Abstract
Aging is not and cannot be programmed. Instead, aging is a continuation of developmental growth, driven by genetic pathways such as mTOR. Ironically, this is often misunderstood as a sort of programmed aging. In contrast, aging is a purposeless quasi-program or, figuratively, a shadow of actual programs.
“The brightest flame casts the darkest shadow.” -George Martin
Keywords: senescence, geroconversion, gerosuppression, rapamycin, rapalogs, evolution, selection
Introduction
Genes regulate lifespan, in some cases, dramatically.1-17 Pro-aging genes encode signaling pathways such as the insulin/PI3K/TOR pathway that accelerate aging.13-16 These signal-transduction pathways are essential for development, growth, and survival early in life.18 Furthermore, the same signaling pathways drive cellular geroconversion: a conversion from cellular quiescence to senescence.19-40 The same PI3K/TOR pathway is also involved in cancer and other age-related diseases.41-44 The mTOR pathway links development and aging,42 cellular growth and senescence,43 robustness early in life and diseases later in life,44,45 puberty and menopause.46-48 Whereas development and growth are programmed, aging and diseases are not. They are aimless continuations of the program that was not switched off upon its completion. Somehow these notions are confused with programmed aging theory. As discussed,41,49-52 it is only development that is programmed for purpose, aging is not. It is a shadow. Natural selection cannot eliminate the shadow. Nature simply selects for the brightest flame, which in turn casts the darkest shadow.
What Are Programmed Theories of Aging
Aging and its diseases are so orderly that the explanation begs for a program. Like development, aging seems to be programmed.53-57 Programmed theories are thought-provocative and inspiring. They brilliantly illuminate limitations of mainstream theories that aging is a stochastic, random process.53,58 Also, while stochastic aging cannot be prevented,59 the program can be switched off.60-63 This makes programmed theories appealing. But why would nature program aging? It was suggested that aging is beneficial for species and groups.53 There are conditions for group selection in humans, given that human groups had the means to exterminate each other, or using modern terms, to commit genocide. But even group selection cannot select for aging and age-related diseases. In contrast, it should select for robust soldiers, who defend the group from extermination (in human societies and social ants). It was also suggested that organisms undergo programmed death, similar to apoptosis in the multicellular organism.64 Still, aging (at least in humans) is a decades-long process of developing age-related diseases (cancer, hypertension, diabetes, blindness) that terminate life. This is an inefficient way to commit suicide. According to programmed theories, aging prevents overpopulation, speeds up evolution, or benefits young animals, by eliminating old (“less valuable”) animals. But old animals seem less “valuable” precisely because of aging. Thus, aging is programmed to eliminate less valuable animals because of aging. This is a circular reasoning. The only way out from this circle is to suggest that the aging process exists independently of a putative “suicidal program”. But if so, then such a putative program is irrelevant to aging.
Is Aging Programmed in Yeast?
Yeast death in stationary cultures, also known as chronologic senescence, may seem to be programmed.53,65-67 Yeast secretes toxic substances (pheromones, acetic acid, etc.). If “altruistic” yeast die, then other yeast may survive. However, so-called “altruistic” yeast may be less resistant to pH and toxic substances. This simply may be a classic case of survival of the fittest (resistant) yeast. Yeast chronological aging is similar to metabolic self-destruction of human cancer cells.68 In stationary culture, cancer cells acidify the medium with lactic acid. When most cancer cells die, a few cells may survive. Are cancer cells altruistic? In yeast and cancer cell stationary cultures, acid-resistant cells survive. The main difference is that yeast produce acetic acid, whereas cancer cells produce lactic acid.68-72 In yeast, “oncogenic” pathways such as Ras and TOR accelerate chronological senescence.73-75 Inhibitors of the TOR pathway, including rapamycin, decelerate chronological senescence in yeast.75-77 Rapamycin decelerates “yeast-like chronological senescence” in overcrowded cancer cell culture.68 The same signaling pathways (such as TOR) that are involved in chronological senescence in yeast are also involved in metabolic self-destruction of cancer cells.68,73,74,78-81 The same pathways are also involved in cellular geroconversion, organismal aging, and age-related diseases (see ref. 68).
Programmed Elements in Non-Programmed (Stochastic) Theories
Programmed theories neither specify nor predict mechanisms of death. Ironically, it was suggested that programmed aging is caused by free radicals.53 And, vice versa, mainstream (stochastic, decay) theories accept special programs (Table 1). For example, it was suggested that menopause in women is purposefully programmed to stop reproduction and to raise grandchildren instead.82 Also, it was suggested that the rate of aging is regulated by allocation of energetic resources:83 paradoxically, the more available, the less used.83 It is also thought that aging is programmed in Pacific salmon,84 yet, salmon die from pathologies similar to mammalian age-related diseases. Neither aging and nor age-related diseases in Pacific salmon (or any other animals) are programmed. Aging in Pacific salmon and menopause in women are quasi-programmed.46,85
Table 1. Comparison of 3 groups of theories of aging: programmed, stochastic, and quasi-programmed.
| Theories | Defining feature | Purposeful? | Programmed? | Caused by ROS? | Kills via age-related diseases? | Causes death directly? | Menopause in women is | Link between aging and diseases | Use of energetic resources |
|---|---|---|---|---|---|---|---|---|---|
| Programmed | functional decline | yes | yes | mostly | unspecified | yes | programmed | unspecified | unspecified |
| Stochastic | functional decline | sometimes* | in some cases* | mostly | sometimes# | yes | programmed | vulnerability to diseases# | slows aging (via repair) |
| Quasi-programmed | hyperfunction | no | no | no | always | no | prototypical disease | manifested by diseases | fuels aging (via TOR) |
According to stochastic theories, aging is caused by random accumulation of damages, errors, and “garbage” due to multiple causes including but not limited to free radicals. *Stochastic theories still accept that aging can be purposefully programmed (e.g., in salmon). #According to stochastic theories, aging can kill directly (by non-specified mechanisms) and also increases the vulnerability to age-related diseases.
Quasi-Programmed Hyperfunction (Aging)
Quasi-programmed aging is not something between “random damage” and “programmed” aging. Instead, quasi-programmed theory is absolutely different from both random damage and programmed theories (Table 1). According to quasi-programmed theory,41,42,44,45,49,50,52,86-90 neither aging nor menopause is programmed, they are manifestations of the aging process, which, in turn, is a pseudo-program of developmental growth. There is a mechanistic link between mTOR-driven geroconversion, aging, and age-related pathologies, explaining how cellular hyperfunctions eventually lead to organismal death.41
Quasi-programmed theory predicts mechanisms of aging that are determined by mechanisms of growth, differentiation, and development. There is no need to guess what might be the mechanisms. Aging is a shadow. Its shape is determined by the developmental growth. This can be modeled in cell culture, revealing how growth can be converted to aging.
Quasi-Program of Cellular Senescence
Nutrients, growth factors, hormones, and cytokines all activate nutrient-sensing and growth-promoting signaling pathways such as mTOR (target of rapamycin). mTOR stimulates growth and anabolic metabolism, inhibits autophagy, and increases cellular functions.91-102 Cells grow in size, progress through the cell cycle, and then divide. In the absence of growth factors, normal cells become quiescent: they neither grow nor cycle. In When the cell is stimulated to grow, while the cell cycle is arrested, then the cell becomes senescent (geroconversion).43 mTOR drives growth (program) and geroconversion (quasi-program) (Fig. 1). Also, cellular senescence can be viewed as a continuation of differentiation. The same cytokines that initially cause growth and proliferation then cause cell cycle arrest and differentiation.103-106 During differentiation, cells acquire and amplify specific functions. One example of cellular function is secretion of cytokines, hormones, matrix, enzymes, metabolites, or lipoproteins, depending on cell type. Other examples include contraction of smooth muscle cells, adhesion, and aggregation of platelets as well as oxidative burst of neutrophils.
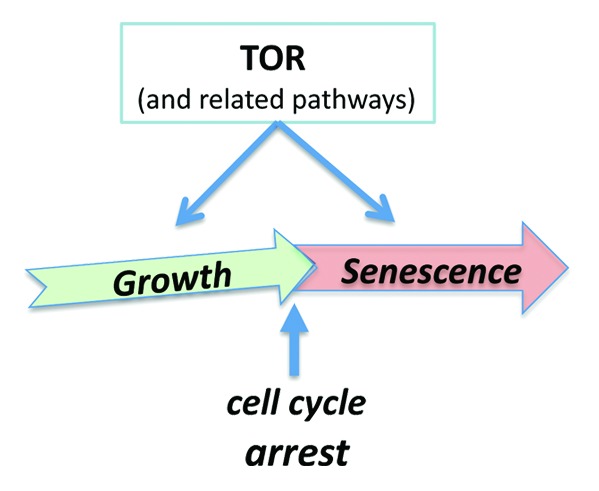
Figure 1. From cellular growth to hypertrophic senescence (geroconversion). Gerogenic conversion (geroconversion) from cellular growth to cellular aging, when the cell cycle is arrested. Geroconversion is a continuation of growth driven by mTOR and related pathways.
The same intracellular signaling pathways that initially drive proliferation, and then differentiation, also stimulate functions in differentiating cells. Cell senescence-associated hypertrophy and hyper-functions are a continuation of growth (Fig. 1).
From Cellular to Organismal Aging
The most relevant hallmark of cellular aging is hypertrophy/hyperfunctions and compensatory signal resistance, such as insulin resistance. Hyper-functions coupled with signal resistance cause loss of homeostasis, malfunction, organ damage, and death. The link between hyper-functions, including hypertrophy, and diseases has been discussed41,42,50,78,107-110 and will be discussed further (“Aging: From fiction to hyperfunction”, in press).
Quasi-Program of Aging
Genetic programs determine developmental growth and the onset of reproduction. When these programs are completed, they are not switched off.
Thus, programs become quasi-programs (Fig. 2). Specific characteristics of quasi-programs of aging and age-related diseases were discussed in detail.41-45,49-52,86-90 The evolutionary theory predicts quasi-programs, like it predicts genes harmful later in life, if they are useful earlier in life.49 I emphasize that the quasi-program does not exist for its own sake: it is a shadow. Aging has no purpose (neither for individuals nor for group), no intention. Nature does not select for quasi-programs. It selects for robust developmental growth. Accelerated aging is the price for robustness.46,50,52,88,111 Although (in some conditions) natural selection works against quasi-programs of aging, it cannot eliminate them without harming development. Genes that drive aging are needed in development. Knockout of PI3K extends the lifespan of C. elegans 10-fold.12 But this comes at a price: prolonged development. Even further, disruption of the mTOR gene leads to post-implantation lethality in mice.112-115 Whereas disruption of S6K1 extends lifespan in mice,14 knockout of both S6K1 and S6K2 causes perinatal lethality.116 In Drosophila, TOR is required for normal growth during larval development.117
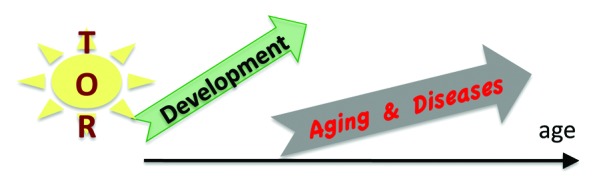
Figure 2. From delelopmental growth (program) to aging (shadow). Quasi-programmed aging is driven by over-activation of signal-transduction pathways such as TOR and exacerbation of normal cellular functions, which become harmful (hyper-function), leading to alterations of homeostasis, malfunctions, diseases, and organ damage.
The Utility of the Model
Mechanisms of aging are not arbitrary but determined by mechanisms of development and growth. Since development and growth are relatively well understood, we can interpolate this knowledge to studying aging. For example, it is known that mTOR drives cellular mass growth. This predicts that p53 and hypoxia, which inhibit mTOR and cellular mass growth, will suppress geroconversion despite causing cell cycle arrest.25,26,118-120 Thus, like other tumor suppressors,43 p53 and hypoxia may play a dual role in aging.121-128 The map of growth-promoting signaling network can be interpolated to aging. Gerogenes (insulin receptor, PI-3K, Akt, mTOR) and gerosuppressors (PTEN, TSC, AMPK) form a network, which (in analogy with the periodic “Mendeleev” table) predicts the effect of a particular gene on aging and diseases.129 Basically, genes that activate the mTOR pathway are gerogenes, and those that antagonize the pathway are gerosuppressors.43,129 As another example, developmental trends, such as an increase in blood pressure, near vision point, and FSH levels (all necessary for development and reproductive functions) cause hypertension, presbyopia, and menopause, respectively, later in life.89 Many predictions of the quasi-programmed aging model42 were confirmed by 2010,87 including the prediction that rapamycin will extend lifespan in mice.130 Numerous recent publications further illuminate the role of the mTOR pathway (and related pathways) in aging.35,131-168
If used properly, rapamycin improves immunity and decreases infections and their complications.148,169,170 Under certain conditions, rapamycin can exert immunostimulatory effects, boosting T-cell responses in the face of pathogen infections and vaccines.170,171 Rapamycin may improve response against pathogens but prevent transplant rejection.172,173
Conclusion
The essence of quasi-program was discussed previously.42,89 Here I addressed a misunderstanding that a quasi-program is a sort of a program. It is not (Table 1). Whereas the growth of the body is programmed, the emergence of the shadow is not. Natural selection cannot eliminate the shadow without hurting the “body”. As a case in point, mTOR knockout is lethal in embryogenesis. However, pharmacologic interventions can be started in post-development, thus extending healthy lifespan. MTOR-driven quasi-program can be suppressed pharmacologically.174 And this is what is actually important. After all, according to Oscar Wilde, “What men call the shadow of the body is not the shadow of the body, but is the body of the soul.”
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27188
References
- 1.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 2.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–62. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 3.Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–75. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- 4.Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–4. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am. 2006;294:48–51, 54-7. doi: 10.1038/scientificamerican0306-48. [DOI] [PubMed] [Google Scholar]
- 6.Gardner MP, Gems D, Viney ME. Extraordinary plasticity in aging in Strongyloides ratti implies a gene-regulatory mechanism of lifespan evolution. Aging Cell. 2006;5:315–23. doi: 10.1111/j.1474-9726.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- 7.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 8.Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. doi: 10.12703/P5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladyshev VN, Zhang G, Wang J. The naked mole rat genome: understanding aging through genome analysis. Aging (Albany NY) 2011;3:1124. doi: 10.18632/aging.100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartke A, Coschigano K, Kopchick J, Chandrashekar V, Mattison J, Kinney B, Hauck S. Genes that prolong life: relationships of growth hormone and growth to aging and lifespan. J Gerontol A Biol Sci Med Sci. 2001;56:B340–9. doi: 10.1093/gerona/56.8.B340. [DOI] [PubMed] [Google Scholar]
- 11.Bartke A. Insulin and aging. Cell Cycle. 2008;7:3338–43. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- 12.Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 13.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian lifespan. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–45. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4:913–20. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann J, Romey R, Fink C, Yong L, Roeder T. Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging (Albany NY) 2013;5:315–27. doi: 10.18632/aging.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009;1:357–62. doi: 10.18632/aging.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blagosklonny MV. Cell senescence and hypermitogenic arrest. EMBO Rep. 2003;4:358–62. doi: 10.1038/sj.embor.embor806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–61. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 21.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–95. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 22.Demidenko ZN, Shtutman M, Blagosklonny MV. Pharmacologic inhibition of MEK and PI-3K converges on the mTOR/S6 pathway to decelerate cellular senescence. Cell Cycle. 2009;8:1896–900. doi: 10.4161/cc.8.12.8809. [DOI] [PubMed] [Google Scholar]
- 23.Demidenko ZN, Blagosklonny MV. Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential. Aging (Albany NY) 2009;1:1008–16. doi: 10.18632/aging.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010;2:344–52. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A. 2010;107:9660–4. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A. 2012;109:13314–8. doi: 10.1073/pnas.1205690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009;8:4112–8. doi: 10.4161/cc.8.24.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010;2:924–35. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Differ. 2013;20:1241–9. doi: 10.1038/cdd.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leontieva OV, Blagosklonny MV. CDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversion. Cell Cycle. 2013;12:3063–9. doi: 10.4161/cc.26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darzynkiewicz Z. Forever young, slim and fit: rapamycin to the rescue. Cell Cycle. 2009;8:1820–1. doi: 10.4161/cc.8.12.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romanov VS, Abramova MV, Svetlikova SB, Bykova TV, Zubova SG, Aksenov ND, Fornace AJ, Jr., Pospelova TV, Pospelov VA. p21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle. 2010;9:3945–55. doi: 10.4161/cc.9.19.13160. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Halicka HD, Li J, Darzynkiewicz Z. Berberine suppresses gero-conversion from cell cycle arrest to senescence. Aging (Albany NY) 2013;5:623–36. doi: 10.18632/aging.100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging (Albany NY) 2012;4:952–65. doi: 10.18632/aging.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C, Liu Y, Liu Y, Zheng P. The axis of mTOR-mitochondria-ROS and stemness of the hematopoietic stem cells. Cell Cycle. 2009;8:1158–60. doi: 10.4161/cc.8.8.8139. [DOI] [PubMed] [Google Scholar]
- 37.Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8:1003–6. doi: 10.4161/cc.8.7.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–70. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young AR, Narita M, Narita M. Spatio-temporal association between mTOR and autophagy during cellular senescence. Autophagy. 2011;7:1387–8. doi: 10.4161/auto.7.11.17348. [DOI] [PubMed] [Google Scholar]
- 40.Fujii S, Hara H, Araya J, Takasaka N, Kojima J, Ito S, Minagawa S, Yumino Y, Ishikawa T, Numata T, et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology. 2012;1:630–41. doi: 10.4161/onci.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blagosklonny MV. Answering the ultimate question “what is the proximal cause of aging?”. Aging (Albany NY) 2012;4:861–77. doi: 10.18632/aging.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- 43.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–65. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle. 2009;8:4055–9. doi: 10.4161/cc.8.24.10310. [DOI] [PubMed] [Google Scholar]
- 45.Blagosklonny MV. Big mice die young but large animals live longer. Aging (Albany NY) 2013;5:227–33. doi: 10.18632/aging.100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blagosklonny MV. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging (Albany NY) 2010;2:265–73. doi: 10.18632/aging.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. 2010;9:1673–4. doi: 10.4161/cc.9.9.11626. [DOI] [PubMed] [Google Scholar]
- 48.Luo LL, Xu JJ, Fu YC. Rapamycin prolongs female reproductive lifespan. Cell Cycle. 2013;12:3353–4. doi: 10.4161/cc.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–6. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 50.Blagosklonny MV. Why the disposable soma theory cannot explain why women live longer and why we age. Aging (Albany NY) 2010;2:884–7. doi: 10.18632/aging.100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011;3:1051–62. doi: 10.18632/aging.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blagosklonny MV. MTOR-driven quasi-programmed aging as a disposable soma theory: blind watchmaker vs. intelligent designer. Cell Cycle. 2013;12:1842–7. doi: 10.4161/cc.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Longo VD, Mitteldorf J, Skulachev VP. Programmed and altruistic ageing. Nat Rev Genet. 2005;6:866–72. doi: 10.1038/nrg1706. [DOI] [PubMed] [Google Scholar]
- 54.Bredesen DE. The non-existent aging program: how does it work? Aging Cell. 2004;3:255–9. doi: 10.1111/j.1474-9728.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 55.Prinzinger R. Programmed ageing: the theory of maximal metabolic scope. How does the biological clock tick? EMBO Rep. 2005;6:S14–9. doi: 10.1038/sj.embor.7400425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Magalhães JP. Programmatic features of aging originating in development: aging mechanisms beyond molecular damage? FASEB J. 2012;26:4821–6. doi: 10.1096/fj.12-210872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Magalhães JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2006;41:1–10. doi: 10.1016/j.exger.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Mitteldorf J. Can experiments on caloric restriction be reconciled with the disposable soma theory for the evolution of senescence? Evolution. 2001;55:1902–5, discussion 1906. doi: 10.1111/j.0014-3820.2001.tb00841.x. [ discussion 1906] [DOI] [PubMed] [Google Scholar]
- 59.Hayflick L. “Anti-aging” is an oxymoron. J Gerontol A Biol Sci Med Sci. 2004;59:B573–8. doi: 10.1093/gerona/59.6.B573. [DOI] [PubMed] [Google Scholar]
- 60.Skulachev VP, Longo VD. Aging as a mitochondria-mediated atavistic program: can aging be switched off? Ann N Y Acad Sci. 2005;1057:145–64. doi: 10.1196/annals.1356.009. [DOI] [PubMed] [Google Scholar]
- 61.Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, et al. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta. 2009;1787:437–61. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Skulachev VP. SkQ1 treatment and food restriction--two ways to retard an aging program of organisms. Aging (Albany NY) 2011;3:1045–50. doi: 10.18632/aging.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skulachev VP. Aging as a particular case of phenoptosis, the programmed death of an organism (a response to Kirkwood and Melov “On the programmed/non-programmed nature of ageing within the life history”) Aging (Albany NY) 2011;3:1120–3. doi: 10.18632/aging.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skulachev VP. Programmed death phenomena: from organelle to organism. Ann N Y Acad Sci. 2002;959:214–37. doi: 10.1111/j.1749-6632.2002.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 65.Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, Büttner S, Fehr M, Sigrist S, Madeo F. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–7. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, Dossen JW, Gralla EB, Longo VD. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol. 2004;166:1055–67. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fabrizio P, Longo VD. Chronological aging-induced apoptosis in yeast. Biochim Biophys Acta. 2008;1783:1280–5. doi: 10.1016/j.bbamcr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leontieva OV, Blagosklonny MV. Yeast-like chronological senescence in mammalian cells: phenomenon, mechanism and pharmacological suppression. Aging (Albany NY) 2011;3:1078–91. doi: 10.18632/aging.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–70. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burtner CR, Murakami CJ, Kaeberlein M. A genomic approach to yeast chronological aging. Methods Mol Biol. 2009;548:101–14. doi: 10.1007/978-1-59745-540-4_6. [DOI] [PubMed] [Google Scholar]
- 71.Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464:513–9. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabrizio P, Wei M. Conserved role of medium acidification in chronological senescence of yeast and mammalian cells. Aging (Albany NY) 2011;3:1127–9. doi: 10.18632/aging.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Longo VD. Ras: the other pro-aging pathway. Sci Aging Knowledge Environ. 2004;2004:pe36. doi: 10.1126/sageke.2004.39.pe36. [DOI] [PubMed] [Google Scholar]
- 74.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological lifespan via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–77. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological lifespan in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–84. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alvers AL, Wood MS, Hu D, Kaywell AC, Dunn WA, Jr., Aris JP. Autophagy is required for extension of yeast chronological lifespan by rapamycin. Autophagy. 2009;5:847–9. doi: 10.4161/auto.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan Y, Shadel GS. Extension of chronological lifespan by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany NY) 2009;1:131–45. doi: 10.18632/aging.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011;3:1130–41. doi: 10.18632/aging.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, Dang N, Fields S, Kennedy BK. Increased lifespan due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2005;1:e69. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Powers T. TOR signaling and S6 kinase 1: Yeast catches up. Cell Metab. 2007;6:1–2. doi: 10.1016/j.cmet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 81.Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–8. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shanley DP, Kirkwood TB. Evolution of the human menopause. Bioessays. 2001;23:282–7. doi: 10.1002/1521-1878(200103)23:3<282::AID-BIES1038>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 83.Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408:233–8. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 84.Austad SN. Is aging programed? Aging Cell. 2004;3:249–51. doi: 10.1111/j.1474-9728.2004.00112.x. [DOI] [PubMed] [Google Scholar]
- 85.Blagosklonny MV. Paradoxes of aging. Cell Cycle. 2007;6:2997–3003. doi: 10.4161/cc.6.24.5124. [DOI] [PubMed] [Google Scholar]
- 86.Blagosklonny MV. Program-like aging and mitochondria: instead of random damage by free radicals. J Cell Biochem. 2007;102:1389–99. doi: 10.1002/jcb.21602. [DOI] [PubMed] [Google Scholar]
- 87.Blagosklonny MV. Rapamycin and quasi-programmed aging: four years later. Cell Cycle. 2010;9:1859–62. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- 88.Blagosklonny MV. Why human lifespan is rapidly increasing: solving “longevity riddle” with “revealed-slow-aging” hypothesis. Aging (Albany NY) 2010;2:177–82. doi: 10.18632/aging.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blagosklonny MV. Rapamycin extends life- and health span because it slows aging. Aging (Albany NY) 2013;5:592–8. doi: 10.18632/aging.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blagosklonny MV. M(o)TOR of aging: MTOR as a universal molecular hypothalamus. Aging (Albany NY) 2013;5:490–4. doi: 10.18632/aging.100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 92.Hands SL, Proud CG, Wyttenbach A. mTOR’s role in ageing: protein synthesis or autophagy? Aging (Albany NY) 2009;1:586–97. doi: 10.18632/aging.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan L, Mieulet V, Lamb RF. Nutrient regulation of mTORC1 and cell growth. Cell Cycle. 2010;9:2473–4. doi: 10.4161/cc.9.13.12124. [DOI] [PubMed] [Google Scholar]
- 94.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 97.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim E, Guan KL. RAG GTPases in nutrient-mediated TOR signaling pathway. Cell Cycle. 2009;8:1014–8. doi: 10.4161/cc.8.7.8124. [DOI] [PubMed] [Google Scholar]
- 100.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hildebrand DG, Lehle S, Borst A, Haferkamp S, Essmann F, Schulze-Osthoff K. α-Fucosidase as a novel convenient biomarker for cellular senescence. Cell Cycle. 2013;12:1922–7. doi: 10.4161/cc.24944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Völkers M, Sussman M. mTOR/PRAS40 interaction: Hypertrophy or proliferation. Cell Cycle. 2013;12 doi: 10.4161/cc.26822. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ogawa M. Hemopoietic stem cells: stochastic differentiation and humoral control of proliferation. Environ Health Perspect. 1989;80:199–207. doi: 10.1289/ehp.8980199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watari K, Tojo A, Nagamura-Inoue T, Matsuoka M, Irie S, Tani K, Yamada Y, Asano S. Hyperfunction of neutrophils in a patient with BCR/ABL negative chronic myeloid leukemia: a case report with in vitro studies. Cancer. 2000;89:551–60. doi: 10.1002/1097-0142(20000801)89:3<551::AID-CNCR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 105.Metcalf D. Implications of the polyfunctionality of hemopoietic regulators. Stem Cells. 1994;12(Suppl 1):259–75. doi: 10.1002/stem.5530120722. [DOI] [PubMed] [Google Scholar]
- 106.Baumann MA, Paul CC, Lemley-Gillespie S, Oyster M, Gomez-Cambronero J. Modulation of MEK activity during G-CSF signaling alters proliferative versus differentiative balancing. Am J Hematol. 2001;68:99–105. doi: 10.1002/ajh.1160. [DOI] [PubMed] [Google Scholar]
- 107.Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol. 2012;181:1142–6. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 108.Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle. 2009;8:1883–7. doi: 10.4161/cc.8.12.8815. [DOI] [PubMed] [Google Scholar]
- 109.Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–8. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- 110.Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012;4:350–8. doi: 10.18632/aging.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: preliminary proposal. Cell Cycle. 2010;9:4788–94. doi: 10.4161/cc.9.24.14360. [DOI] [PubMed] [Google Scholar]
- 112.Hentges KE, Sirry B, Gingeras AC, Sarbassov D, Sonenberg N, Sabatini D, Peterson AS. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc Natl Acad Sci U S A. 2001;98:13796–801. doi: 10.1073/pnas.241184198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–16. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–8. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shor B, Cavender D, Harris C. A kinase-dead knock-in mutation in mTOR leads to early embryonic lethality and is dispensable for the immune system in heterozygous mice. BMC Immunol. 2009;10:28. doi: 10.1186/1471-2172-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–24. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–24. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santoro R, Blandino G. p53: The pivot between cell cycle arrest and senescence. Cell Cycle. 2010;9:4262–3. doi: 10.4161/cc.9.21.13853. [DOI] [PubMed] [Google Scholar]
- 119.Loayza-Puch F, Drost J, Rooijers K, Lopes R, Elkon R, Agami R. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol. 2013;14:R32. doi: 10.1186/gb-2013-14-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Serrano M. Shifting senescence into quiescence by turning up p53. Cell Cycle. 2010;9:4256–7. doi: 10.4161/cc.9.21.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng Z, Hu W, Rajagopal G, Levine AJ. The tumor suppressor p53: cancer and aging. Cell Cycle. 2008;7:842–7. doi: 10.4161/cc.7.7.5657. [DOI] [PubMed] [Google Scholar]
- 122.Roemer K. Are the conspicuous interdependences of fecundity, longevity and cognitive abilities in humans caused in part by p53? Cell Cycle. 2010;9:3438–41. doi: 10.4161/cc.9.17.13001. [DOI] [PubMed] [Google Scholar]
- 123.Bauer JH, Helfand SL. Sir2 and longevity: the p53 connection. Cell Cycle. 2009;8:1821. doi: 10.4161/cc.8.12.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hasty P, Sharp ZD, Curiel TJ, Campisi J. mTORC1 and p53: clash of the gods? Cell Cycle. 2013;12:20–5. doi: 10.4161/cc.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Viña J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–9. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 126.Berkers CR, Maddocks OD, Cheung EC, Mor I, Vousden KH. Metabolic Regulation by p53 Family Members. Cell Metab. 2013;18:617–33. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tower J. The genetic architecture of aging: sexual antagonistic pleiotropy of p53 and foxo. Cell Cycle. 2010;9:3840–1. doi: 10.4161/cc.9.19.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaeberlein M, Kapahi P. The hypoxic response and aging. Cell Cycle. 2009;8:2324. doi: 10.4161/cc.8.15.9126. [DOI] [PubMed] [Google Scholar]
- 129.Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov Today. 2007;12:218–24. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends lifespan of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11:326–35. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheng XF. Chemoprevention of age-related macular regeneration (AMD) with rapamycin. Aging (Albany NY) 2012;4:375–6. doi: 10.18632/aging.100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khanna A, Kapahi P. Rapamycin: killing two birds with one stone. Aging (Albany NY) 2011;3:1043–4. doi: 10.18632/aging.100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–13. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, et al. If started early in life, metformin treatment increases lifespan and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–57. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, Strong R, Bokov A, Javors MA, Ikeno Y, et al. Rapamycin extends lifespan of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging (Albany NY) 2013;5:100–10. doi: 10.18632/aging.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011;3:1039–40. doi: 10.18632/aging.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao C, Vollrath D. mTOR pathway activation in age-related retinal disease. Aging (Albany NY) 2011;3:346–7. doi: 10.18632/aging.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang X, Liu J, Withers BR, Samide AJ, Leggas M, Dickson RC. Reducing signs of aging and increasing lifespan by drug synergy. Aging Cell. 2013;12:652–60. doi: 10.1111/acel.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Santini E, Valjent E, Fisone G. mTORC1 signaling in Parkinson’s disease and L-DOPA-induced dyskinesia: A sensitized matter. Cell Cycle. 2010;9:2713–8. doi: 10.4161/cc.9.14.12180. [DOI] [PubMed] [Google Scholar]
- 143.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–62. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene. 2013;523:82–7. doi: 10.1016/j.gene.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 145.Glazer HP, Osipov RM, Clements RT, Sellke FW, Bianchi C. Hypercholesterolemia is associated with hyperactive cardiac mTORC1 and mTORC2 signaling. Cell Cycle. 2009;8:1738–46. doi: 10.4161/cc.8.11.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, Baur JA. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY) 2013;5:539–50. doi: 10.18632/aging.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol. 2012;47:958–65. doi: 10.1016/j.exger.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 149.Wang M, Miller RA. Fibroblasts from long-lived mutant mice exhibit increased autophagy and lower TOR activity after nutrient deprivation or oxidative stress. Aging Cell. 2012;11:668–74. doi: 10.1111/j.1474-9726.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol. 2012;181:472–7. doi: 10.1016/j.ajpath.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 151.Gems D, de la Guardia Y. Alternative Perspectives on Aging in Caenorhabditis elegans: Reactive Oxygen Species or Hyperfunction? Antioxid Redox Signal. 2013;19:321–9. doi: 10.1089/ars.2012.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gems D, Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol. 2013;75:621–44. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- 153.Moskalev AA, Shaposhnikov MV. Pharmacological inhibition of phosphoinositide 3 and TOR kinases improves survival of Drosophila melanogaster. Rejuvenation Res. 2010;13:246–7. doi: 10.1089/rej.2009.0903. [DOI] [PubMed] [Google Scholar]
- 154.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of lifespan extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Garelick MG, Mackay VL, Yanagida A, Academia EC, Schreiber KH, Ladiges WC, Kennedy BK. Chronic rapamycin treatment or lack of S6K1 does not reduce ribosome activity in vivo. Cell Cycle. 2013;12:2493–504. doi: 10.4161/cc.25512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, et al. Rapamycin Extends Life and Health in C57BL/6 Mice. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fang Y, Bartke A. Prolonged rapamycin treatment led to beneficial metabolic switch. Aging (Albany NY) 2013;5:328–9. doi: 10.18632/aging.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Passtoors WM, Beekman M, Deelen J, van der Breggen R, Maier AB, Guigas B, Derhovanessian E, van Heemst D, de Craen AJ, Gunn DA, et al. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell. 2013;12:24–31. doi: 10.1111/acel.12015. [DOI] [PubMed] [Google Scholar]
- 159.Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 2012;11:401–14. doi: 10.1016/j.stem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Iglesias-Bartolome R, Gutkind SJ. Exploiting the mTOR paradox for disease prevention. Oncotarget. 2012;3:1061–3. doi: 10.18632/oncotarget.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, et al. GSK-3α is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–32. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging (Albany NY) 2012;4:709–14. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, Gudkov AV, Antoch MP. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging (Albany NY) 2012;4:715–22. doi: 10.18632/aging.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497:211–6. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang Y, Cai D. Hypothalamic inflammation and GnRH in aging development. Cell Cycle. 2013;12:2711–2. doi: 10.4161/cc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Perkey E, Fingar D, Miller RA, Garcia GG. Increased Mammalian target of rapamycin complex 2 signaling promotes age-related decline in CD4 T cell signaling and function. J Immunol. 2013;191:4648–55. doi: 10.4049/jimmunol.1300750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu X, Cao Y, Nie J, Liu H, Lu S, Hu X, Zhu J, Zhao X, Chen J, Chen X, et al. Genetic and pharmacological inhibition of Rheb1-mTORC1 signaling exerts cardioprotection against adverse cardiac remodeling in mice. Am J Pathol. 2013;182:2005–14. doi: 10.1016/j.ajpath.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y, Xu X, Ren J. MTOR overactivation and interrupted autophagy flux in obese hearts: a dicey assembly? Autophagy. 2013;9:939–41. doi: 10.4161/auto.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rao RR, Li Q, Shrikant PA. Fine-tuning CD8(+) T cell functional responses: mTOR acts as a rheostat for regulating CD8(+) T cell proliferation, survival and differentiation? Cell Cycle. 2010;9:2996–3001. doi: 10.4161/cc.9.15.12359. [DOI] [PubMed] [Google Scholar]
- 170.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nicoletti F, Lapenta C, Donati S, Spada M, Ranazzi A, Cacopardo B, Mangano K, Belardelli F, Perno C, Aquaro S. Inhibition of human immunodeficiency virus (HIV-1) infection in human peripheral blood leucocytes-SCID reconstituted mice by rapamycin. Clin Exp Immunol. 2009;155:28–34. doi: 10.1111/j.1365-2249.2008.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185:2004–8. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ferrer IR, Araki K, Ford ML. Paradoxical aspects of rapamycin immunobiology in transplantation. Am J Transplant. 2011;11:654–9. doi: 10.1111/j.1600-6143.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blagosklonny MV. How to save Medicare: the anti-aging remedy. Aging (Albany NY) 2012;4:547–52. doi: 10.18632/aging.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY) 2009;1:281–8. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, Ford ML, Ahmed R, Kirk AD, Larsen CP. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant. 2011;11:613–8. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hill JA, Hummel M, Starling RC, Kobashigawa JA, Perrone SV, Arizón JM, Simonsen S, Abeywickrama KH, Bara C. A lower incidence of cytomegalovirus infection in de novo heart transplant recipients randomized to everolimus. Transplantation. 2007;84:1436–42. doi: 10.1097/01.tp.0000290686.68910.bd. [DOI] [PubMed] [Google Scholar]
- 178.Kobashigawa J, Ross H, Bara C, Delgado JF, Dengler T, Lehmkuhl HB, Wang SS, Dong G, Witte S, Junge G, et al. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis. 2013;15:150–62. doi: 10.1111/tid.12007. [DOI] [PubMed] [Google Scholar]
- 179.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104:643–52. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]


