Abstract
Nodulin-35 (N-35), a subunit of nodule-specific uricase (uricase II) of soybean (Glycine max), is shown to be preferentially synthesized on free polysomes during nodule development and is localized in peroxisomes of the uninfected cells of this tissue. A cDNA clone, isolated by using mRNA from immunoprecipitated polysomes, revealed the primary structure of this protein with a molecular mass of 35,100. That this clone represents N-35 was confirmed by comparing the deduced amino acid sequence with the partial sequence of a CNBr-cleaved peptide of purified N-35. Southern blot hybridizations with genomic DNA suggest that there are several EcoRI fragments containing N-35 sequences. Three of these sequences were isolated from a genomic library of soybean. Nucleotide sequence analysis showed that the complete gene extends almost 5000 base pairs on two EcoRI fragments and the coding region (309 codons) is interrupted by seven introns ranging in size from 154 to 1341 base pairs. Lack of a signal sequence and its translation on free polysomes suggest that N-35 is posttranslationally transported to the peroxisomes. Furthermore, there is no cross-hybridization of N-35 cDNA with RNA from young (3- to 4-day) roots and leaves, indicating that the observed “uricase” activity in these tissues is due to the product of a different gene.
Keywords: nucleotide sequence, gene organization, immunocytochemistry, in vitro translation, symbiotic nitrogen fixation
Full text
PDF
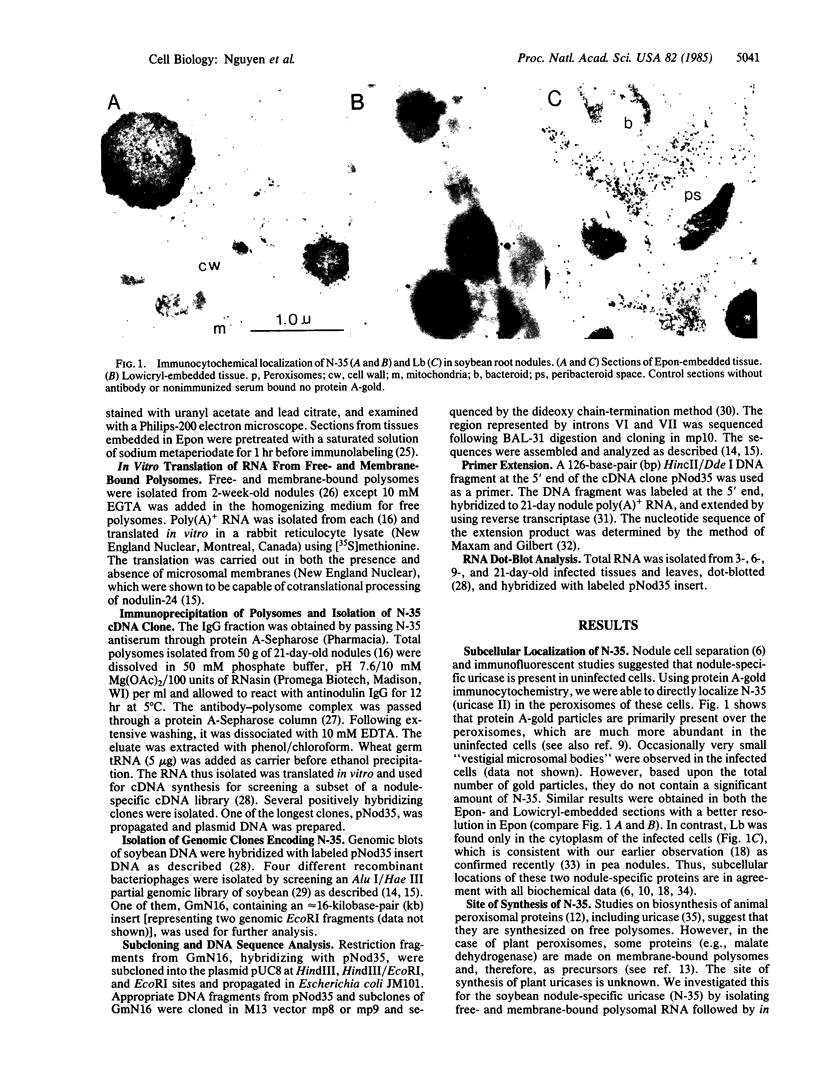
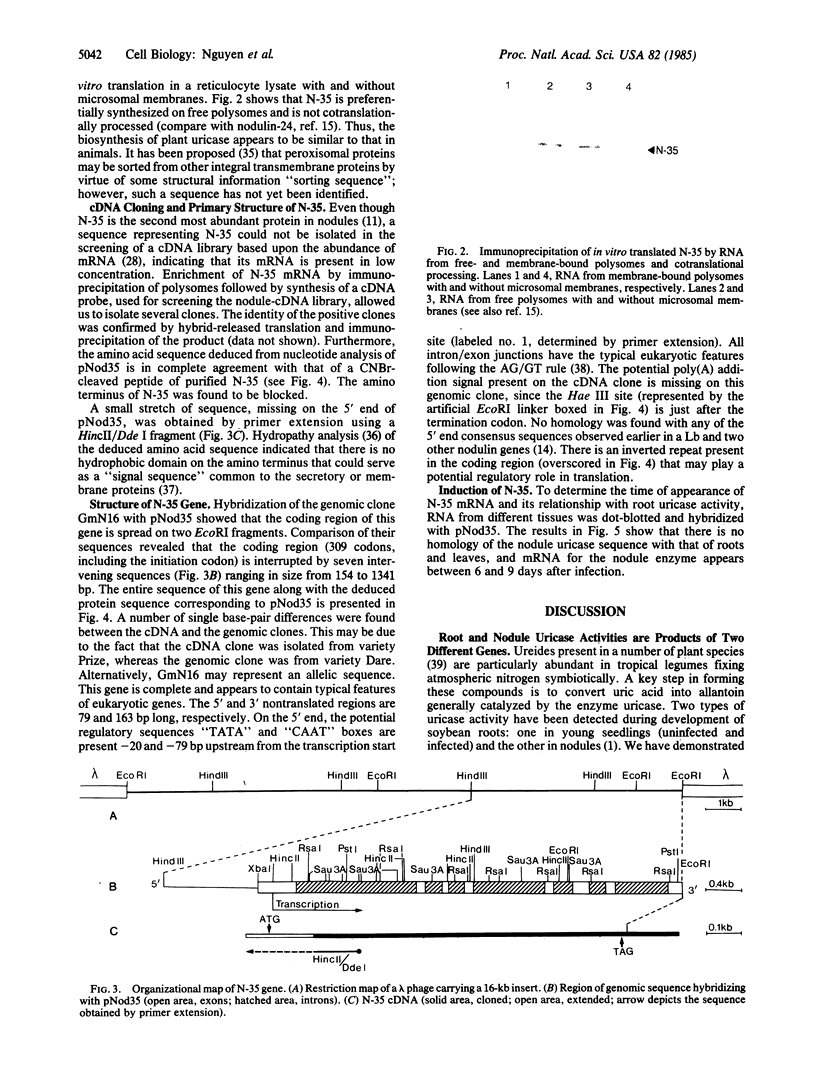


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbruster B. L., Garavito R. M., Kellenberger E. Dehydration and embedding temperatures affect the antigenic specificity of tubulin and immunolabeling by the protein A-colloidal gold technique. J Histochem Cytochem. 1983 Dec;31(12):1380–1384. doi: 10.1177/31.12.6195215. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Roth J., Perrelet A., Orci L. Quantitative immunocytochemical localization of pancreatic secretory proteins in subcellular compartments of the rat acinar cell. J Histochem Cytochem. 1980 Feb;28(2):149–160. doi: 10.1177/28.2.7354212. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Fischer R. L., Goldberg R. B. Structure and flanking regions of soybean seed protein genes. Cell. 1982 Jun;29(2):651–660. doi: 10.1016/0092-8674(82)90181-7. [DOI] [PubMed] [Google Scholar]
- Fuller F., Künstner P. W., Nguyen T., Verma D. P. Soybean nodulin genes: Analysis of cDNA clones reveals several major tissue-specific sequences in nitrogen-fixing root nodules. Proc Natl Acad Sci U S A. 1983 May;80(9):2594–2598. doi: 10.1073/pnas.80.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Tolbert N. E., Schubert K. R. Localization of enzymes of ureide biosynthesis in peroxisomes and microsomes of nodules. Plant Physiol. 1981 Jul;68(1):65–69. doi: 10.1104/pp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinakis P., Verma D. P. Nodulin-24 gene of soybean codes for a peptide of the peribacteroid membrane and was generated by tandem duplication of a sequence resembling an insertion element. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4157–4161. doi: 10.1073/pnas.82.12.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. A nodule-specific plant protein (nodulin-35) from soybean. Science. 1979 Jul 13;205(4402):190–193. doi: 10.1126/science.205.4402.190. [DOI] [PubMed] [Google Scholar]
- Mauro V. P., Nguyen T., Katinakis P., Verma D. P. Primary structure of the soybean nodulin-23 gene and potential regulatory elements in the 5'-flanking regions of nodulin and leghemoglobin genes. Nucleic Acids Res. 1985 Jan 11;13(1):239–249. doi: 10.1093/nar/13.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Newcomb E. H., Tandon S. R. Uninfected cells of soybean root nodules: ultrastructure suggests key role in ureide production. Science. 1981 Jun 19;212(4501):1394–1396. doi: 10.1126/science.212.4501.1394. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R. Enzymes of Purine Biosynthesis and Catabolism in Glycine max: I. COMPARISON OF ACTIVITIES WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING NODULE DEVELOPMENT. Plant Physiol. 1981 Nov;68(5):1115–1122. doi: 10.1104/pp.68.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Varsanyi-Breiner A., Gusella J. F., Keys C., Housman D. E., Sullivan D., Brisson N., Verma D. P. The organization of a nuclear DNA sequence from a higher plant: molecular cloning and characterization of soybean ribosomal DNA. Gene. 1979 Nov;7(3-4):317–334. doi: 10.1016/0378-1119(79)90051-9. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Bal A. K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3843–3847. doi: 10.1073/pnas.73.11.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Ball S., Guérin C., Wanamaker L. Leghemoglobin biosynthesis in soybean root nodules. Characterization of the nascent and released peptides and the relative rate of synthesis of the major leghemoglobins. Biochemistry. 1979 Feb 6;18(3):476–483. doi: 10.1021/bi00570a016. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A. Metabolism of Poly(A) in Plant Cells: Discrete Classes Associated with Free and Membrane-bound Polysomes. Plant Physiol. 1976 Sep;58(3):405–410. doi: 10.1104/pp.58.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]





