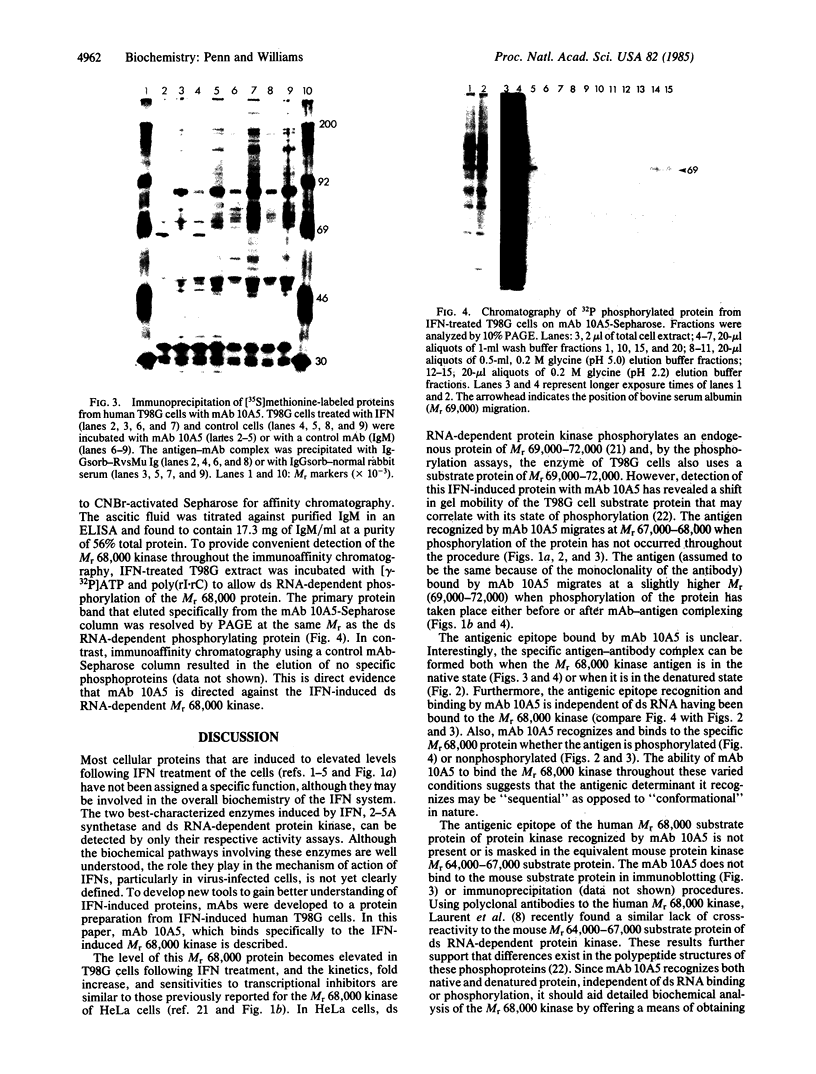
Abstract
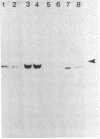
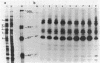
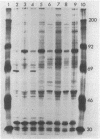
One of the interferon-induced proteins thought to be involved in the antiviral effects of interferon is a double-stranded RNA-dependent protein kinase. This paper reports the development of a monoclonal antibody, 10A5, that recognizes a protein that co-migrates with the double-stranded RNA-dependent protein kinase at an approximate molecular weight of 68,000. Levels of this protein and of the protein kinase activity increase 3-fold on interferon treatment of T98G cells. The specificity of the monoclonal antibody was determined by ELISA, immunoblotting, and immunoprecipitation procedures. Furthermore, immunoaffinity chromatography of an interferon-induced T98G cell extract previously phosphorylated in the presence of double-stranded RNA and radiolabeled ATP resulted in the specific elution of a phosphorylated Mr 68,000 protein from the monoclonal antibody 10A5-Sepharose column. Monoclonal antibody 10A5 recognizes both native and denatured protein kinase, independent of double-stranded RNA binding or phosphorylation, and should therefore serve as a useful tool in analyzing the role of the double-stranded RNA-dependent protein kinase in the mechanism of interferon action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Fantes K. H., Burke D. C., Morser J. Analysis and purification of human lymphoblastoid (Namalwa) interferon using a monoclonal antibody. J Gen Virol. 1982 Nov;63(Pt 1):207–212. doi: 10.1099/0022-1317-63-1-207. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Maroney P. A., West D. K. 2'5'Oligo(A) polymerase activity and inhibition of viral RNA synthesis in interferon-treated HeLa cells. Biochemistry. 1979 May 1;18(9):1765–1770. doi: 10.1021/bi00576a020. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Pang R. H. Induction of unique mRNAs by human interferons. J Biol Chem. 1982 Aug 25;257(16):9234–9237. [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. The (2'-5') oligoadenylate (pppA2'-5'A2'-5'A) synthetase and protein kinase(s) from interferon-treated cells. Eur J Biochem. 1979 Feb 1;93(3):515–526. doi: 10.1111/j.1432-1033.1979.tb12850.x. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Meurs E., Aujean O., Vaquero C., Stefanos S., Falcoff E. Antiviral response and induction of specific proteins in cells treated with immune T (type II) interferon analogous to that from viral interferon (type I)-treated cells. Virology. 1980 Jul 15;104(1):195–204. doi: 10.1016/0042-6822(80)90377-3. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. Fibroblast interferon induces synthesis of four proteins in human fibroblast cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1824–1827. doi: 10.1073/pnas.76.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust B., Galabru J., Hovanessian A. G. Further characterization of the protein kinase activity mediated by interferon in mouse and human cells. J Biol Chem. 1984 Jul 10;259(13):8494–8498. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurent A. G., Krust B., Svab J., Hovanessian A. G. Characterisation of the interferon-mediated protein kinase by polyclonal antibodies. Biochem Biophys Res Commun. 1984 Nov 30;125(1):1–7. doi: 10.1016/s0006-291x(84)80324-1. [DOI] [PubMed] [Google Scholar]
- Penn L. J., Williams B. R. Interferon-induced 2-5A synthetase activity in human peripheral blood mononuclear cells after immunization with influenza virus and rubella virus vaccines. J Virol. 1984 Mar;49(3):748–753. doi: 10.1128/jvi.49.3.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Interferon-induced proteins in human fibroblasts and development of the antiviral state. J Virol. 1980 May;34(2):446–454. doi: 10.1128/jvi.34.2.446-454.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Analysis of the oligosaccharides on the HLA-DR and DC1 B cell antigens. J Immunol. 1983 Jan;130(1):274–282. [PubMed] [Google Scholar]
- Stein G. H. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J Cell Physiol. 1979 Apr;99(1):43–54. doi: 10.1002/jcp.1040990107. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Swyryd E. A., Stark G. R. An improved method for purifying 2',5'-oligoadenylate synthetases. J Biol Chem. 1984 Jan 25;259(2):1363–1370. [PubMed] [Google Scholar]
- Williams B. R., Brown R. E., Gilbert C. S., Golgher R. R., Wreschner D. H., Roberts W. K., Silverman R. H., Kerr I. M. Assay of (2'-5')-oligo(A) synthesized in vitro and the analysis of naturally occurring (2'-5')-oligo(A) from intact cells. Methods Enzymol. 1981;79(Pt B):199–208. doi: 10.1016/s0076-6879(81)79030-x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]