (p>0.05).
3.3.3. Brain activation to visual cannabis cues versus food cues
3.3.3.1. Cannabis cures>food cues
There were 5 clusters in which activation during viewing cannabis cues was greater than activation during food images (Table 4; Figure 5). These activated clusters included frontal regions (cluster 1in Table 4), temporal regions (clusters 2, 3, 4, and 5 in Table 4; inferior, middle, and superior temporal gyri and fusiform gyrus) and occipital regions (clusters 2 and 4 in Table 4; middle occipital gyrus).
Table 4.
Run 1 brain activation to cannabis cues>food cues: correlation with cannabis craving
| MNI Coordinates |
Correlation Coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clus ter # |
Side | Regions | Brodmann’s Area | x | y | z | ES | Pre- Run |
Run 1 | Run 2 | Run 3 |
| Cannabis greater than food | |||||||||||
| 1 | R | Medial orbital frontal gyrus | 10, 11 | 6 | 58 | −6 | 3.72 | 0.702 | 0.674 | 0.715 | 0.790 |
| 2 | R | Middle temporal gyrus (superior temporal gyrus, middle occipital gyrus, inferior temporal gyrus) | 19, 22, 37, 39 | 54 | −64 | 12 | 3.28 | 0.019 | 0.171 | −0.106 | 0.003 |
| 3 | L | Inferior temporal gyrus (fusiform gyrus) | 19, 20 | −46 | −46 | −24 | 3.16 | −0.414 | −0.312 | −0.491 | −0.396 |
| 4 | L | Middle occipital gyrus (middle temporal gyrus, inferior temporal gyrus) | 19, 22, 37, 39 | −44 | −82 | 4 | 2.87 | −0.026 | 0.106 | −0.129 | 0.034 |
| 5 | R | Fusiform gyrus (inferior temporal gyrus) | 37 | 40 | −50 | −22 | 2.76 | −0.474 | −0.441 | −0.465 | −0.444 |
Statistical Parametric Analysis (SPM) was performed using a 1-sample t-test for all subjects across all runs with the contrast of activation during cannabis cues>activation during food images (n=16). Voxel threshold p=0.001, extent threshold k=30, family-wise corrected p= 0.05. BOLD signal intensity (as the measure of activation) was extracted from activated regions for all runs. Montreal Neurological Institute (MNI) coordinates and effect size (ES; Cohen’s d) correspond to peak voxel in each region.
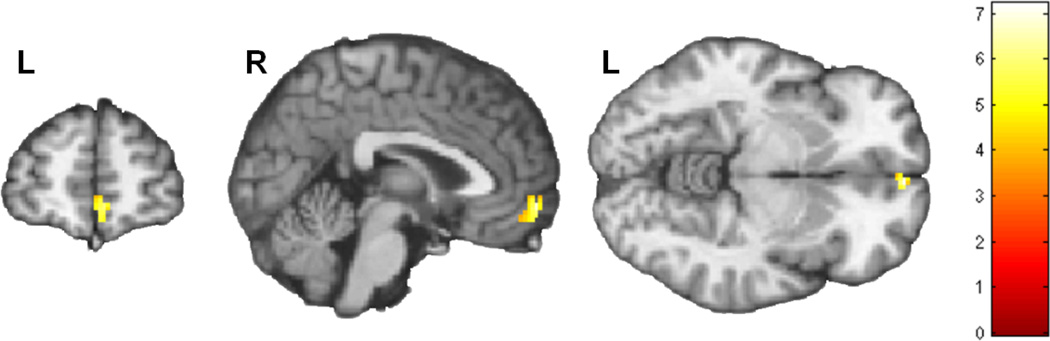
Figure 5. Brain regions activated during comparison of visual cannabis cues > food cues.
Statistical Parametric Analysis (SPM) was performed using a 1-sample t-test for all subjects across all runs with the contrast of activation during cannabis cues versus food cues (n=16). This contrast revealed 5 clusters as shown in Table 4. Voxel threshold p=0.001, extent threshold k=30; for display, gray matter mask removed to show regional continuity. Montreal Neurological Institute (MNI) coordinates used for labeling. Color bar represents t-scores.
Correlation with craving
For the 5 clusters in which activation was greater during viewing cannabis cues than food images, MCQ craving score at baseline and at the end of runs 1, 2, and 3 was positively correlated (r values ranging from 0.674 to 0.790; p values ranging from 0.004 to 0.001; bolded in Table 4) with Run 1 activation in cluster 1, which had peak activation in the right medial orbital frontal gyrus. There were no statistically significant associations for any MCQ craving score with activation during Run 1 in the other 4 regions activated during cannabis cues>food images (Table 4). There were no significant associations of craving score at any time with brain activation in any of the 5 regions during runs 2 and 3 (not shown). All correlations were Spearman’s rho, using p≤0.05 as significance threshold.
3.3.3.2. Cannabis cures<food cues
There were no significant clusters for the contrast of cannabis cues<food cues (p>0.05).
4. Discussion
Our major finding is that visual cannabis cues increased craving in cannabis users who had refrained from cannabis use for at least 8 hours and that cue-induced craving significantly correlated with activation in limbic, occipital, and temporal regions. These brain regions overlap partially with brain regions previously associated generally with craving and relapse (Weiss, 2005) and overlap somewhat with the regions we predicted (orbitofrontal and visual cortices) to show an association with visual cue-induced craving; however, we did not find a predicted relationship with striatal and anterior cingulate regions.
4.1. Visual cannabis cues increase craving and activate frontal, limbic, occipital and temporal regions
That exposure to visual cannabis cues resulted in a significant increase in self-reported craving is in line with recent reports (Gray et al., 2008; Bordnick et al., 2009). Visual cannabis cues produced increased activation in frontal, limbic, temporal, occipital, and parietal cortices as well as in the thalamus. These regions overlap partially with findings from other studies of cue-induced activation and craving for drugs other than marijuana (e.g. Childress et al., 1999; Franklin et al., 2007); Kilts et al., 2004; Koob and Volkow, 2010). Similarly, our findings overlap partially with those reported by Filbey and colleagues (Filbey et al., 2009) in their study using a combined visual and tactile drug cue (marijuana pipe). Notably, the ventral tegmental area (VTA) and dorsal anterior cingulate cortex were activated by cannabis cues in the Filbey et al., study and those regions were not detected in our cohort. Filbey and colleagues further reported (Filbey et al., 2010) that genetic variations in genes encoding the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) influenced cue-induced activation. Since we did not obtain genetic data on our cohort, we cannot comment on the role of genetic influences. However, it seems likely that genetic effects, cohort differences (heavy cannabis users in Filbey et al. and cannabis-dependent participants in the current study), and paradigm differences, especially cue type (visual and tactile) and craving rating methods may account for differential findings between the present study and that of Filbey et al., (2009). The visual cues used in our study are perhaps less ecologically valid in terms of the actual cannabis use experience because the combined visual/tactile cue employed by Filbey and colleagues (Filbey et al., 2010;Filbey et al., 2009) may represent a cue that is more proximate to actual cannabis use. However, the fact that purely visual cues are less reliable or more distant indicators of the actual availability of cannabis could possibly enhance their ability to induce craving, suggesting that cue choice and relevance may be critical aspects of study designs examining the neurobiology of cue-associated craving.
The cingulate gyrus has been strongly implicated in activation during drug cue exposure or craving. Anterior cingulate activation is also often found in imaging studies during drug cue viewing and drug craving (Childress et al., 1999;Garavan et al., 2000; Kilts et al., 2004; Koob and Volkow, 2010). We found posterior cingulate activation in response to cannabis cues but did not detect anterior cingulate activation in our cohort. Posterior cingulate activation to drug cues has been found in prior studies (Kilts et al., 2004; Garavan et al., 2000) posterior cingulate perfusion correlates with cue-induced nicotine craving; and greater posterior cingulate activation to drug cues predicted a lower risk for relapse in cocaine-dependent patients (Kosten et al., 2006).
Activation of the ventral striatum/nucleus accumbens region has been reported in some studies of drug craving, reviewed in (Yalachkov et al., 2013) and in a recent report of cannabis craving (Goldman et al., 2012) We did not detect activation in this region in our study, a finding in line with the study of Filbey and colleagues (Filbey et al., 2009) and Cousijn and colleagues (Cousijn et al., 2012). The reasons for these discrepancies are unclear but may relate to different levels of cannabis use, different levels of craving, or other methodological differences across studies. Additionally, we did not assess for the contribution of genetic variants potentially influencing cannabis craving or cue-evoked brain activation (Schacht et al., 2012; Haughey et al., 2008; Haughey et al., 2008; Filbey et al., 2010).
4.2. Craving scores correlate with brain activation in frontal, limbic, temporal, and occipital regions
To our knowledge, this report is among a handful of reports (Goldman et al., 2013; Cousijn et al., 2012) linking self-reported cannabis craving to activation in specific brain regions. Notably, Filbey and colleagues specifically examined the association of craving with brain activation to 3-dimensional tactile marijuana cues (while we used only 2-dimensional visual cues), and did not find an association between craving and brain activation. However, tactile cue-associated brain activation was found to correlate with problems associated with marijuana use. Differences in the association of craving score with brain activation in our report and that of Filbey and colleagues may be related to experimental differences, including the use of tactile versus visual cues, duration of abstinence, and method for assessing cannabis craving. Cousijn and colleagues reported that brain activation to visual cannabis cues in right dorsolateral prefrontal cortex and right putamen was negatively correlated with cannabis craving in a group of frequent cannabis users, but no regions were reported to show a positive association between craving and cue-induced brain activation (Cousijn et al., 2012). We found a negative association of craving and brain activation in the contrast of cannabis>nature cues in a region of fusiform gyrus for later runs. This suggests the possibility that this brain region which has a role in visual salience may be initially involved in cue associated craving but with sustained cue exposure, it may be inhibited by higher brain regions. In contrast, Goldman and colleagues (Goldman et al., 2013) examined the relationship of brain activation and marijuana cue exposure in treatment seeking cannabis dependent individuals. Unlike our current study, cannabis cue exposure did not increase craving above the pre-cue exposure baseline. However, baseline craving scores were found to correlate positively with cue-associated activation in ventral striatum and medial and lateral orbitofrontal cortex. With regard to orbitofrontal cortex, we found a non-significant positive correlation of craving score and Run 1 brain activation in the region of orbitofrontal cortex activated more strongly in the contrast of cannabis cues>Gaussian baseline images, and strongly significant correlations for the correlation of craving score and Run1 brain activation in the region of orbitofrontal cortex activated more strongly in the contrast of cannabis cues>food cues. This range of findings suggests that the association of cannabis cue viewing and cannabis craving is dependent on cue type, level of craving, and other individual differences, as well as the type of contrast comparison. We had anticipated that the relationship of craving with orbitofrontal activation would be strongest for the contrast of cannabis cues>Gaussian baseline images and weakest for the contrast of cannabis cues>food images because we expected some degree of overlap in food and cannabis-associated craving, given their role as reinforcers. However, our findings suggest the possibility that the use of a food cue comparison increased specificity of orbitofrontal activation for cannabis craving.
In our study, craving scores both at baseline (MCQ-baseline) and following cannabis cue exposure for each run (MCQ-post run 1, MCQ post run 2, MCQ post run 3) were correlated positively with activation of the parahippocampal gyrus, amygdala and hippocampus, and the superior temporal pole. Cannabis cue exposure increased craving from baseline, with a modest effect size (0.29), while the correlation coefficients for BOLD signal change and MCQ score in significant clusters were generally above 0.5, consistent with a large observed effect size (Cohen, 1992). The amygdala and hippocampus are limbic structures with key roles in the neural mechanisms of learning and memory affecting drug-related behaviors--the amygdala is central to conditioned incentive learning and promotes interaction with drug-related cues while the hippocampus is central to the declarative memory system and the acquisition of information about the relationship between external cues and internal affective states (White, 1996).The temporal pole is a paralimbic associational structure having multimodal perceptual features, memory functions, and that also plays a role in social and emotional processing (Olson et al., 2007). In addition to the findings from limbic and temporal pole regions, craving scores both at baseline and following cue-induced increases in craving were correlated positively with cue-related activation in occipital cortical regions, including the cuneus and the middle occipital, calcarine, lingual, and fusiform gyri (a temporal lobe structure continuous with occipital cortex).
Activation in these brain regions during the initial fMRI cue-exposure run was correlated with craving scores prior to cannabis cue exposure and across subsequent runs; however, activation in these regions in subsequent cue exposure runs (Runs 2 and 3) did not correlate significantly with cannabis craving scores. This result suggests that the cannabis cue-activated limbic, occipital and temporal regions may have a role in the initial response to visual cannabis cues that is not sustained, despite the persistence of the increase in craving. It is possible that the lack of a sustained correlation between BOLD signal intensity in cannabis cue-activated regions during late runs (runs 2 and 3) and craving at the end of runs 2 and 3 is due to adaptation of the BOLD response, as has been demonstrated for other brain regions and tasks (Grill-Spector et al., 2006). It is also possible that the level of craving may influence or predict the brain activation in these regions upon the initial exposure to cannabis cues, such that heightened craving may lead to heightened activation in these regions in the face of initial cue exposure. We speculate that these regions therefore may link higher craving and subsequent behavioral repertoires associated with cue-induced craving and drug seeking, leading to relapse.
We believe that this finding in part validates our analytic design, that is, to identify cue-activated regions and then determine how activation in these regions correlates with craving. These findings highlight the fact that the simultaneous occurrence of craving and brain activation in response to drug cues does not necessarily imply an association between the two, as we found no association of craving and brain activation in the cue-activated regions during later runs (runs 2 and 3). The bulk of the brain regions (14 of 19) activated during cannabis cue exposure do not appear to be directly correlated with craving, but may be related to other aspects of the cues, such as affective memory, cue salience, taste or smell memory. This finding aligns in part with the clinical findings regarding craving in that cue-triggered craving is not a dichotomous phenomena but instead may wax and wane in intensity (Franken, 2003). As such, additional studies targeted at more precisely identifying the regional and network patterns associated with the intensification and decline in craving in the presence of cannabis cues seem essential. In particular, understanding the relationship of brain activation, craving, and subsequent drug use will be critical to interventions targeted at altering brain activity mediating cue-induced drug use.
4.3. Limitations
Limitations of this study include a relatively small sample size and brief duration of cannabis abstinence—however; these limitations would be expected to reduce the likelihood of detecting effects so are unlikely to account for the positive findings in this study. We relied on each subject to serve as their own control for the difference in reaction to cannabis and neutral cues and did not include a control group of non-cannabis users. Several subjects reported alcohol or other drug use disorders. While this may have impacted brain activation if we had used other drug or alcohol cues as baseline images, it is unclear if prior drug or alcohol use interacted with cannabis craving to influence brain activation. A larger sample size with subjects excluded for other drug dependence would help address this issue. Our sample was primarily female, which may have influenced our results, given evidence that addiction, including cannabis dependence, shows sex-specific differences and that females may be more sensitive to drug cues, reviewed in (Fattore, 2013). We did not include a cannabis naïve control group, thus, we cannot address differences in brain activation for cannabis users and non-users. Small sample size and brief abstinence period that was not empirically confirmed may account for the subthreshold correlation of craving with brain activation in the orbitofrontal cortex for our primary analysis, although this relationship was found for the contrast with food cues. However, since we relied on self-report of craving score in our correlation analysis, the duration of abstinence is expected to be factored into the impact on craving, that is, by using craving as a metric, abstinence is relevant only in relation to its ability to drive craving. It is possible also that using longer fMRI runs or an overall greater number of cue exposure events might have detected additional brain regions related to cannabis craving. The fact that craving was present prior to cue onset and during the baseline periods used to determine brain activation suggests that the modeling methods that we used in SPM likely underestimate the degree of brain activation produced by cannabis cues because the component of activation due to craving that persisted outside the period of cue exposure would have been modeled out of the activation data. Future study designs, incorporating fMRI image acquisition during craving induction and return to baseline, with more frequent craving assessment and manipulation of the duration of abstinence may permit a more detailed analysis of the time course of brain activation patterns in relation to cue exposure and craving. While we used corrected statistics to determine activation within SPM to define cue-associated activations, we did not correct for the large number of correlation analyses performed in this exploratory analysis. Instead, we focused on the pattern and regional specificity of the correlations.
4.4. Conclusions
Cannabis cues increased craving in cannabis-dependent individuals and this increase in craving was associated with activation in the in the limbic, paralimbic, and visual systems, suggesting that these regions may mediate visually cued aspects of drug craving. This study provides preliminary evidence for the neural basis of cue-induced cannabis craving and suggests possible neural targets for interventions targeted at treating cannabis dependence. Understanding the neurobiology of craving may be specifically important to developing interventions, such as psychotherapy or meditation interventions that augment the function of specific brain regions. In addition, pharmacotherapies or emerging therapies such as transcranial magnetic stimulation that influence regional brain function may be specifically employed to target brain regions or brain networks involved in craving.
Acknowledgements
This study was supported in part by the Vanderbilt Institute for Clinical and Translational Research (VICTR) grant 1UL1 RR024975 from the National Center for Research Resources (NCRR/NIH), and in part from grants R01 DA01537 and R21 DA020149 from the National Institute on Drug Abuse (NIDA/NIH) (to RLC), 1R01 DA015713 from NIDA/NIH (to PRM), and from the Vanderbilt Addiction Center. MMB was supported in part by DK69465; JUB was supported in part by grant K01 MH083052. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research Location: Vanderbilt University Medical Center
Author’s contribution
EJC, MSD, MMB, SP, PRM, MSB, MB and RLC were responsible for the study concept and design. EJC, MSD, AC, TJW, JUB, MMB, PRM, MB and RLC assisted with data analysis and interpretation of the findings. EJC, TJW, MSB, and RLC performed the experiments. EJC, MSD, SP, JUB, MMB, PRM, MB, and RLC provided critical revision of the manuscript important for intellectual content. All authors critically reviewed content and approved the final version for publication.
Financial Disclosures
Within the past 3 years, Dr. Cowan has received publication royalties from Lippincott Williams and Wilkins, consultant income from the Southwest Michigan First Life Science Fund and the University of West Alabama, and research and salary support from Shire Pharmaceuticals and Novo Nordisk for projects not overlapping with this report.
Reference List
- Bordnick PS, Copp HL, Traylor A, Graap KM, Carter BL, Walton A, Ferrer M. Reactivity to cannabis cues in virtual reality environments. Journal of Psychoactive Drugs. 2009;41:105–112. doi: 10.1080/02791072.2009.10399903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. Journal of Neural Transmission. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, Martin PR. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011 Mar 8;6(3):e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addiction Science and Clinical Practice. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. The American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addiction Biology Epub ahead of print. 2012 doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L. Considering gender in cannabinoid research: a step towards personalized treatment of marijuana addicts. Drug Testing and Analysis. 2013;5:57–61. doi: 10.1002/dta.1401. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proceedings of the National Academy of Sciences, U.S.A. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuropsychopharmacology and Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. The American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldman M, Szucs-Reed RP, Jagannathan K, Ehrman RN, Wang Z, Li Y, Suh JJ, Kampman K, O’brien CP, Childress AR, Franklin TR. Reward-related Brain Response and Craving Correlates of Marijuana Cue Exposure: A Preliminary Study in Treatment-seeking Marijuana-dependent Subjects. Journal of Addiction Medicine. 2012 doi: 10.1097/ADM.0b013e318273863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Upadhyaya HP. Cue reactivity in young marijuana smokers: a preliminary investigation. Psychology of Addictive Behaviors. 2008;22:582–586. doi: 10.1037/a0012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berlin) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and Alcohol Dependence. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. Methods in Molecular Medicine. 2006;123:209–216. doi: 10.1385/1-59259-999-0:209. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. The American Journal of Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Macdonald S, Hall W, Roman P, Stockwell T, Coghlan M, Nesvaag S. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408–416. doi: 10.1111/j.1360-0443.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology (Berlin) 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37:2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Gorres A, Seehaus A, Naumer MJ. Sensory modality of smoking cues modulates neural cue reactivity. Psychopharmacology (Berlin) 2013;225:461–471. doi: 10.1007/s00213-012-2830-x. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neuroscience and Biobehavioral Reviews. 2012;36:825–835. doi: 10.1016/j.neubiorev.2011.12.004. [DOI] [PubMed] [Google Scholar]