Abstract
Background
There is scant evidence on the effect that chronic kidney disease (CKD) confers on clinically meaningful outcomes among patients with heart failure with preserved left ventricular ejection fraction (HF-PEF).
Methods and Results
We identified a community-based cohort of patients with HF. Electronic medical record data were used to divide into HF-PEF and reduced left ventricular EF on the basis of quantitative and qualitative estimates. Level of CKD was assessed by estimated glomerular filtration rate (eGFR) and by dipstick proteinuria. We followed patients for a median of 22.1 months for outcomes of death and hospitalization (HF-specific and all-cause). Multivariable Cox regression estimated the adjusted relative-risk of outcomes by level of CKD, separately for HF-PEF and HF with reduced left ventricular EF. We identified 14 579 patients with HF-PEF and 9762 with HF with reduced left ventricular EF. When compared with patients with eGFR between 60 and 89 mL/min per 1.73 m2, lower eGFR was associated with an independent graded increased risk of death and hospitalization. For example, among patients with HF-PEF, the risk of death was nearly double for eGFR 15 to 29 mL/min per 1.73 m2 and 7× higher for eGFR<15 mL/min per 1.73 m2, with similar findings in those with HF with reduced left ventricular EF.
Conclusions
CKD is common and an important independent predictor of death and hospitalization in adults with HF across the spectrum of left ventricular systolic function. Our study highlights the need to develop new and effective interventions for the growing number of patients with HF complicated by CKD.
Keywords: chronic kidney disease, heart failure, hospitalization, mortality
Heart failure (HF) currently affects ≈5.7 million adults in the United States and is associated with an estimated $29 billion in hospital charges annually.1 Driven by a variety of factors, the prevalence of HF is a current and increasing public health problem nationally and internationally. Many patients with HF also have chronic kidney disease (CKD), most frequently manifest as a reduced glomerular filtration rate (GFR), and the risk of developing HF is substantially increased with worsening stage of CKD.2 Many of the same factors contribute to the development of both chronic diseases, including age, diabetes mellitus, and hypertension.2,3 Although patients with HF suffer poor outcomes, including a death rate of ≈50% within 5 years of diagnosis,1 the co-occurrence of CKD and HF seems to confer an even higher rate of poor outcomes, especially in those with HF and reduced left ventricular ejection fraction (HF-REF).4
The physiological relations between CKD and HF are multifactorial and causally intertwined. For example, kidney dysfunction contributes to HF by increased salt retention and volume expansion, upregulation of neurohormonal pathways, proinflammatory mechanisms, and likely other mechanisms. HF worsens CKD by decreasing renal perfusion and activation of the catecholaminergic and renin–angiotensin–aldosterone system.5–7 In addition, both CKD and HF can cause or worsen other comorbid conditions, including anemia,8 coronary and peripheral atheroschlerosis,9 and malnutrition.10
Because the population prevalence of HF has increased, so has the proportion of patients with HF preserved left ventricular EF (HF-PEF).11 Few studies have, however, examined how CKD affects clinically meaningful outcomes among patients with HF-PEF. Existing data have largely come from studies that are modest in size12,13 and with limited patient populations in terms of range of age, racial/ethnic diversity, comorbidity, or treatment.14–16
To address this knowledge gap, we assembled a large, contemporary, multicenter, community-based cohort of adults with HF to examine the association between CKD and adverse outcomes in those with HF-PEF versus HF-REF. We focused on evaluating the independent association between measures of renal function (using both estimated GFR [eGFR] and documented proteinuria) and relevant clinical outcomes (all-cause mortality, hospitalization for HF, and any hospitalization) and whether these associations varied by type of HF (HF-PEF versus HF-REF).
Methods
Source Population
We used data from a geographically and demographically diverse set of 4 health plans that are members of the National Heart, Lung and Blood Institute–sponsored Cardiovascular Research Network, including Kaiser Permanente Northern California, Kaiser Permanente Colorado, Kaiser Permanente Northwest (Oregon and Washington), and Fallon Community Health Plan (Massachusetts).21 These 4 healthcare delivery systems each have long-standing research divisions that have created site-specific, coordinated Virtual Data Warehouses (VDWs) to facilitate interinstitutional research studies.16 These and related systems have been shown to reflect the population makeup of the communities they serve.17,18 The VDW served as the primary data source for patient identification and covariate characterization in our study. The VDW at each site consists of standardized data tables, including linked demographic, administrative, ambulatory pharmacy, outpatient lab tests and results, and health-care use (including both in-plan and out-of-plan ambulatory visits and hospitalizations with diagnoses and procedures). This study was reviewed and approved by institutional review boards at each site. Waiver of informed consent was obtained because of the nature of the study.
Cohort Assembly
We identified patients aged ≥21 years who had diagnosed HF between January 1, 2005, and December 31, 2008. We included patients who were hospitalized with a primary discharge diagnosis of HF or having ≥3 ambulatory, non–emergency department visits coded for HF. To increase specificity for the outpatient HF criteria, we required the visits had to take place within the study period and that ≤1 of the visits be with a cardiologist. We used International Classification of Diseases, Ninth Edition (ICD-9) codes to define HF, including 398.91, 428.x, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, and 404.93. A high positive predictive value (>95%) has been noted for these inpatient ICD-9 codes when compared against chart review and Framingham clinical criteria.17–19
Patients’ left ventricular EF status was determined by reviewing assessments of echocardiograms, radionuclide scintigraphy, other nuclear imaging modalities, and left ventriculography test results from both electronic databases and from reviews of patient medical records. PEF was defined as either a reported left ventricular EF ≥50% or based on a qualitative assessment of normal systolic function.20 We defined reduced EF as a reported left ventricular EF ≤40% or based on qualitative assessment of moderate, moderate to severe, or severe systolic dysfunction.
To ensure adequate baseline to characterize patient’s clinical status, we excluded patients with <12 months of continuous health plan membership and pharmacy drug benefit before index date. We also excluded patients without a documented left ventricular EF assessment, patients with a reported EF between 41% and 49%, and those patients with a baseline eGFR >130 mL/min per 1.72 m2. We excluded patients (n=13) with a baseline eGFR >130 mL/min per 1.72 m2 over concern that it was reflective of acute physiological changes (eg, malnutrition, volume increases) and not actual GFR. But because we used time-varying covariates in our model, those higher eGFR values (and their prognostic information) may occur during follow-up. Our cohort is thus a community-based HF population with nonacute renal function measurements at baseline, similar to what most clinicians see in practice.
Predictors
The primary predictor was the presence and severity of CKD, as assessed by eGFR and documented proteinuria. Estimated GFR was determined using the CKD–Epidemiology Collaboration formula19 and ambulatory, non–emergency department serum creatinine measurements from participating site lab databases. We categorized eGFR on the basis of stages of CKD:20 90 to 130, 60 to 89, 45 to 59, 30 to 44, 15 to 29, <15 mL/min per 1.72 m2 not on dialysis, and dialysis or renal transplant (referred to collectively as dialysis). Using previously described methods,21 we also used data from ambulatory lab databases at each site to ascertain for the presence of urine dip-stick proteinuria, which was categorized as negative or trace, 1+, 2+, and 3 to 4+.
Outcomes
We followed patients through December 31, 2008, for death from any cause, hospitalization for HF, and hospitalization for any cause. Patients were censored if they disenrolled from their health plan or reached the end of study follow-up. To investigate whether findings varied by potential length of follow-up, we performed a sensitivity analysis, restricting to 1 year of follow-up. Dates of death were identified using a combination of state death certificate records, Social Security Administration files, hospitalization databases, and administrative files. Hospitalizations for HF were identified using VDW hospital files and the same ICD-9 codes used for cohort assembly. All-cause hospitalizations were also identified from the VDW hospital files.
Covariates
We used VDW files to obtain data on comorbidities and procedures from inpatient and ambulatory healthcare encounters using ICD-9 codes, lab test results, as well as ambulatory pharmacy databases and site-specific diabetes mellitus and cancer registries.
Prevalent HF was defined by any hospitalization or ambulatory HF diagnosis during the 5 years before the index date. During the 5 years before cohort entry and throughout the follow-up period, we also assessed patient records for diagnoses of acute myocardial infarction, unstable angina, coronary artery revascularization, stroke or transient ischemic attack, cerebrovascular disease, other thromboembolism, atrial fibrillation or flutter, ventricular fibrillation or tachycardia, mitral or aortic valvular heart disease, peripheral arterial disease, rheumatic heart disease, receipt of a pacemaker, receipt of cardiac resynchronization therapy, receipt of an implantable cardioverter defibrillator, dyslipidemia, hypertension, diabetes mellitus, hospitalized bleed, diagnosed dementia, diagnosed depression, chronic lung disease, chronic liver disease, mechanical fall, and systemic cancer using relevant ICD-9 codes and current procedural terminology codes that have been previously described.21
At baseline and during the follow-up period, ambulatory systolic and diastolic blood pressure measurements were identified from VDW vital sign files, whereas information on serum low-density lipoprotien and high-density lipoprotein cholesterol measurements and blood hemoglobin levels were ascertained from site ambulatory lab databases.
Statistical Analyses
All analyses were conducted using Statistical Analysis System software, version 9.1 (Cary, NC).
We used analysis of variance or nonparametric tests for continuous variables and χ2 tests for categorical variables to compare characteristics across renal function groups. We calculated rates (per 100 population-years) and plotted Kaplan–Meier survival curves separately for each outcome across groups of renal function, with comparison of survival curves using a log-rank test. We fitted multivariable extended Cox regression models allowing for time-varying characteristics on all variables for each outcome to examine the independent association between measures of renal function and outcomes. To be consistent with the baseline exclusions, we censored patients when their eGFR reached a level >130 and undertook sensitivity analyses without that censoring. Separate models were built for patients with HF-PEF and for those with HF-REF. Models were adjusted for age, sex, calendar year of index date, and any other variables that differed across groups with a P≤0.2; in addition, we applied a robust sandwich estimator to account for clustering of multiple observations within the same subject and explored whether additional adjustment for clustering at the site level was necessary. We also explored but did not find significant effect modification within HF-PEF and HF-REF groups for several important covariates, including age (<75, ≥75), systolic blood pressure (>140, ≥140), and anemia (hemoglobin <12, ≥12), so only main results are described in the present findings. When modeling proteinuria, we combined patients with undocumented proteinuria and those with negative or trace findings because previous work has established that patients with a missing dipstick have few CKD risk factors and thus a very low probability of having proteinuria.21 For other variables, we used a missing category when values were not available; this occurred infrequently (hemoglobin and blood pressure <4%; high-density lipoprotein and low-density lipoprotein <9%). Data were checked for consistency across sites (eg, range and frequency) and clinical plausibility, with suspect data points being inspected and corrected if necessary. We found no qualitative evidence of violation of proportional hazards by comparing coefficients from analyses using earlier censoring times.22
Results
Baseline Characteristics
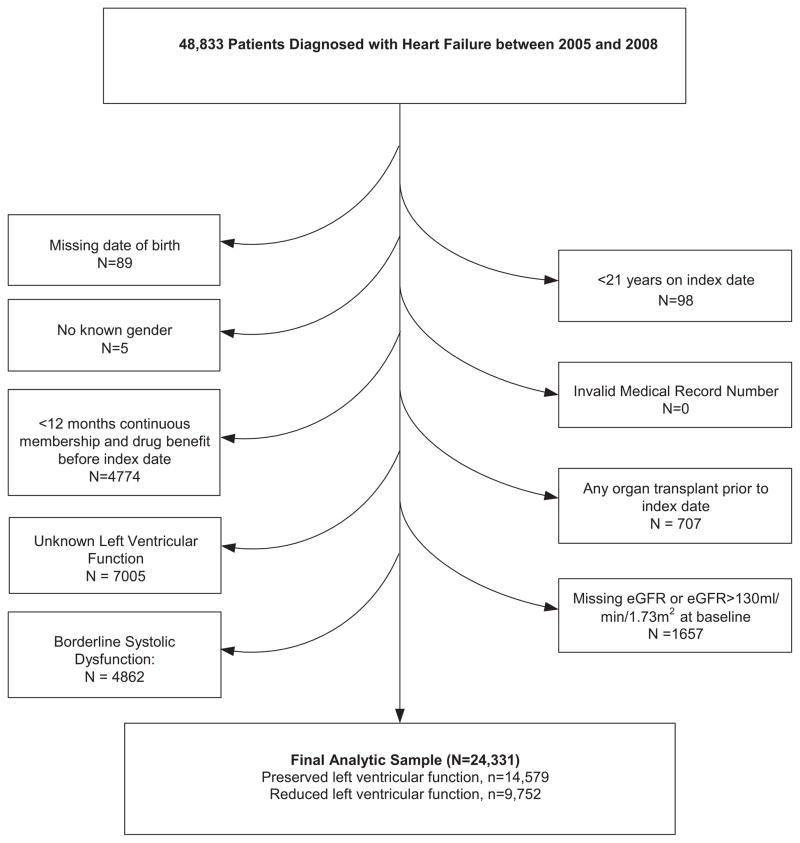
Between 2005 and 2008, we identified 43 833 patients with HF as a diagnosis; of these 24 331 patients were included, 14 579 patients with HF-PEF and 9752 with HF-REF. The most common reasons for exclusion were unknown left ventricular function (16%), borderline systolic function (11%), and <12 months health plan membership (11%; Figure 1). Very few patients were lost to follow-up (<10% HF-PEF and <12% HF-REF). Patients at lower levels of eGFR at entry tended to be older and have a higher comorbidity burden (Table 1). However, patients with an eGFR <15 mL/min per 1.73 m2 were closer in age to patients with an eGFR of 60 to 89 mL/min per 1.73 m2. In the overall cohort, median follow-up was 22.1 (interquartile range [IQR], 9.0–37.1) months, 21.5 (IQR, 8.7–36.1) months in patients with HF-PEF, and 22.9 (IQR, 9.5–38.7) months in patients with HF-REF. Most patients’ EF was assessed using echocardiogram (68%), followed by nuclear imaging (26%), and other or unknown (6%; data not shown). We found that individuals identified in the inpatient setting had a longer time between baseline eGFR and their index date (median, 72 days; IQR, 19, 205) than did individuals identified in the outpatient setting (median, 29 days; IQR, 2, 130; data not shown).
Figure 1.
Patient flow diagram. eGFR indicates estimated glomerular filtration rate.
Table 1.
Baseline Characteristics Among 24 331 Adults With Heart Failure and Preserved or Reduced Left Ventricular Systolic Function, Stratified by eGFR Category
| Overall (n=24 331) | eGFR, 90–130 (n=2443) | eGFR, 60–89 (n=8688) | eGFR, 45–59 (n=5782) | eGFR, 30–44 (n=4457) | eGFR, 15–29 (n=1988) | eGFR <15 (n=251) | Dialysis (n=722) | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD), age, y | 74.1 (12.1) | 61.5 (12.6) | 72.5 (11.8) | 77.3 (9.80) | 79.1 (9.6) | 78.1 (10.5) | 74.5 (11.6) | 68.3 (12.0) | <0.001 |
| Female sex, n (%) | 11 590 (47.6) | 1353 (55.4) | 3843 (44.2) | 2674 (46.2) | 2232 (50.1) | 1035 (52.1) | 125 (49.8) | 328 (45.4) | <0.001 |
| Medical history, n (%) | |||||||||
| Prevalent heart failure | 14 745 (60.6) | 1173 (48.0) | 4695 (54.0) | 3585 (62.0) | 3081 (69.1) | 1491 (75.0) | 167 (66.5) | 553 (76.6) | <0.001 |
| Left ventricular systolic function category, n (%) | |||||||||
| Preserved EF | 14 579 (59.9) | 1336 (54.7) | 4965 (57.1) | 3477 (60.1) | 2835 (63.6) | 1313 (66.0) | 184 (73.3) | 469 (65.0) | <0.001 |
| Reduced EF | 9752 (40.1) | 1107 (45.3) | 3723 (42.9) | 2305 (39.9) | 1622 (36.4) | 675 (34.0) | 67 (26.7) | 253 (35.0) | <0.001 |
| Acute myocardial infarction | 3239 (13.3) | 257 (10.5) | 975 (11.2) | 801 (13.9) | 655 (14.7) | 348 (17.5) | 44 (17.5) | 159 (22.0) | <0.001 |
| Percutaneous coronary intervention | 2482 (10.2) | 206 (8.4) | 853 (9.8) | 594 (10.3) | 481 (10.8) | 189 (9.5) | 23 (9.2) | 136 (18.8) | <0.001 |
| Ischemic stroke or transient ischemic attack | 2020 (8.3) | 128 (5.2) | 602 (6.9) | 558 (9.7) | 401 (9.0) | 215 (10.8) | 24 (9.6) | 92 (12.7) | <0.001 |
| Cerebrovascular disease | 5204 (21.4) | 288 (11.8) | 1553 (17.9) | 1331 (23.0) | 1168 (26.2) | 573 (28.8) | 75 (29.9) | 216 (29.9) | <0.001 |
| Atrial fibrillation or flutter | 9275 (38.1) | 651 (26.6) | 3294 (37.9) | 2411 (41.7) | 1940 (43.5) | 721 (36.3) | 55 (21.9) | 203 (28.1) | <0.001 |
| Mitral or aortic valvular disease | 6048 (24.9) | 493 (20.2) | 2117 (24.4) | 1542 (26.7) | 1243 (27.9) | 488 (24.5) | 39 (15.5) | 126 (17.5) | <0.001 |
| Dyslipidemia | 16 475 (67.7) | 1427 (58.4) | 5610 (64.6) | 4016 (69.5) | 3187 (71.5) | 1487 (74.8) | 208 (82.9) | 540 (74.8) | <0.001 |
| Hypertension | 19 423 (79.8) | 1588 (65.0) | 6410 (73.8) | 4765 (82.4) | 3887 (87.2) | 1826 (91.9) | 246 (98.0) | 701 (97.1) | <0.001 |
| Diabetes mellitus | 5870 (24.1) | 611 (25.0) | 2012 (23.2) | 1389 (24.0) | 1110 (24.9) | 499 (25.1) | 64 (25.5) | 185 (25.6) | 0.16 |
| Diagnosed depression | 4550 (18.7) | 562 (23.0) | 1649 (19.0) | 1000 (17.3) | 813 (18.2) | 361 (18.2) | 45 (17.9) | 120 (16.6) | <0.001 |
| Chronic lung disease | 10 169 (41.8) | 1069 (43.8) | 3515 (40.5) | 2427 (42.0) | 1938 (43.5) | 864 (43.5) | 77 (30.7) | 279 (38.6) | <0.001 |
| Baseline dipstick proteinuria, n (%) | <0.001 | ||||||||
| Negative or trace 0 | 15 949 (65.6) | 1821 (74.5) | 6459 (74.3) | 3979 (68.8) | 2705 (60.7) | 795 (40.0) | 35 (13.9) | 155 (21.5) | |
| 1+ | 4312 (17.7) | 358 (14.7) | 1336 (15.4) | 1041 (18.0) | 933 (20.9) | 518 (26.1) | 43 (17.1) | 83 (11.5) | |
| 2+ | 2607 (10.7) | 197 (8.1) | 662 (7.6) | 539 (9.3) | 560 (12.6) | 386 (19.4) | 68 (27.1) | 195 (27.0) | |
| 3+ | 1463 (6.0) | 67 (2.7) | 231 (2.7) | 223 (3.9) | 259 (5.8) | 289 (14.5) | 105 (41.8) | 289 (40.0) | |
| Baseline hemoglobin, g/dL, n (%) | <0.001 | ||||||||
| ≥11 | 20 342 (83.6) | 2124 (86.9) | 7662 (88.2) | 4965 (85.9) | 3575 (80.2) | 1347 (67.8) | 137 (54.6) | 532 (73.7) | |
| <11 | 3169 (13.0) | 189 (7.7) | 663 (7.6) | 645 (11.2) | 768 (17.2) | 609 (30.6) | 114 (45.4) | 181 (25.1) | |
| Missing | 820 (3.4) | 130 (5.3) | 363 (4.2) | 172 (3.0) | 114 (2.6) | 32 (1.6) | 0 | 9 (1.2) | |
| Systolic blood pressure, mm Hg, n (%) | <0.001 | ||||||||
| ≥180 | 546 (2.2) | 47 (1.9) | 163 (1.9) | 104 (1.8) | 106 (2.4) | 77 (3.9) | 12 (4.8) | 37 (5.1) | |
| 160–179 | 1493 (6.1) | 139 (5.7) | 473 (5.4) | 334 (5.8) | 254 (5.7) | 158 (7.9) | 39 (15.5) | 96 (13.3) | |
| 140–159 | 4251 (17.5) | 443 (18.1) | 1502 (17.3) | 952 (16.5) | 753 (16.9) | 369 (18.6) | 66 (26.3) | 166 (23.0) | |
| 130–139 | 4659 (19.1) | 478 (19.6) | 1716 (19.8) | 1096 (19.0) | 858 (19.3) | 350 (17.6) | 41 (16.3) | 120 (16.6) | |
| 121–129 | 3688 (15.2) | 373 (15.3) | 1382 (15.9) | 906 (15.7) | 644 (14.4) | 267 (13.4) | 32 (12.7) | 84 (11.6) | |
| 110–120 | 6796 (27.9) | 694 (28.4) | 2436 (28.0) | 1678 (29.0) | 1268 (28.4) | 527 (26.5) | 39 (15.5) | 154 (21.3) | |
| 100–109 | 1317 (5.4) | 158 (6.5) | 475 (5.5) | 328 (5.7) | 235 (5.3) | 86 (4.3) | 8 (3.2) | 27 (3.7) | |
| <100 | 747 (3.1) | 87 (3.6) | 254 (2.9) | 166 (2.9) | 151 (3.4) | 63 (3.2) | 6 (2.4) | 20 (2.8) | |
| Missing | 834 (3.4) | 24 (1.0) | 287 (3.3) | 218 (3.8) | 188 (4.2) | 91 (4.6) | 8 (3.2) | 18 (2.5) | |
| Low-density lipoprotein cholesterol, g/dL, n (%) | <0.001 | ||||||||
| Mean (SD) | 96.5 (34.0) | 105.2 (35.2) | 98.8 (33.0) | 94.9 (32.7) | 92.5 (33.2) | 92.7 (35.1) | 94.3 (39.5) | 88.8 (41.6) | |
| Baseline medication use (from 120 before index date up to, but not including, index date) | |||||||||
| Ace inhibitors/angiotensin II receptor blockers | 13 983 (57.5) | 1360 (55.47) | 5102 (58.7) | 3493 (60.4) | 2668 (59.9) | 951 (47.8) | 97 (38.6) | 312 (43.2) | <0.001 |
| β-Blocker | 15 239 (62.6) | 1319 (54.0) | 5248 (60.4) | 3690 (63.8) | 2975 (66.7) | 1383 (69.6) | 178 (70.9) | 446 (61.8) | <0.001 |
| Diuretic (loop) | 12 177 (50.0) | 913 (37.4) | 3788 (43.6) | 3013 (52.1) | 2701 (60.6) | 1373 (69.1) | 168 (66.9) | 221 (30.6) | <0.001 |
| Diuretic (thiazide) | 4282 (17.6) | 371 (15.2) | 1486 (17.1) | 1101 (19.0) | 896 (20.1) | 359 (18.1) | 42 (16.7) | 27 (3.7) | <0.001 |
EF indicates ejection fraction; and eGFR, estimated glomerular filtration rate.
Renal Function and Mortality in HF-PEF and HF-REF
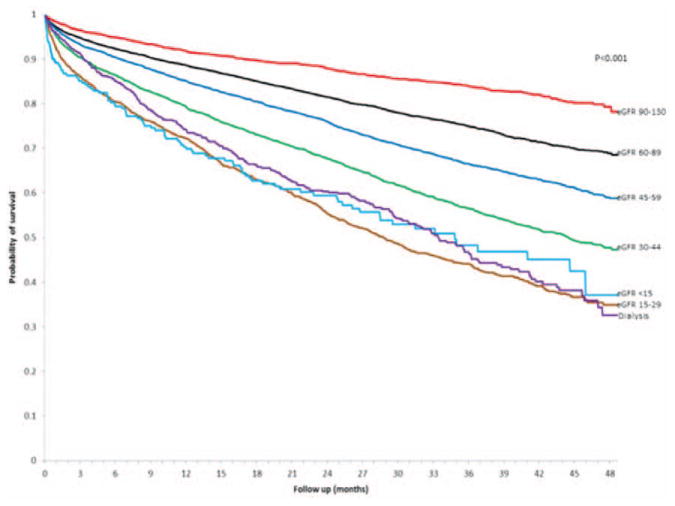
During follow-up, a total of 6768 patients (27.2%) died from any cause, with higher crude rates in population with lower levels of eGFR (Figure 2). Compared with eGFR values of 60 to 89 mL/min per 1.73 m2, we found that there was a graded increased risk of death with lower eGFR level that was similar in patients with HF-PEF and HF-REF, after adjustment for potential confounders and the presence and severity of proteinuria (Table 2). Dialysis was associated with a multivariable adjusted 90% to 151% increased relative rate of death. Compared with no documented proteinuria, patients with any level of proteinuria were 23% to 61% more likely to die (Table 2).
Figure 2.
Crude probability of survival among 24 331 adults with heart failure and preserved or reduced left ventricular ejection fraction stratified by levels of renal function. eGFR indicates estimated glomerular filtration rate.
Table 2.
Multivariable Association Between Kidney Function and Death From Any Cause Among 24 331 Adults With Heart Failure Stratified by Preserve and Reduced Left Ventricular Systolic Function (2005–2008)
| Death From Any Cause Adjusted Hazard Ratio (95% Confidence Interval)
|
||
|---|---|---|
| Preserved Systolic Function* (n=14 579) | Reduced Systolic Function† (n=9752) | |
| eGFR (mL/min per 1.73 m2) category, n (%) | ||
| 90–130 | 1.32 (1.11–1.56) | 0.93 (0.73–1.18) |
| 60–89 | Reference | Reference |
| 45–59 | 0.99 (0.90–1.09) | 1.08 (0.96–1.22) |
| 30–44 | 1.16 (1.05–1.27) | 1.29 (1.14–1.46) |
| 15–29 | 1.57 (1.41–1.76) | 2.15 (1.87–2.48) |
| <15 | 3.22 (2.60–3.98) | 3.69 (2.81–4.84) |
| Dialysis | 1.90 (1.61–2.23) | 2.51 (2.05–3.07) |
| Urine dipstick protein excretion | ||
| Negative/trace or undocumented | Reference | Reference |
| 1+ | 1.53 (1.41–1.67) | 1.41 (1.27–1.57) |
| 2+ | 1.54 (1.39–1.71) | 1.44 (1.27–1.63) |
| 3+ | 1.61 (1.39–1.87) | 1.23 (1.01–1.49) |
eGFR indicates estimated glomerular filtration rate.
Adjusted for age, sex, prevalent heart failure, acute myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass surgery, ischemic stroke or transient ischemic attack, other thromboembolic event, atrial fibrillation or flutter, mitral or aortic valve disease, peripheral arterial disease, rheumatic heart disease, implantable cardioverter defibrillator, pacemaker, dyslipidemia, diabetes mellitus, hospitalized bleeds, diagnosed dementia, chronic liver disease, chronic lung disease, mechanical fall, systemic cancer, hemoglobin, systolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, year of study entry, and sites.
Adjusted for age, sex, prevalent heart failure, acute myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass surgery, ischemic stroke or transient ischemic attack, other thromboembolic event, atrial fibrillation or flutter, ventricular tachycardia or fibrillation, mitral or aortic valve disease, peripheral arterial disease, rheumatic heart disease, cardiac resynchronization therapy, pacemaker, dyslipidemia, hypertension, diabetes mellitus, hospitalized bleeds, diagnosed dementia, diagnosed depression, chronic lung disease, mechanical fall, systemic cancer, hemoglobin, systolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, year of study entry, and sites.
Renal Function and Hospitalization in HF-PEF and HF-REF
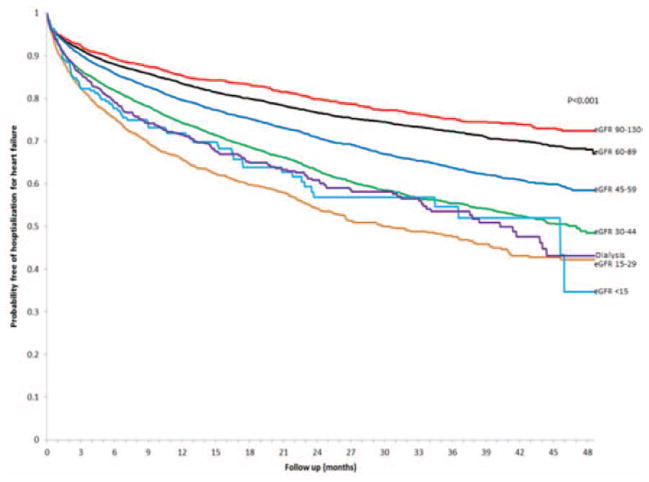
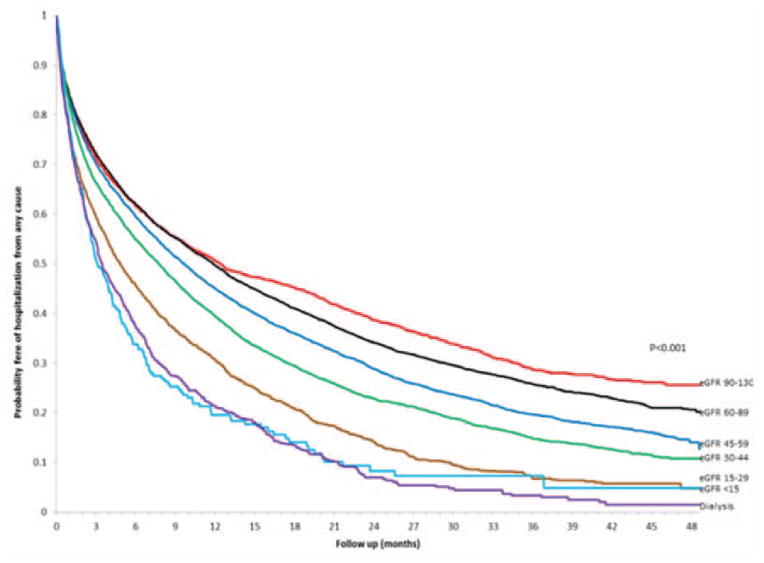
During follow-up, 6691 patients (26.9%) were hospitalized for HF and 16 711 (67.2%) were hospitalized for any cause, with higher crude rates noted with lower levels of eGFR (Figures 3 and 4). Compared with eGFR findings between 60 and 89 mL/min per 1.73 m2, we found that there was a graded increased rate of being hospitalized for HF with lower eGFR level that was similar in patients with HF-PEF and HF-REF after adjustment for potential confounders and the presence and severity of proteinuria (Table 3). Dialysis was associated with an adjusted 35% increased relative rate of being hospitalized for HF in those with HF-PEF but was not a significant predictor in those with HF-REF after adjustment for confounders. Compared with no documented proteinuria, patients with any level of proteinuria were 35% to 64% more likely to have a hospitalization for HF (Table 3). In addition, similar relationships were observed for eGFR level and proteinuria for the outcome of all-cause hospitalization in those with either HF-PEF or HF-REF (Table 4). We found similar results with analyses restricted to 1 year of follow-up, and when patients were not censored when their eGFR increased >130 mL/min per 1.73 m2 (data not shown).
Figure 3.
Crude probability of survival free of hospitalization for heart failure among 24 331 adults with heart failure and preserved or reduced left ventricular ejection fraction stratified by levels of renal function. eGFR indicates estimated glomerular filtration rate.
Figure 4.
Crude probability of survival free of hospitalization from any cause among adults with heart failure and preserved or reduced left ventricular ejection fraction stratified by levels of renal function. eGFR indicates estimated glomerular filtration rate.
Table 3.
Multivariable Association Between Kidney Function and Hospitalization for Heart Failure Among 24 331 Adults With Heart Failure Stratified by Preserved and Reduced Left Ventricular Systolic Function (2005–2008)
| Hospitalization for Heart Failure Adjusted Hazard Ratio (95% Confidence Interval)
|
||
|---|---|---|
| Preserved Systolic Function* (n=14 579) | Reduced Systolic Function† (n=9752) | |
| eGFR (mL/min per 1.73 m2) category, n (%) | ||
| 90–130 | 0.99 (0.83–1.17) | 1.04 (0.82–1.32) |
| 60–89 | Reference | Reference |
| 45–59 | 1.17 (1.07–1.29) | 1.24 (1.12–1.38) |
| 30–44 | 1.54 (1.40–1.69) | 1.39 (1.24–1.55) |
| 15–29 | 1.91 (1.71–2.13) | 2.05 (1.79–2.35) |
| <15 | 2.28 (1.83–2.84) | 1.95 (1.45–2.64) |
| Dialysis | 1.35 (1.14–1.60) | 1.19 (0.97–1.46) |
| Urine dipstick protein excretion | ||
| Negative/trace or undocumented | Reference | Reference |
| 1+ | 1.40 (1.29–1.53) | 1.35 (1.22–1.50) |
| 2+ | 1.60 (1.45–1.77) | 1.43 (1.25–1.64) |
| 3+ | 1.64 (1.44–1.86) | 1.52 (1.29–1.79) |
eGFR indicates estimated glomerular filtration rate.
Adjusted for age, sex, prevalent heart failure, acute myocardial infarction, coronary artery bypass surgery, ischemic stroke or transient ischemic attack, atrial fibrillation or flutter, mitral or aortic valve disease, peripheral arterial disease, pacemaker, dyslipidemia, hypertension, diabetes mellitus, hospitalized bleeds, diagnosed depression, chronic lung disease, mechanical fall, hemoglobin, systolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, year of study entry, and sites.
Adjusted for age, sex, prevalent heart failure, acute myocardial infarction, unstable angina, ischemic stroke or transient ischemic attack, other thromboembolic event, atrial fibrillation or flutter, mitral or aortic valve disease, peripheral arterial disease, cardiac resynchronization therapy, pacemaker, dyslipidemia, hypertension, diabetes mellitus, hospitalized bleeds, diagnosed depression, chronic liver disease, chronic lung disease, hemoglobin, systolic blood pressure, cholesterol, cholesterol, year of study entry, and sites.
Table 4.
Multivariable Association Between Kidney Function and Hospitalization From Any Cause Among 24 331 Adults With Heart Failure Stratified by Preserved and Reduced Left Ventricular Systolic Function (2005–2008)
| Hospitalization From Any Cause Adjusted Hazard Ratio (95% Confidence Interval)
|
||
|---|---|---|
| Preserved Systolic Function* (n=14 579) | Reduced Systolic Function† (n=9752) | |
| eGFR (mL/min per 1.73 m2) category, n (%) | ||
| 90–130 | 1.15 (1.05–1.25) | 1.04 (0.94–1.16) |
| 60–89 | Reference | Reference |
| 45–59 | 1.08 (1.02–1.13) | 1.07 (1.01–1.14) |
| 30–44 | 1.16 (1.09–1.22) | 1.09 (1.02–1.17) |
| 15–29 | 1.32 (1.24–1.41) | 1.47 (1.35–1.60) |
| <15 | 1.73 (1.50–2.00) | 1.85 (1.52–2.25) |
| Dialysis | 1.87 (1.71–2.04) | 1.71 (1.53–1.92) |
| Urine dipstick protein excretion | ||
| Negative/trace or undocumented | Reference | Reference |
| 1+ | 1.28 (1.22–1.34) | 1.30 (1.23–1.38) |
| 2+ | 1.33 (1.26–1.40) | 1.37 (1.27–1.48) |
| 3+ | 1.36 (1.26–1.47) | 1.42 (1.27–1.57) |
eGFR indicates estimated glomerular filtration rate.
Adjusted for age, sex, prevalent heart failure, acute myocardial infarction, unstable angina, percutaneous coronary intervention, ischemic stroke or transient ischemic attack, atrial fibrillation or flutter, mitral or aortic valve disease, peripheral arterial disease, pacemaker, dyslipidemia, hypertension, diabetes mellitus, hospitalized bleeds, diagnosed depression, chronic lung disease, mechanical fall, hemoglobin, systolic blood pressure, high-density lipoprotein cholesterol, year of study entry, and sites.
Adjusted for age, sex, prevalent heart failure, acute myocardial infarction, unstable angina, percutaneous coronary intervention, ischemic stroke or transient ischemic attack, other thromboembolic event, atrial fibrillation or flutter, ventricular tachycardia or fibrillation, mitral or aortic valve disease, peripheral arterial disease, pacemaker, cardiac resynchronization therapy, implantable cardioverter defibrillator, dyslipidemia, hypertension, diabetes mellitus, hospitalized bleeds, diagnosed depression, chronic lung disease, mechanical fall, hemoglobin, systolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, year of study entry, and sites.
Discussion
Among a large, diverse multicenter cohort of adults with HF, we found a consistent relationship between lower levels of eGFR and higher rates of adverse outcomes in those with either HF-PEF or HF-REF. When compared with patients with an index eGFR between 60 and 89 mL/min per 1.73 m2, lower eGFR was associated with an independent, graded increased risk of death from any cause and hospitalization for HF or other causes. For example, among patients with HF-PEF, the 4-year risk of death was 60% higher for CKD stage 4 (eGFR, 15–29 mL/min per 1.73 m2) and 3× higher for CKD stage 5 (eGFR <15 mL/min per 1.73 m2), with similar findings in those with HF-REF (Table 2). Documented urine dipstick proteinuria was also found to be a consistent independent predictor of clinical outcomes in HF-PEF and HF-REF, regardless of level of proteinuria.
Ahmed et al16 found that patients with CKD (defined as an eGFR <60 mL/min per 1.73 m2) were 22% more likely to die than their propensity score-matched, non-CKD counterparts (hazard ratio, 1.22; 95% confidence interval, 1.09, 1.36) during a median of 38 months of follow-up in Digitalis Investigation Group trial HF participants during 1991–1993 (2389 matched pairs). The increased risk of death with lower eGFR was observed across left ventricular EF categories but was higher for patients with a left ventricular EF >45% (hazard ratio, 1.71; 95% confidence interval, 1.21, 2.41) than for patients with left ventricular EF ≤45% (hazard ratio, 1.19; 95% confidence interval, 1.07, 1.32; interaction P=0.03). Our study confirms and extends these findings in a much larger, contemporary, and generalizable cohort to demonstrate a similarly worse prognosis across the wide range of reduced eGFR levels in those with either HF-PEF or HF-REF. Importantly, we found no evidence of clinically meaningful effect modification of eGFR level with targeted characteristics (ie, age, presence of proteinuria, systolic blood pressure level, and hemoglobin level) on the risk of adverse outcomes in HF-PEF or HF-REF.
Reduced kidney function could contribute to poor outcomes in patients with HF in several ways. The physiological relationship between HF and CKD is, however, quite complex, and a variety of cardiorenal regulatory systems unravel as each organ system’s function declines. The impact of CKD on patients with HF likely operates through pathways common to both diseases, including increased inflammatory cytokines,23 malnutrition,24 and neurohormonal changes.23 For example, CKD contributes to HF by volume expansion through increased renin production and decreased erythropoietin production; HF worsens CKD by decreasing renal perfusion. HF is a cause of renal impairment, 15,25,26 and HF causes CKD progression.26 In addition, the presence of HF is more common among patients with CKD than the general population, and decreased renal function is linearly associated with increased prevalence of congestive HF.27,28
For patients with HF-PEF, we observed a U-shaped relationship between level of renal function and death,29 and to a lesser extent between level of renal function and all-cause hospitalization (Tables 2 and 4), even though we excluded individuals with baseline eGFR >130 mL/min per 1.73 m2 and censored patients when their eGFR increased beyond that level. Our findings confirm that the effect of eGFR on outcomes is not linear, highlighting the need for investigators to allow for this nonlinearity when modeling eGFR. Development of eGFR >130 mL/min per 1.73 m2 during follow-up was independently associated with worse outcomes, and the low serum creatinine concentrations that drive these high GFR estimates likely represent either malnutrition or fluid overload and more impaired ventricular function, which would contribute to the poorer prognosis.
Our study had several strengths. We assembled a large, contemporary, community-based HF cohort that reflects real-world outcomes. We were also able to longitudinally characterize level of eGFR across a wide range of kidney function and examine its association with multiple clinically and public health-relevant outcomes after accounting for a large set of potential confounders and the presence and severity of documented proteinuria. We used the CKD–Epidemiology Collaboration formula19 to estimate eGFR, an estimating equation recently shown to more accurately categorize end-stage renal disease risk and mortality risk, compared with the Modification of Diet in Renal Disease formula.30 Using the older estimating equation would likely have attenuated our relative-risk estimates.
Our study also had several limitations. We relied on information collected as part of routine healthcare encounters rather than through a structured data collection protocol, which could contribute to some misclassification of key study predictor and control variables. However, we used an approach on the basis of validated algorithms to ascertain relevant comorbid conditions and other exposures from each site’s VDW. Our cohort’s racial and ethnic makeup reflects the communities from which they are drawn, but we did not include an investigation of those factors; this may be an important issue for future work because the epidemiology of kidney disease is known to vary with race. In addition, because our study was conducted among integrated healthcare delivery systems, we were less susceptible to missing data given that nearly all clinical care is captured through each site’s electronic health records and clinical data systems. Our analysis does not include the 11% of patients with borderline systolic function (ie, those with EF between 40% and 50%), so our results may not apply to those patients. We also acknowledge that, despite the large population and statistical control of a wide array of potential confounders, our analysis may suffer from residual confounding. Finally, because our sample included only insured patients, our findings may not be completely generalizable to the uninsured or other types of practice settings.
In summary, reduced renal function is a common and important independent predictor of death and hospitalization in adults with HF across the spectrum of left ventricular EF. Although the relationship between congestive HF and CKD is well defined, it is unclear what differences exist between patients with HF-REF and HF-PEF. Patients with HF are treated differently on the basis of their EF, and these treatments (eg, diuretics, renin-angiotensin system agents) are often dependent on renal function. Our study highlights the need to delineate the mechanisms through which lower levels of renal function contributes to worse outcomes in HF and to develop new and effective interventions for the growing number of patients with HF complicated by CKD. Thus, although beyond the scope of our analysis, investigations that examine the relationship between treatment-related outcomes and the presence of CKD are an important next step.
Supplementary Material
WHAT IS KNOWN
Chronic kidney disease confers a greater risk of poor outcomes in patients with reduced systolic function heart failure.
Little is known about how and whether chronic kidney disease modifies outcomes in those with preserved systolic function heart failure.
WHAT THIS STUDY ADDS
Using data from a large, contemporary, multicenter, community-based cohort, the impact of chronic kidney disease is compared between reduced and preserved systolic function heart failure.
There was a consistent relationship between lower levels of estimated glomerular filtration rate and higher rates of adverse outcomes in those with either reduced or preserved systolic function heart failure.
Documented urine dipstick proteinuria was a consistent independent predictor of clinical outcomes in reduced and preserved systolic function heart failure, regardless of level of proteinuria.
Acknowledgments
Sources of Funding
This study was conducted within the Cardiovascular Research Network sponsored by the National Heart, Lung, and Blood Institute (U19 HL91179-01) and the American Recovery and Reinvestment Act of 2009 (National Heart, Lung, and Blood Institute grant #1RC1HL099395-01).
Footnotes
The online-only Data Supplement is available at http://circoutcomes.ahajournals.org/lookup/suppl/doi:10.1161/CIRCOUTCOMES.113.000221/-/DC1.
This manuscript was handled independently by Peter W. Groeneveld, MD, MS, as Guest Editor. The Editors had no role in the evaluation of the manuscript in the or in the decision about its acceptance.
Disclosures
Partial salary support was provided by National Institutes of Health grants KL2RR031981 (D.D.M.) and and 1K23HL105896 (L.A.A.). The other authors report no conflicts.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Renal Data System, USRDS. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: 2010. 2009 Annual Data Report. [Google Scholar]
- 3.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D Framingham Heart Study Investigators. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 4.Galil AG, Pinheiro HS, Chaoubah A, Costa DM, Bastos MG. Chronic kidney disease increases cardiovascular unfavourable outcomes in outpatients with heart failure. BMC Nephrol. 2009;10:31. doi: 10.1186/1471-2369-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis BM, Parfrey PS. Congestive heart failure in chronic kidney disease: disease-specific mechanisms of systolic and diastolic heart failure and management. Cardiol Clin. 2005;23:275–284. doi: 10.1016/j.ccl.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Bruch C, Rothenburger M, Gotzmann M, Wichter T, Scheld HH, Breithardt G, Gradaus R. Chronic kidney disease in patients with chronic heart failure: impact on intracardiac conduction, diastolic function and prognosis. Int J Cardiol. 2007;118:375–380. doi: 10.1016/j.ijcard.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 7.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 8.Colombo PC, Ganda A, Lin J, Onat D, Harxhi A, Iyasere JE, Uriel N, Cotter G. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17:177–190. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 10.von Haehling S, Anker SD. Cachexia as a major underestimated and un-met medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 12.Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) Circ Heart Fail. 2011;4:27–35. doi: 10.1161/CIRCHEARTFAILURE.109.932996. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 14.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 15.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenlick M, Freeborn D, Pope C. Health Care Research in an HMO: Two Decades of Discovery. Baltimore: Johns Hopkins University Press; 1998. [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) Investigators. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:4–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.K/DOQI. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 21.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 22.Altman DG, De Stavola BL. Practical problems in fitting a proportional hazards model to data with updated measurements of the covariates. Stat Med. 1994;13:301–341. doi: 10.1002/sim.4780130402. [DOI] [PubMed] [Google Scholar]
- 23.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 24.Iversen PO, Woldbaek PR, Tønnessen T, Christensen G. Decreased hematopoiesis in bone marrow of mice with congestive heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R166–R172. doi: 10.1152/ajpregu.2002.282.1.R166. [DOI] [PubMed] [Google Scholar]
- 25.Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325:163–167. doi: 10.1097/00000441-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Das M, Aronow WS, McClung JA, Belkin RN. Increased prevalence of coronary artery disease, silent myocardial ischemia, complex ventricular arrhythmias, atrial fibrillation, left ventricular hypertrophy, mitral annular calcium, and aortic valve calcium in patients with chronic renal insufficiency. Cardiol Rev. 2006;14:14–17. doi: 10.1097/01.crd.0000148162.88296.9f. [DOI] [PubMed] [Google Scholar]
- 27.Varma R, Ying-Lai M, Klein R, Azen SP Los Angeles Latino Eye Study Group. Prevalence and risk indicators of visual impairment and blindness in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1132–1140. doi: 10.1016/j.ophtha.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Varma R, Garrick R, McClung J, Frishman WH. Chronic renal dysfunction as an independent risk factor for the development of cardiovascular disease. Cardiol Rev. 2005;13:98–107. doi: 10.1097/01.crd.0000132600.45876.d0. [DOI] [PubMed] [Google Scholar]
- 29.Cox HJ, Bhandari S, Rigby AS, Kilpatrick ES. Mortality at low and high estimated glomerular filtration rate values: a ‘U’ shaped curve. Nephron Clin Pract. 2008;110:c67–c72. doi: 10.1159/000151720. [DOI] [PubMed] [Google Scholar]
- 30.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS Chronic Kidney Disease Prognosis Consortium. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.