SUMMARY
The bone marrow (BM) microenvironment regulates survival and maintenance of normal hematopoietic stem cells. Within the endosteal niche, hypoxia has an essential role in maintenance of the primitive quiescent hematopoietic stem cell. We and others have demonstrated that in the context of hematologic malignancies the BM is highly hypoxic, and that progression of the disease is associated with expansion of hypoxic niches and stabilization of the oncogenic HIF-1α. This review will provide an overview of the normal and leukemic BM microenvironment with a special emphasis on pathological hypoxia including the development of hypoxia-activated prodrugs and their applicability in hematological malignancies.
Hematopoietic stem cells residing in the bone marrow (BM) are responsible for producing both lymphoid and myeloid cells that in turn carry out essential functions including maintaining the body’s immune integrity, O2 delivery, blood clotting, waste removal and a wide variety of physiologic processes that are necessary for survival. Early on Curry, Trentin and Wolf pointed out the indispensable role of the BM micro environment in supporting hemato poiesis [1], while in 1978 Schofield coined the term ‘hematopoietic niche’ [2]; however, due to the difficulty in accessing the BM, the intracellular regulatory mechanisms of hematopoietic stem cells (HSCs) have been studied in isolation neglecting their confines in the BM, with little emphasis on the effects this environment might have on this essential process [3]. Development and improvement of the in vivo imaging tools provided real-time visualization of cellular interactions [4, 5] and showed that HSCs depend on the microenvironment where they reside, supporting the ‘stem cell niche’ hypothesis [4].
Within the complex BM microenvironment HSCs are believed to reside in two distinct physical niches, the endosteal and vascular (sinusoidal) niches (Figure 1) [6–9]. Components of these niches include bone lining cells (osteoblasts and osteoclasts), mesenchymal stem cells, sinusoidal endothelium and perivascular stromal cells, immune cells and other cell types that contribute to HSC homeostasis [10]. More recently, a reticular niche comprised of CXCL12 abundant reticular cells and nestin-positive mesenchymal cells, has also been implicated as an important element of the BM microenvironment supporting HSCs [11]. Elucidating the functions of these three niches is crucial to understanding the behavior of HSCs and to exploit them for clinical applications.
Figure 1. Organization of osteoblastic, vascular and reticular niches in the bone marrow microenvironment.
Both hematopoietic stem cells and leukemia stem cells establish niches around the bone marrow endosteum and sinusoids. The endosteal niche is formed by osteoblasts, osteoclasts and stromal cells. Sinusoidal niches are regulated by the sinusoidal endothelial cells and perivascular stromal cells. The more recently described reticular niche is composed of CAR cells and nestin-positive mesenchymal cells. The oxygen gradient decreases from the sinusoids to the endosteum, and the most primitive hematopoietic stem cells and possibly leukemia stem cells are thought to be sequestered in a hypoxic microenvironment. CAR: CXCL12 abundant reticular.
The endosteum, formed by both cortical and trabecular bone, is lined by bone cells such as osteoblasts and osteoclasts, constituting the inner surface of the BM cavity. As determined by intravital microscopy, fluorescently labeled HSCs home to the BM endosteum, suggesting a preference for this anatomical site for their survival and maintenance [10]. Several lines of evidence suggest that osteoblast-associated HSCs are quiescent in nature, giving them the ability to survive and contribute to hematopoiesis over a long period of time [12, 13]. Osteoblasts have been suggested to express several cytokines, chemokines and adhesion molecules such as CXCL12, angiopoietin 1, stem cell factor (SCF) and thrombopoietin, all of which seem to be important for HSCs regulation.
The BM vascular niche is formed mainly by thin-walled vessels called sinusoids that connect the marrow cavity with the systemic circulation. Kiel et al. showed that most HSCs reside adjacent to sinusoidal endothelium in the spleen and BM, while few showed preference for the BM endosteum [8]. As in the endosteal niche, cells supporting the vascular compartment also secrete cytokines, including SCF and CXCL12, which are critical for HSC regulation. The functional and anatomical distinctions between the endosteal and vascular niches remain elusive. To elucidate the specific contribution of each BM niche to hematopoiesis, Ding et al. used Cre-lox conditional knockout mice to specifically delete SCF from osteoblasts, sinusoidal endothelium, perivascular stromal cells and nestin-positive mesenchymal stem cells [7]. The results suggest that sinusoidal endothelium and leptin receptor (Lepr)-expressing perivascular stromal cells but not osteoblasts or nestin-Cre- or nestin-CreER-expressing cells, are directly responsible for the expression of SCF and functionally regulate HSCs in the sinusoidal niche. Recently, Ding and Morrison went further and showed that HSCs depend on CXCL12 (also known as SDF-1α) produced by perivascular and endothelial cells while lymphoid progenitors rely on that provided by osteoblasts [14]. These observations appear to override the model in which HSCs are believed to reside in hypoxic areas of the endos-teal niche. Findings by Greenbaum et al. generated by selective deletion of CXCL12 in target stromal populations supported these conclusions, whereby expression of CXCL12 in perivascular stromal cells, including endothelial cells and mesenchymal progenitors, supports HSCs function, while CXCL12-abundant reticular cells and osteoblasts play a role in support of B-lymphoid progenitors [11]. These findings point to the complexity of the BM micro environment and the interplay between its different components to sustain HSCs homeostasis.
Physiologic BM hypoxia
High or low O2 levels can cause detrimental consequences and therefore, in normal tissues, O2 levels are tightly regulated. Partial O2 pressure (pO2) in human tissues exhibit great variations and are far below the atmospheric values of 21% (or 160 mmHg). Hypoxia is defined as a drop in O2 levels below the physiological values [15]. pO2 varies in arterial blood, between 75 and 100 mmHg (equivalent to 10 and 13.3% partial atmospheric O2 pressure [16–18]) while in venous blood it is between 30 and 40 mmHg (4% and 5.3% partial atmospheric O2 pressure [19]). In tissues, O2 concentration depends on vascular-ization, ranging from 4–14% in well perfused tissues and between 0.7–7% in low-perfused organs [15,20–24]. Owing to the difficulty in accessing the tissue, direct O2 measurements in the BM are difficult to make and to interpret. In patients with chronic pulmonary disease or in healthy volunteers mean O2 values in the BM were 48 and 54.9 mmHg, respectively [25,26]. Utilizing Hoechst 33342 (Ho) perfusion dye, Parmar et al. suggested the existence of an O2 gradient from the vasculature to the endosteal niche, with the majority of HSCs having the lowest Ho intensity [27]. In turn, these HSC were labeled by the hypoxia marker pimonidazole (PIMO) and were sensitive to tirapazamine, a hypoxia-activated prodrug. This study and work done by others led to the notion that hypoxia promotes maintenance of HSCs in a quiescent and pluripotent state [28], while HSCs that reside in close proximity to the vascular niche are short-term, actively cycling and are responsible for replenishing circulating cells [29]. Endosteal niche residents, the long-term HSCs, depend on HIF-1, the master transcriptional regulator of hypoxia response, to maintain them in a quiescent state by promoting expression of different target genes including VEGFα, Cripto/GRP7 8 and CXCR4 [30–32]. In the presence of O2, HIF-1α subunits are normally degraded by the protein encoded by the von Hippel–Lindau tumor suppressor gene VHL (pVHL) but are stabilized under hypoxic conditions. A vast array of gene products controlling angiogenesis, apoptosis, energy metabolism and the cell cycle [33] are induced by HIF-1.
Hypoxia in hematologic malignancies
In solid tumors, hypoxia is a common phenomenon often associated with poor prognosis. In this context, increased aggressiveness and invasiveness, metastatic behavior and resistance to chemotherapy [33,34] are linked to pathophysiological hypoxia. As a matter of fact, in a vast number of human cancers [35], HIF-1α or HIF-2α protein (or both) are overexpressed relative to the normal tissue counterparts. Downstream of HIF-1, a long list of genes involved in key processes lead-ing to tumor progression including angiogenesis [36] and metabolism regulation [37–44] support the notion of the detrimental effect of hypoxia.
Even though the importance of hypoxia in cancer was proposed over 50 years ago [45] and is deeply understood in solid tumors, studying its significance in hematological malignancies has been slowed by the lack of reliable methods to unequivocally measure O2 levels in BM. Despite the difficulties, early findings indicate the previously unrecognized role of hypoxia in the hematologic malignancies. Jensen et al. showed in a rat leukemia model, via in vivo pulse-labeling with a theophylline-linked 2-nitroimidazole hypoxia probe that leukemic cells infiltrating the BM were markedly hypoxic [46]. In a study where conventional gas assay methodology was used to measure pO2 values in the BM aspirates from 19 acute myeloid leukemia (AML) patients, levels were found to be 6.06% on average [47]. However this value likely overestimates the O2 levels within the endosteal niches. We recently reported that hypoxia constitutes a general component of the leukemic BM environment. As such, vast expansion of hypoxic areas was detected by labeling of the BM of mice bearing xenografts or syngeneic acute leukemia models with the 2-nitroimidazole hypoxia probe PIMO, which increased with disease progression (Figure 2) [48]. HIF-1α, one of the best characterized markers of hypoxia, was shown to be overexpressed in clusters of BM-resident leukemic cells in pediatric acute lymphoblastic leukemia (ALL) cases while absent in normal BM biopsies [49]. Likewise, BMs from adult patients with ALL frequently exhibit HIF-1 expression, which in turn is associated with inferior out-comes [50]. Consistent with these findings, in paired BM samples from ALL patients, HIF-1α was expressed at the time of diagnosis, however, it routinely became undetectable at the time when remission was obtained following induction chemotherapy [48]. Different mechanisms can explain hypoxia contribution to leukemia progression, including induction of chemoresistance [48,50] and expression of chemokine receptor CXCR4, which in turn induces homing of leukemic blasts to the protective BM environment [47,48]. Recently, findings by Matsunaga et al. suggested that hypoxia, via HIF-1α could play a role in the maintenance of minimal residual disease in AML [51]. They observed that HIF-1α promotes quiescence of leukemic cells residing in the endosteal niches and speculated that this could contribute to persistence of residual leukemic cells in AML [51].
Figure 2. Expansion of the hypoxic bone marrow niche in a syngeneic chronic myeloid leukemia xenograft model.
Representative images from one healthy control mouse (A) or a mouse transplanted with GFP/YFP labeled cells expressing the oncogenes BCR/ABL and Nup98 (cells were kindly provided by Craig Jordan, University of Rochester, NY, USA) (B). After 6 days, mice were injected with pimonidazole 3 h prior to sacrifice. Areas of hypoxia were detected by pimonidazole antibody. Original magnification is shown next to each panel. bcCML: Blast crisis chronic myeloid leukemia.
Among hematological malignancies, multiple myeloma (MM [52]) has received special attention with regards to the importance of hypoxia and of HIFs in disease progression. Asosingh et al. utilized PIMO and an in vivo murine MM model (5T2MM), and showed that a heavily infiltrated MM BM is less hypoxic than the normal counterpart owing to the presence of active angio genesis stimulated by initial hypoxia [53]. In addition, they pointed to the role that hypoxia may have in disease establishment given that the hypoxic BM environment selects for tumor-initiating and apoptosis-resistant CD45-positive MM cells. By contrast, in a more recent study, Hu et al., showed significantly higher positive PIMO areas in the BM of mice harboring 5T33MM cells compared with control animals [54], suggesting that hypoxia increases in association with MM progression. In a recent provocative study, Azab et al. reported that BM hypoxia promotes dissemination and rehoming of MM cells by inducing epithelial to mesenchymal transition-like features in MM cells [55]. Finally, shRNA-mediated HIF-1α silencing in MM cells led to downregulation of pro angiogenic as well as pro-osteoclastogenic cytokines resulting in tumor suppression due to decreased angiogenesis and bone destruction [56].
Its widespread contribution to solid and liquid tumors had made hypoxia a desirable target for anticancer therapy. In solid tumors, two general approaches are being explored for targeting hypoxic cells, both of which are potentially applicable to hypoxia in hematologic malignancies. One approach focuses on inhibiting mole cular targets required for survival of hypoxic cells, particularly HIF-1 and its downstream or upstream partners. However, targeting a transcription factor such as HIF-1 has proved difficult and so far has not yielded specific druggable pharmacophores [57]. By contrast, development of inhibitors of CAIX, a HIF-1 target acting as an important regulator of intracellular pH and cell survival in hypoxic microenvironments [58], is well advanced with several antibodies and small molecule inhibitors currently in preclinical and clinical development for solid tumors [59]. HIF-independent pathways involved in tolerance of hypoxia such as PERK/eIF2α signaling [60] also offer druggable and promising molecular targets [61]. Rouschop et al. demonstrated that the PERK/eIF2α arm of the unfolded protein response is essential for the survival of a group of radioresistant tumor hypoxic cells [60]. At the same time, Atkins et al. developed a PERK competitive inhibitor that exhibited excellent anti tumor activity in several xenograft murine models [61]. However, the importance of this mechanism in acute leukemia remains to be shown.
In the second approach, a set of drugs named hypoxia-activated prodrugs (HAPs) have been developed in an effort to eliminate cancer cells residing in hypoxic microenvironments. In the next section we will provide a brief update on the progress of these HAPs within the hematological malignancies field.
Hypoxia-activated prodrugs
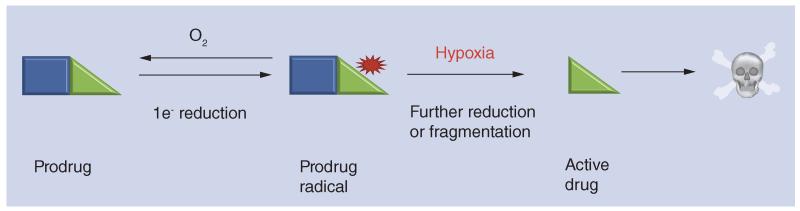
Even though HAP development is a relatively mature field, it still harbors its own significant challenges as discussed in several reviews [62–64]. These prodrugs function as O2 sensors that are activated by enzymatic reduction in hypoxic cells. Typically (most nitro compounds, quinones and aromatic N-oxide HAPs) hypoxia selectivity is achieved through one-electron reduction of the prodrug by POR and other flavoprotein reductases [65––]. In the presence of O2, the resulting prodrug radical undergoes redox cycling [68], while in its absence the prodrug radical has a much longer lifetime and can fragment, or be reduced further, to generate the active drug in hypoxic cells (Figure 3). Two-electron reductases such as NAD(P)H dehydrogenase [quinone]-1 (NQO-1; DT-diaphorase) can bypass the O2-sensitive free radical inter-mediate in some cases, resulting in prodrug activation independently of hypoxia [69]. In addition, hypoxic selectivity can be the result of a competition between the HAP and O2 for reductants rather than redox cycling [70,71].
Figure 3. General mechanism of activation of hypoxia-activated prodrugs.
The first step consists of one-electron reduction of the prodrug by POR and other flavoprotein reductases [95]. In the presence of oxygen, the resulting prodrug radical undergoes redox cycling [68], while in its absence the prodrug radical has a much longer lifetime and can fragment, or be reduced further, to generate the active drug killing the hypoxic cells.
PR-104, a well-studied embodiment of this group, was developed at the University of Auck-land (Auckland, New Zealand). A water-soluble phosphate ester preprodrug, PR-104, is rapidly hydrolyzed systemically to the corresponding alcohol, PR-104A [72], which acts as a HAP through one-electron reduction of the nitro group para to its nitrogen mustard moiety, in a reaction catalyzed in hypoxic cells by POR and other diflavin reductases. The resulting hydroxylamine (PR-104H) and amine (PR-104M) metabolites are activated nitrogen mustards that kill hypoxic cells through formation of DNA interstrand crosslinks [73]. Later, it was found that PR-104A is a substrate for an O2-insensitive two-electron reductase, aldo-keto reductase 1C3 (AKR1C3), which had not previously been known to act as a nitroreductase and which does not activate other HAP classes. Activation by AKR1C3 appears to be a major contributor to the monotherapy activity of PR-104 in preclinical models [74].
Initial Phase I clinical trials of PR-104 in patients with advanced solid tumors established a maximum tolerated dose (MTD) of 1100 mg/m2 for a once every three weeks schedule [75] and 675 mg/m2/week for dosing weekly for 3 weeks of a 4-week cycle, with little evidence of monotherapy activity in these unselected tumors. Hematological toxicity was dose limiting in the above Phase I studies, which together with the emerging evidence for hypoxia in human leukemias (see above) suggested that evaluation against leukemias might be warranted. Despite the fact that use of alkylators in the treatment of AML has declined with the advent of anthracyline/cytarabine combinations, their activity in this setting is well attested and they are still often used in conditioning regimens for allogeneic or autologous stem cell transplantation; thus imposing additional selectivity through targeting hypoxia in BM (and potentially disease in peripheral tissue) is attractive. In preclinical studies, PR-104 demonstrated impressive single agent activity in xenograft models of human pre-B ALL and AML providing rationale for exploiting HAPs in leukemia setting [48].
Other factors tilting the balance for potential utility of PR-104 in a leukemia setting include sensitivity of some AML subgroups to cross-linking agents [76] given the ability of active metabolites of PR-104A to exploit defects in DNA crosslink repair [77]; and the possibility of activation of PR-104A by AKR1C3, whose mRNA was found to be highly expressed by AML blasts in some patients [78], as well as hypoxic (one-electron) reductases. In fact, recent studies demonstrate consistently higher expression and enzyme activity of AKR1C3 in cells from a panel of T-ALL than B-ALL xenografts [79] suggesting possible utility in this subtype.
The preclinical data made moving PR-104 into early Phase I/II clinical trial in relapsed and/or refractory acute leukemias (AML/ALL) possible [80] (PR104 2009-0772: clinicaltrials.gov ID: NCT01037556 [101]). PR-104 was administered as a 1-h intravenous infusion every 2 weeks, at doses of 1.1, 1.6, 2.2, 3 and 4 g/m2, with cohort expansion at 3 and 4 g/m2. Interim ana lysis demonstrated that PR-104 was tolerated at doses three- to four-times higher than the solid tumor MTD; however, myelosuppression, primarily neutropenia and thrombocytopenia, can be prolonged at doses ≥3 g/m2. Evidence of activity at doses of 3 or 4 g/m2 support continued evaluation of this regimen in acute leukemias.
In a similar fashion to PR-104, TH-302 generates a DNA-crosslinking mustard alkylating agent following reduction of a nitro group in the prodrug, but differs in several other respects. Development of symmetrical (achiral) analogs of nitroheterocyclic phosphoramidate prodrugs [81] at Threshold Pharmaceuticals (CA, USA) led to the 2-nitroimidazole TH-302, which showed a 400-fold anoxia/oxic potency differential for H460 cells and promising activity with gemcitabine in a xenograft model [82]. Later, high hypoxic selectivity of TH-302 was confirmed in a wide range of tumor cell lines, and its fragmentation on reduction to release the potent cytotoxin bromo-isophosphoramide mustard (Br-iPM) has been shown to be responsible for the generation of DNA crosslinks in hypoxic cells [83]. The ability of Br-iPM to diffuse from the cells in which it is generated is supported by its detection in plasma of nonclinical species [84] and humans, and is also suggested by evidence for a bystander effect when TH-302 is activated by an O2-insensitive bacterial nitroreductase in multicellular layer cultures [83]. Impressive activity of TH-302 has been reported in combination with conventional chemotherapy in multiple preclinical solid tumor models [85]. In multiple myeloma models, TH-302 induced apoptosis of 5T33MM cells, under anoxia in vitro and within the BM microenvironment in an in vivo murine model [54]. TH-302 also had hypoxia-selective antileukemia activity against acute leukemia cell lines, primary leukemia samples and in the in vivo AML xenograft model [86].
When TH-302 was administered in humans as monotherapy on a once every three week or once a week for 3 weeks schedule, the dose-limiting toxicity was skin and mucosal toxicity with minimal hematological toxicity [87], however, the later was more prominent when TH-302 was given in combination with gemcitabine in advanced pancreatic cancer [88] or with doxorubicin in soft tissue sarcomas [89]. Licensed by Threshold Pharmaceuticals to Merck Serono (Geneva, Switzerland), TH-302 is currently in clinical development in a broad series of indications (including AML/ALL and MM). A Phase I clinical trial of single agent TH-302 in patients with advanced leukemias (n = 34) is ongoing. Preliminary results were reported at the American Society of Clinical Oncology annual meeting 2012 [90]. TH-302 was administered intravenously over 30–60 min daily on days 1–5 of a 21-day cycle, at escalating doses of 120, 170, 240, 330, 460 or 550 mg/m2. MTD was determined to be 460 mg/m2, with dose-limiting toxicity of esophagitis at higher doses (TH-302 2010-0268: clinicaltrials.gov ID: NCT01149915 [102]). Clinical activity has been noted with a few objective responses, but the majority of cytoreductions were transient, arguing for future strategies in which TH-302 is combined with chemotherapeutics targeting oxic AML and ALL cells.
Many other prodrugs are currently being investigated for their potential to eradicate cells residing in hypoxic niches. Preclinical and clinical testing showed promising results, however their relevance in the context of hematological malignancies has not been evaluated. These include the chloroethylhydrazine prodrugs KS119 and its more soluble phosphate ester KS119W [91]; and the benzotriazine di-N-oxide, tirapazamine (TPZ) whose cytotoxicity derives from oxidative DNA damage by free radicals that arise from the initial one-electron reduction product, rather than via DNA alkylation. TPZ is the most intensively studied HAP and has been instructive in several respects [92]. Importantly, benzotriazine di-N-oxide agents have significant myelotoxicity in mice and humans [93] and TPZ is selectively toxic to presumptively hypoxic HSC in the BM in mice [27]. Therefore, expanded hypoxia in the BM and other involved tissues in advanced leukemia with high leukemia burden may be exploitable by these agents, with a cautionary note of expected toxicity to normal hematopoiesis.
Conclusion
Recently, hypoxia has been shown as a prominent feature of the BM microenvironment in different hematological malignancies. In this context and similarly to solid tumors, hypoxia can mediate chemoresistance through regulation of angiogenesis, metabolic switching, modulation of microenvironment and other mechanisms, which represent active areas of ongoing investigation. While targeting of transcription factors such as HIF-1/2 remains a challenge, exploiting tumor hypoxia with HAPs appears to be feasible and has already moved from preclinical experiments into early human clinical trials exhibiting promising antitumor activity.
Future perspective
Identifying the key downstream targets and pathways mediating hypoxia responses of hematopoietic tissue-derived tumors will facilitate development of molecularly targeted therapies addressing this major component of diseased microenvironments. In the long term, studies are underway to generate HAP that are triggered by hypoxia to release molecularly targeted inhibitors (rather than DNA-reactive cytotoxins) that potentially would provide better tolerated strategies for exploiting hypoxia. An example is HAPs that release irreversible pan-ErbB kinase inhibitors when reduced in hypoxic tissue [94]. Similar approaches may have potential for addressing key pathways in hypoxic leukemic cells to provide highly selective hypoxia-dependent targeted therapy in the future.
Practice Points.
-
■
Studying the bone marrow (BM) microenvironment has been challenging due to the difficulty in accessing it.
-
■
Two main components of the BM microenvironment are the endosteal and vascular niches. These compartments are formed by different cell types (osteoblasts and osteoclasts, and endotheliar and perivascular cells, respectively) and they have been shown to be equally important for the maintenance of hematopoietic stem cell homeostasis.
-
■
An oxygen gradient exists from the vascular to the endosteal niche. Most primitive hematopoietic stem cells appear to reside in most hypoxic areas where low oxygen keeps them in a quiescent state.
-
■
Expansion of hypoxic areas in the BM microenvironment is a prevalent feature observed across hematological malignancies.
-
■
Preclinical and clinical studies are being actively carried out to explore the potential of targeting cells residing in hypoxic areas with hypoxia-activated prodrugs.
Acknowledgments
The following grants funded this work: NIH/NCI CA153019-02, CA155056-03, CA100632-08; Leukemia and Lymphoma Society: Scholar in Clinical Research 2189-12.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■of interest
■■of considerable interest
- 1.Curry JL, Trentin JJ, Wolf N. Hemopoietic spleen colony studies. II. Erythropoiesis. J. Exp. Med. 1967;125(4):703–720. doi: 10.1084/jem.125.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1-2):7–25. [PubMed] [Google Scholar]
- 3.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 4.Schofield R. The stem cell system. Biomed. Pharmacother. 1983;37(8):375–380. [PubMed] [Google Scholar]
- 5.Lo Celso C, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457(7225):92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 7.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 10.Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 13.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14■.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. Importance of endothelial and osteoblastic niches is shown. Hematopoietic stem cells depend on CXCL12 produced by perivascular and endothelial cells, while lymphoid progenitors rely on that provided by osteoblasts.
- 15.Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. J. Cell Physiol. 2009;219(2):271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro BA. Temperature correction of blood gas values. Respir. Care Clin. N. Am. 1995;1(1):69–76. [PubMed] [Google Scholar]
- 17.Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg. Med. J. 2007;24(8):569–571. doi: 10.1136/emj.2007.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu YC, Chen CZ, Lee CH, Chen CW, Chang HY, Hsiue TR. Prediction of arterial blood gas values from venous blood gas values in patients with acute respiratory failure receiving mechanical ventilation. J. Formos. Med. Assoc. 2003;102(8):539–543. [PubMed] [Google Scholar]
- 19.Walkey AJ, Farber HW, O’Donnell C, Cabral H, Eagan JS, Philippides GJ. The accuracy of the central venous blood gas for acid-base monitoring. J. Intensive Care Med. 2010;25(2):104–110. doi: 10.1177/0885066609356164. [DOI] [PubMed] [Google Scholar]
- 20.Wolfle D, Jungermann K. Long-term effects of physiological oxygen concentrations on glycolysis and gluconeogenesis in hepatocyte cultures. Eur. J. Biochem. 1985;151(2):299–303. doi: 10.1111/j.1432-1033.1985.tb09100.x. [DOI] [PubMed] [Google Scholar]
- 21.Jungermann K, Kietzmann T. Role of oxygen in the zonation of carbohydrate metabolism and gene expression in liver. Kidney Int. 1997;51(2):402–412. doi: 10.1038/ki.1997.53. [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Khanna S, Wallace WA, et al. Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. J. Biol. Chem. 2003;278(47):47129–47135. doi: 10.1074/jbc.M308703200. [DOI] [PubMed] [Google Scholar]
- 23.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001;59(1):230–237. doi: 10.1046/j.1523-1755.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 24.Mik EG, van Leeuwen TG, Raat NJ, Ince C. Quantitative determination of localized tissue oxygen concentration in vivo by two-photon excitation phosphorescence lifetime measurements. J. Appl. Physiol. 2004;97(5):1962–1969. doi: 10.1152/japplphysiol.01399.2003. [DOI] [PubMed] [Google Scholar]
- 25.Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99(1):394–394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 26.Skouby AR. Haematologic adaptation in patients with chronic bronchitis and pulmonary insufficiency. Acta Med. Scand. 1976;199(3):185–190. doi: 10.1111/j.0954-6820.1976.tb06714.x. [DOI] [PubMed] [Google Scholar]
- 27■■.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA. 2007;104(13):5431–5436. doi: 10.1073/pnas.0701152104. Utilizing Hoechst 33342 (Ho) perfusion dye, Parmar et al.suggested the existence of an oxygen gradient from the vasculature to the endosteal niche, with the majority of hematopoietic stem cells having the lowest Ho intensity, that is, being hypoxic in nature.
- 28.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 2007;405(1):1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 29.Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–385. doi: 10.1182/blood-2009-07-233437. [DOI] [PubMed] [Google Scholar]
- 30.Miharada K, Karlsson G, Rehn M, et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. 2011;9(4):330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown JM, William WR. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 35.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5835. [PubMed] [Google Scholar]
- 36.Chen Y, Hu L. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 2009;29(1):29–64. doi: 10.1002/med.20137. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin. Cancer Biol. 2009;19(1):12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Chen JQ, Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim. Biophys. Acta. 2012;1826(2):370–384. doi: 10.1016/j.bbcan.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev. 2007;26(2):273–279. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- 40.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26(7):1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 41.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin. Cancer Res. 2010;16(24):5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Zhao T, Itasaka S, et al. Involvement of decreased hypoxia-inducible factor 1 activity and resultant G(1)-S cell cycle transition in radioresistance of perinecrotic tumor cells. Oncogene. 2012;32(16):2058–2068. doi: 10.1038/onc.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updat. 2011;14(3):191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Schults MA, Timmermans L, Godschalk RW, et al. Diminished carcinogen detoxification is a novel mechanism for hypoxia-inducible factor 1-mediated genetic instability. J. Biol. Chem. 2010;285(19):14558–14564. doi: 10.1074/jbc.M109.076323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 1955;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46■■.Jensen PO, Mortensen BT, Hodgkiss RJ, et al. Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Prolif. 2000;33(6):381–395. doi: 10.1046/j.1365-2184.2000.00183.x. First report associating hypoxia and leukemia using a rat leukemia model, via in vivo pulse-labeling with a theophylline-linked 2-nitroimidazole hypoxia probe.
- 47.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113(7):1504–1512. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48■■.Benito J, Shi Y, Szymanska B, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS ONE. 2011;6(8):e23108. doi: 10.1371/journal.pone.0023108. Expansion of hypoxia was found in the bone marrow microenvironment of several xenograft leukemia models. Antileukemia effect of hypoxia activated prodrugs is shown.
- 49.Wellmann S, Guschmann M, Griethe W, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18(5):926–933. doi: 10.1038/sj.leu.2403332. [DOI] [PubMed] [Google Scholar]
- 50.Frolova O, Samudio I, Benito J, et al. Regulation of HIF-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol. Ther. 2012;13(10):1–13. doi: 10.4161/cbt.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsunaga T, Imataki O, Torii E, et al. Elevated HIF-1α expression of acute myelogenous leukemia stem cells in the endosteal hypoxic zone may be a cause of minimal residual disease in bone marrow after chemotherapy. Leuk. Res. 2012;36(6):e122–e124. doi: 10.1016/j.leukres.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 52.Martin SK, Diamond P, Gronthos S, Peet DJ, Zannettino AC. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia. 2011;25(10):1533–1542. doi: 10.1038/leu.2011.122. [DOI] [PubMed] [Google Scholar]
- 53.Asosingh K, De Raeve H, De Ridder M, et al. Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica. 2005;90(6):810–817. [PubMed] [Google Scholar]
- 54.Hu J, Handisides DR, Van Valckenborgh E, et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood. 2010;116(9):1524–1527. doi: 10.1182/blood-2010-02-269126. [DOI] [PubMed] [Google Scholar]
- 55.Azab AK, Hu J, Quang P, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–5794. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56■.Storti P, Bolzoni M, Donofrio G, et al. Hypoxia-inducible factor (HIF)-1α suppression in myeloma cells blocks tumoral growth in vivo inhibiting angiogenesis and bone destruction. Leukemia. 2013 doi: 10.1038/leu.2013.24. doi:10.1038/leu.2013.24. Epub ahead of print. Importance of hypoxia in multiple myeloma. Silencing HIF-1α via shRNA results in tumor suppression due to downregulation of proangiogenic as well as pro-osteoclastogenic cytokines.
- 57.Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur. J. Med. Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 58.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumour pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 59.McDonald PC, Winum JY, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3(1):84–97. doi: 10.18632/oncotarget.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rouschop KM, Dubois LJ, Keulers TG, et al. PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc. Natl Acad. Sci. USA. 2013;110(12):4622–4627. doi: 10.1073/pnas.1210633110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atkins C, Liu Q, Minthorn E, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73(6):1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Hu L. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 2009;29(1):29–64. doi: 10.1002/med.20137. [DOI] [PubMed] [Google Scholar]
- 63.Denny WA. Hypoxia-activated prodrugs in cancer therapy: progress to the clinic. Future Oncol. 2010;6(3):419–428. doi: 10.2217/fon.10.1. [DOI] [PubMed] [Google Scholar]
- 64■■.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. Provides an extensive review of the alternative aproaches to target hypoxia including bioreductive agents and inhibitors of molecular targets. Discusses mechanisms of actions, challenges and up-to-date results of the clinical trials carried out to exploit hypoxia as a therapeutic target.
- 65.Belcourt MF, Hodnick WF, Rockwell S, Sartorelli AC. Differential toxicity of mitomycin C and porfiromycin to aerobic and hypoxic Chinese hamster ovary cells overexpressing human NADPH:cytochrome c (P-450) reductase. Proc. Natl Acad. Sci. USA. 1996;93(1):456–460. doi: 10.1073/pnas.93.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guise CP, Wang AT, Theil A, Bridewell DJ, Wilson WR, Patterson AV. Identification of human reductases that activate the dinitrobenzamide mustard prodrug PR-104A: A role for NADPH:cytochrome P450 oxidoreductase under hypoxia. Biochem. Pharmacol. 2007;74(6):810–820. doi: 10.1016/j.bcp.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Foehrenbacher A, Su J, et al. The 2-nitroimidazole EF5 is a biomarker for oxidoreductases that activate bioreductive prodrug CEN-209 under hypoxia. Clin. Cancer Res. 2012;18(6):1684–1695. doi: 10.1158/1078-0432.CCR-11-2296. [DOI] [PubMed] [Google Scholar]
- 68.Mason RP, Holtzman JL. The role of catalytic superoxide formation in the O2 inhibition of nitroreductase. Biochem. Biophys. Res. Commun. 1975;67(4):1267–1274. doi: 10.1016/0006-291x(75)90163-1. [DOI] [PubMed] [Google Scholar]
- 69.Plumb JA, Gerritsen M, Workman P. DT-diaphorase protects cells from the hypoxic cytotoxicity of indoloquinone EO9. Br. J. Cancer. 1994;70(6):1136–1143. doi: 10.1038/bjc.1994.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patterson LH. Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab. Rev. 2002;34(3):581–592. doi: 10.1081/dmr-120005659. [DOI] [PubMed] [Google Scholar]
- 71.Anderson RF, Denny WA, Ware DC, Wilson WR. Pulse radiolysis studies on the hypoxia-selective toxicity of a colbalt-mustard complex. Br. J. Cancer. 1996;(Suppl. 27):S48–S51. [PMC free article] [PubMed] [Google Scholar]
- 72.Patel K, Choy SF, Hicks KO, Melink TJ, Holford NHG, Wilson WR. A combined pharmacokinetic model for the hypoxia-targeted prodrug PR-104A in humans, dogs, rats and mice predicts species differences in clearance and toxicity. Cancer Chemother. Pharmacol. 2011;67:1145–1155. doi: 10.1007/s00280-010-1412-z. [DOI] [PubMed] [Google Scholar]
- 73.Singleton RS, Guise CP, Ferry DM, et al. DNA crosslinks in human tumor cells exposed to the prodrug PR-104A: relationships to hypoxia, bioreductive metabolism and cytotoxicity. Cancer Res. 2009;69(9):3884–3891. doi: 10.1158/0008-5472.CAN-08-4023. [DOI] [PubMed] [Google Scholar]
- 74.Guise CP, Abbattista M, Singleton RS, et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010;70(4):1573–1584. doi: 10.1158/0008-5472.CAN-09-3237. [DOI] [PubMed] [Google Scholar]
- 75.Jameson MB, Rischin D, Pegram M, et al. A Phase I trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldo-keto reductase 1C3, in patients with solid tumors. Cancer Chemother. Pharmacol. 2010;65(4):791–801. doi: 10.1007/s00280-009-1188-1. [DOI] [PubMed] [Google Scholar]
- 76.D’Andrea AD. Targeting DNA repair pathways in AML. Best Pract. Res. Clin. Haematol. 2010;23(4):469–473. doi: 10.1016/j.beha.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Gu Y, Patterson AV, Atwell GJ, et al. Roles of DNA repair and reductase activity in the cytotoxicity of the hypoxia-activated dinitrobenzamide mustard PR-104A. Mol. Cancer Ther. 2009;8(6):1714–1723. doi: 10.1158/1535-7163.MCT-08-1209. [DOI] [PubMed] [Google Scholar]
- 78.Birtwistle J, Hayden RE, Khanim FL, et al. The aldo-keto reductase AKR1C3 contributes to 7,12-dimethylbenz(a)anthracene-3,4-dihydrodiol mediated oxidative DNA damage in myeloid cells: implications for leukemogenesis. Mutat. Res. 2009;662(1-2):67–74. doi: 10.1016/j.mrfmmm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 79.Manesh DM, Szymanska B, Carol H, et al. Molecular determinants of T-cell acute lymphoblastic leukaemia sensitivity to the pre-prodrug PR-104; Presented at: 24th Lorne Cancer Conference 2012; Lorne, Victoria, Australia. 2012.Feb 9-11, [Google Scholar]
- 80■■.Konopleva M, Borthakur G, Thall PF, et al. Phase I/II study of PR104, a bioreductive prodrug, in patients with relapsed/refractory acute myeloid leukemia (AML) using patient-specific adaptive dose selection. Blood. 2011;118(21):661–661. Provides an update of the Phase I/II trial of PR-104, a hypoxia-activated prodrug, in the setting of acute leukemias.
- 81.Borch RF, Liu J, Joswig C, Baggs RB, Dexter DL, Mangold GL. Antitumor activity and toxicity of novel nitroheterocyclic phosphoramidates. J. Med. Chem. 2001;44(1):74–77. doi: 10.1021/jm000359y. [DOI] [PubMed] [Google Scholar]
- 82.Duan JX, Jiao H, Kaizerman J, et al. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J. Med. Chem. 2008;51(8):2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 83.Meng F, Evans JW, Bhupathi D, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol. Cancer Ther. 2012;11(3):740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- 84.Jung D, Lin L, Jiao H, Cai X, Duan JX, Matteucci M. Pharmacokinetics of TH-302: a hypoxically activated prodrug of bromo-isophosphoramide mustard in mice, rats, dogs and monkeys. Cancer Chemother. Pharmacol. 2012;69(3):643–654. doi: 10.1007/s00280-011-1741-6. [DOI] [PubMed] [Google Scholar]
- 85.Liu Q, Sun JD, Wang J, et al. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother. Pharmacol. 2012;69(6):1487–1498. doi: 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benito J, Lu H, Shi Y, et al. Hypoxia activated pro-drug TH-302 induces hypoxia-dependent anti-leukemia activity in vitro and in vivo. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research. Cancer Res. 2012;72(8 Suppl.) Abstract 4641. [Google Scholar]
- 87.Saunders P, Cisterne A, Weiss J, Bradstock KF, Bendall LJ. The mammalian target of rapamycin inhibitor RAD001 (everolimus) synergizes with chemotherapeutic agents, ionizing radiation and proteasome inhibitors in pre-B acute lymphocytic leukemia. Haematologica. 2011;96(1):69–77. doi: 10.3324/haematol.2010.026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borad MJ, Chiorean EG, Molina JR, et al. Clinical benefits of TH-302, a tumor selective hypoxia-activated prodrug, and gemcitabline in first-line pancreatic cancer (PanC) J. Clin. Oncol. 2011;29(Suppl. 4) Abstract. [Google Scholar]
- 89.Ganjoo KN, Cranmer LD, Butrynski JE, et al. A Phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology. 2011;80(1-2):50–56. doi: 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- 90.Konopleva M, Handisides D, Lorente GA, et al. A Phase 1 study of TH-302, a hypoxia-activated cytotoxic prodrug, in subjects with advanced leukemias. J. Clin. Oncol. 2012;30(15) Abstract 6585. [Google Scholar]
- 91.Seow HA, Penketh PG, Shyam K, Rockwell S, Sartorelli AC. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy] carbonyl]hydrazine: an anticancer agent targeting hypoxic cells. Proc. Natl Acad. Sci. USA. 2005;102(26):9282–9287. doi: 10.1073/pnas.0409013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reddy SB, Williamson SK. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin. Investig. Drugs. 2009;18(1):77–87. doi: 10.1517/13543780802567250. [DOI] [PubMed] [Google Scholar]
- 93.Rischin D, Peters L, Fisher R, et al. Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized Phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02) J. Clin. Oncol. 2005;23(1):79–87. doi: 10.1200/JCO.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 94.Patterson AV, Jagdish J, Syddall SP, et al. Cellular metabolism, murine pharmacokinetics and preclinical antitumor activity of SN29966, a novel hypoxia-activated irreversible pan-HER inhibitor. Mol. Cancer Ther. 2009;8(12 Suppl. 1):B79. Abstract. [Google Scholar]
- 95.Guise CP, Abbattista MR, Tipparaju SR, et al. Diflavin oxidoreductases activate the bioreductive prodrug PR-104A under hypoxia. Mol. Pharmacol. 2012;81(1):31–40. doi: 10.1124/mol.111.073759. [DOI] [PubMed] [Google Scholar]
Websites
- 101.PR104 in Treating Patients With Refractory/Relapsed Acute Leukemia. http://clinicaltrials.gov/show/NCT01037556.
- 102.Study of Hypoxia-Activated Prodrug TH-302 to Treat Advanced Leukemias. http://clinicaltrials.gov/show/NCT01149915.