Abstract
The maintenance of genome stability depends on the DNA damage response (DDR), which is a functional network comprising signal transduction, cell cycle regulation and DNA repair. The metabolism of DNA double-strand breaks governed by the DDR is important for preventing genomic alterations and sporadic cancers, and hereditary defects in this response cause debilitating human pathologies, including developmental defects and cancer. The MRE11 complex, composed of the meiotic recombination 11 (MRE11), RAD50 and Nijmegen breakage syndrome 1 (NBS1; also known as nibrin) proteins is central to the DDR, and recent insights into its structure and function have been gained from in vitro structural analysis and studies of animal models in which the DDR response is deficient.
Genomic instability, defined as a reduction in the fidelity with which genetic information is transmitted to daughter cells, is caused by the failure to recognize and repair parental DNA lesions before cell division. DNA double-strand breaks (DSBs) represent a particularly acute danger as they are the underlying cause of chromosomal rearrangements, and can trigger cytotoxic responses or cytostatic responses. DSB formation is intrinsic to normal cell growth, with DSBs arising spontaneously during DNA replication and as intermediates in programmed DNA rearrangements that occur during meiosis and immune system development. DSBs also result from exposure to DNA-damaging agents used in therapeutic settings, including ionizing radiation (IR) and topoisomerase poisons, such as etopo-side and camptothecin, used in cancer treatment. Therefore, the process of DSB metabolism is an integral part of organismal development and the aetiology of myriad disease states; it also determines the response to clastogenic cancer therapies that act by inducing DNA strand breaks.
The DNA damage response (DDR) is initiated upon recognition of the DNA lesion by sensor proteins, followed by rapid and, in many cases, reversible changes in cell behaviour. The DDR can also trigger specialized programmes, such as apoptosis and senescence, to remove or minimize the risk posed by cells with genetic instability. DDR activation is evident in preneoplastic lesions, leading to the hypothesis that it is an inducible barrier to tumorigenesis1,2. Consistent with this, hereditary cancer predisposition, as well as other severe pathologies, results from mutations in DDR genes3,4.
The MRE11 complex consists of meiotic recombination 11 (MRE11), RAD50 and Nijmegen breakage syndrome 1 (NBS1; also known as nibrin), a homologue of Xrs2 in Saccharomyces cerevisae, and is a sensor of DSBs that also controls the DDR by governing the activation of the central transducing kinase ataxia-telangiectasia mutated (ATM). In addition, the MRE11 complex regulates DSB repair, through the homology directed repair (HDR), non-homologous end-joining (NHEJ; also known as classical (C)-NHEJ) and alternative non-homologous end-joining (A-NHEJ) pathways (fig. 1; for reviews, see refs 5,6). On balance, its primary role in mitotic cells seems to be the promotion of HDR between sister chromatids to resolve damage that arises during DNA replication7 (box 1). During meiotic recombination, the HDR functions of the MRE11 complex promote DSB repair events between homologous chromosomes8,9. During both meiotic and mitotic repair, the MRE11 complex influences DSB repair structurally, by forming a bridge between the participating DNA molecules, and enzymatically, by promoting the resection of DSB ends10. The MRE11 complex is highly conserved, with readily identifiable orthologues of MRE11 and RAD50 evident in eubacterial, archaeal and eukaryal genomes. NBS1 appears to be confined to eukarya and, within that domain, is somewhat less conserved than MRE11 or RAD50.
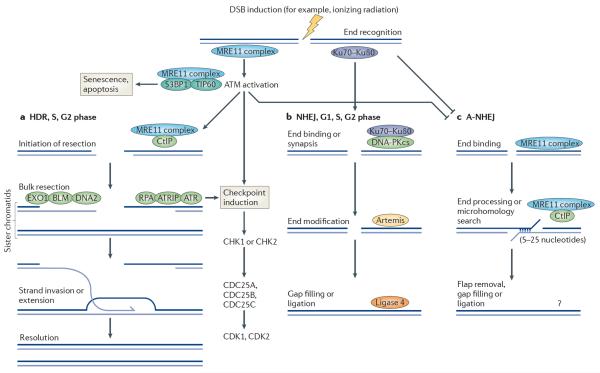
Figure 1. The MRE11 complex regulates the mammalian DNA damage response.
Double-stranded DNA (dsDNA) breaks are recognized by the MRE11 complex, which catalyses the activation of ataxia-telangiectasia mutated (ATM) in conjunction with other proteins such as the tat-interactive protein 60 kDa (TIP60; also known as KAT5) acetyltransferase and p53-binding protein 1 (53BP1)159,160. ATM activation promotes cell-cycle checkpoint induction, influences DNA repair, and can activate apoptosis and senescence in certain cellular contexts3. Depending on the cell-cycle phase and end-binding complexes or end modifications, breaks can be directed into two major repair pathways: homology-directed repair (HDR) or non-homologous end-joining (NHEJ; also known as classical (C)-NHEJ). a | HDR requires the 5′–3′ resection of dsDNA to generate single-stranded DNA (ssDNA)–dsDNA junctions. This is initiated by the MRE11 complex and CtBP-interacting protein (CtIP) and further bulk resection is carried out by exonuclease 1 (EXO1), BLM and DNA2 (refs 10,70,161–163). 3′ ssDNA tails generated by resection are bound by replication protein A (RPA), which activates ATR via ATR-interacting protein (ATRIP) binding to influence the checkpoint response164. RPA on these 3′ tails is exchanged for RAD51 to promote strand invasion, HDR repair and resolution of repair intermediates. b | Ends bound by the Ku70–Ku80 heterodimer can be repaired by NHEJ in conjunction with the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), Artemis nuclease and DNA ligase 4, with the help of additional factors involved in end-modifications, gap filling and ligation165. This NHEJ pathway is independent of the MRE11 complex. c | The MRE11 complex, in conjunction with CtIP, also regulates the poorly defined alternative NHEJ (A-NHEJ) pathway, which is characterized by large deletions and the frequent use of short microhomologies128–132,166–169. This pathway is resection-dependent and requires several enzymatic activities for resection, flap trimming, synthesis and ligation6. CDK, cell division protein kinase; DSB, double-strand break.
In this Review, we discuss recent advances that have defined the molecular and structural bases of the MRE11 complex's role in DSB metabolism, telomere homeostasis, meiosis, apoptosis and immune system development. These advances are founded upon approaches ranging from structural analysis to the derivation of new in vivo models in yeast and mice. Together, these studies have provided important insights into how this complex influences DDR signalling, DNA repair and tumour suppression.
Structural insights into the MRE11 complex
The MRE11 complex comprises a large central globular domain, in which MRE11 and NBS1 associate with the Walker A and B domains of RAD50, and the extended coiled-coil domain of RAD50, in which the amino-terminal and carboxy-terminal portions of the coils associate in an antiparallel manner (fig. 2a,b). At the apex of the RAD50 coils, where the N-terminal and C-terminal stretches fold back on themselves, is a domain called the RAD50 hook4,11,12. Recent insights have been gained into the structural features of the globular domain and the hook domain from crystallographic analyses. The physical properties of the coiled-coil regions in RAD50 have been interrogated with scanning force microscopy13–16, whereas relatively limited structural information on that domain is available at atomic resolution. As the bulk of structural data have been obtained from Pyrococcus furiosus, an archaeon that seems to lack an NBS1 orthologue, some aspects of current models are likely to be revised once structural data from the eukaryotic complexes are obtained. In the following sections, the salient points of these recent studies are summarized.
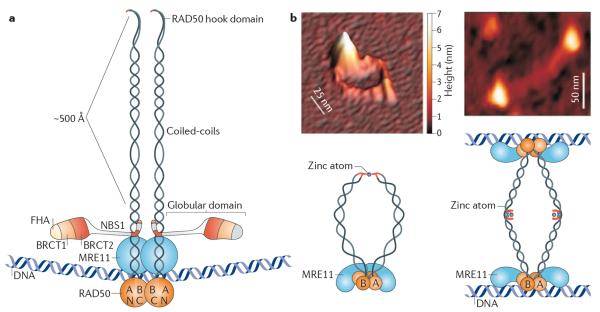
Figure 2. The MRE11 complex consists of a globular domain and extended coiled-coils.
a | The MRE11 complex consists of a large globular domain, in which meiotic recombination 11 (MRE11), and Nijmegen breakage syndrome 1 (NBS1; also known as nibrin) associate with RAD50 and DNA, and extended coiled-coil domains of RAD50 in which the amino-terminal and carboxy-terminal regions of the coils associate in an antiparallel manner. At the apex of the RAD50 coils, the N-terminal and C-terminal regions fold back on themselves to form the `RAD50 hook' domain (image is not to scale). The RAD50 hook domain mediates formation of MRE11 complex assemblies. b | A dimeric MRE11 complex without DNA is shown by scanning force microscopy and in a schematic (left panel; the white bar represents scale along the horizontal plane, whereas the colour gradient on the right represents scale on the vertical plane). The RAD50 hook domain coordinates binding to a zinc atom. Upon DNA binding, the coiled-coil domains adopt a rigid parallel structure that bridges two DNA strands with a distances of ~1,000 Å (right panel). A, Walker A; B, Walker B; BRCT, BRCA1 C-terminal; FHA, Forkhead-associated. Images in panel b are reproduced, with permission, from ref. 37 © (2005) Macmillan Publishers Ltd. All rights reserved.
DNA binding through the globular domain
The MRE11 complex binds DNA via its globular domain, and usually in the context of a higher-order assembly14,16–20. This activity primarily requires MRE11 and RAD50, although some data suggest that NBS1 (and Xrs2 in yeast) may bind DNA and also influence the DNA-binding properties of MRE11 and RAD5019,21,22. Although structural analyses of each component have provided important insights, an integrated picture of how they assemble into the globular domain and affect DNA binding and enzymatic functions has not yet been established.
The crystal structure of P. furiosus Mre11 bound to DNA reveals its binding to DSB ends with two to three base overhangs and branched DNA structures, and provides a basis for understanding the complex's role in NHEJ and DNA end processing17. Mre11 dimerization is critical for DNA binding and is mediated by conserved domains in its N terminus. DNA binding by Mre11 is mediated by six DNA recognition loops, in which 17 residues form sugar–phosphate contacts in the minor groove of DNA. The absence of any base interactions is consistent with the lack of sequence preference for Mre11 in DNA binding. In branched DNA substrates, the single-stranded DNA (ssDNA) is bound similarly by contacts to the phosphodiester backbone. The DNA-binding site also easily accommodates ssDNA, but the double-stranded DNA (dsDNA) and branched substrates appear to be preferred19. Rad50 also binds DNA23, but the relative contributions of Mre11 and Rad50 to DNA binding at the structural level remain to be established.
Mre11 specifies di-manganese-dependent ssDNA endonuclease and 3′–5′ dsDNA exonuclease activities24,25. Accordingly, the active site of the P. furiosus enzyme (and by extension, orthologous MRE11 species) is structured to accommodate both ssDNA and dsDNA. The exo nuclease function of Mre11 seems to be exerted via melting of the dsDNA terminus, followed by endonucleolytic-type cleavage of the 3′ strand-releasing mononucleotides17,24–26, suggesting that the extent of melting required for incision is limited.
Homodimerization through the Rad50 hook domain
X-ray crystallographic data from the P. furiosus Rad50 homologue suggests that the hook domain functions as a zinc-dependent homodimerization cassette that mediates formation of Mre11 complex assemblies. This domain is conserved in the known Rad50 orthologues, and is characterized by a central sequence motif of CXXC. Alignment of hook domains from 132 species (Pfam database ID: Pf04423) reveals marked preferences for the residues at these sites: most (95%) have either Pro (85%) or Tyr (10%) at the first X position and 80% have Leu or Val at the second X position, indicating that the residues between the invariant Cys are constrained. The two Cys residues from one Rad50 protomer coordinate a zinc atom with the two Cys from a second protomer, resembling the intramolecular coordination of zinc in zinc finger domains27. Zinc-dependent interaction within the hook domains of the two proto mers orients their respective coils away from each other at an approximately 140 ° angle, so that the globular domains of each protomer lie at the distal ends of the assembly28 (fig. 2b).
A non-enzymatic, `structural' function of the MRE11 complex in HDR was initially inferred from genetic analyses indicating that the complex promoted recombination-mediated DNA repair between sister chromatids7,29,30. The configuration of MRE11 complexes joined at the hook resonates well with those genetic data, as do the DNA bridging functions inferred from scanning force microscopy of the MRE11 complex associated with DNA15 (fig. 2b). The replacement of the hook domain with an artificial FKBP domain that can induce homodimerization provided direct evidence that the RAD50 hook domain functions as an interaction interface in vivo, and further supported the idea that the complex serves to bridge molecules during HDR31. These experiments also revealed that the hook domain is required for MRE11 complex-dependent telomere maintenance in vegetatively growing cells and for the induction of DSBs by the DNA topoisomerase 2 (TOP2)-like enzyme, SPO11, during meiotic recombination31,32. These functions are not readily attributable to DNA bridging and so this outcome remains somewhat perplexing. Is it simply the case that loss of the hook interaction causes global disruption of the complex? Resolution of this issue will require separation-of-function mutations that distinguish telomeric and meiotic functions from other roles of the MRE11 complex in the DDR.
The coiled-coil domain
The coiled-coil domain of RAD50 remains somewhat enigmatic, as does its functional significance. First, why is it so large? The extended coiled-coil architecture is common to the known RAD50 orthologues, as well as to the analogous structural maintenance of chromosomes (SMC) protein family that regulates sister chromatid cohesion and chromosome condensation (for recent reviews, see refs 33–36). If fully extended, the coiled-coil domains of eukaryotic RAD50 orthologues could span as much as 500 Å; in the hook-mediated dimeric state, this would be nearly 1,000 Å16,28 (fig. 2b), a distance that roughly equals three sister chromatids side-by-side. The domains have highly flexible regions embedded within them13,16 and so the distances spanned may be significantly shorter. Nevertheless, it is surprising that the MRE11 complex would require such long-range actions to affect its diverse functions, and the basis for this apparent requirement is unclear.
Second, it is remarkable that even isosteric mutants of the Cys residues in the CXXC motif have a global effect on MRE11 complex stability, disrupting the association of RAD50 with MRE11 (ref. 11). This observation indicates that the hook domain influences activities at the globular domain, and that the coiled-coil domains, which connect the two, communicate structural perturbations between them. These influences may occur in both directions: upon DNA binding by the human MRE11 complex, the RAD50 coiled-coils seem to become less flexible and long-range interactions with distal RAD50 protomers are favoured37 (fig. 2b; left versus right panels).
Regulation of the complex by NBS1
NBS1 is important for regulation of the MRE11 complex, influencing DNA binding as well as MRE11 nuclease activity. The N-terminal region of NBS1 contains two phosphopeptide-binding modules commonly found in DDR proteins: a Forkhead-associated domain (FHA domain) and a tandem BRCA1 C-terminal domain (BRCT domain)4,38,39. Structural analyses of these N-terminal domains in the Schizosaccharomyces pombe Nbs1 protein have revealed a novel modular architecture that allows diverse phosphorylation-dependent protein interactions40,41. FHA and tandem BRCT domains generally function as `stand-alone' phosphopeptide-binding domains42–44. Thus, Nbs1 is atypical, in that both FHA and tandem BRCT domains are present and could in principle allow three modes of binding to partners: FHA only, BRCT only, or FHA plus BRCT. However, structural analysis has revealed that the FHA domain is fused directly to the tandem BRCT domain40,41, creating a structural inter-dependence between them that makes it less likely that interactions would occur through the BRCT domain alone. Moreover, engagement of the Nbs1 FHA domain by a phosphorylated partner leads to a dramatic structural transition of the BRCT domains. Whether this potentiates BRCT domain interactions has not been established, but the possibility is appealing. The dynamic behaviour of the S. pombe Nbs1 N-terminal domain was revealed by co-crystallization with its binding partner, Ctp1, the orthologue of Sae2 in S. cerevisae and CtBP-interacting protein (CtIP) in mammals40,41,45,46. Ctp1 contains casein kinase 2 phosphorylation sites (SXT clusters) that mediate binding to the Nbs1 FHA domain. Engagement of the FHA domain is associated with a 20 ° rotation at the BRCT1–BRCT2 interface, causing a 10 Å movement of the C-terminal portion of BRCT2 (ref. 40). Based on the role of the Mre11 complex and Ctp1 in DSB end resection, this structural transition is likely to influence HDR. Genetic evidence suggests that Ctp1 deficiency does not impair activation of the S phase checkpoint in S. pombe, supporting the idea that its binding to the Nbs1 FHA domain primarily influences DNA repair47.
In mammals, mediator of DNA damage checkpoint 1 (MDC1) also binds NBS1 via its FHA domain48. As with Ctp1, MDC1 contains SXT phosphorylation site clusters that are required for this interaction and influence MRE11 complex retention at sites of DNA damage48–51. Biochemical evidence suggests that MDC1 SXT clusters engage the FHA and BRCT domains simultaneously41,48–50; thus, this interaction appears to represent an FHA plus BRCT binding mode. It is an appealing possibility that the phospho-binding-induced dynamic structural transitions in NBS1 form the basis of its regulatory influence on the MRE11 complex. Also, this structural information lays a solid foundation for testing this idea at the molecular level.
The MRE11 complex in DNA metabolism
The MRE11 complex has multiple roles in the metabolism of DSBs that involve both its enzymatic and structural functions. The 3′–5′ exonuclease and ssDNA endonuclease activities of MRE11 do not depend on RAD50 and NBS1 but are enhanced when MRE11 is in the holocomplex52. Although a comprehensive view of the physiological significance of the MRE11 nuclease activity remains to be established, the MRE11 complex and its orthologues are clearly important for both the clearance of covalently attached proteins from DNA termini and promotion of DSB end resection en route to the production of 3′ ssDNA tails required in HDR and checkpoint activation.
Biochemical analysis of the MRE11 and RAD50 orthologues SbcC and SbcD of Escherichia coli, as well as the influence of the MRE11 complex on adenovirus replication intermediates, has implicated the MRE11 complex in the removal of covalently attached proteins to promote repair53,54. Some of the most-detailed evidence for the MRE11 complex's role in this process has come from studies of meiosis in yeast. During meiotic recombination, the requisite first step of DSB induction is catalysed by Spo11 (refs 32,55,56), which cleaves dsDNA and remains covalently bound to the 5′ strands of the ensuing breaks. The Mre11 complex subsequently mediates endonucleolytic removal of two differently sized Spo11-DNA oligo species, suggesting that Mre11 cleaves Spo11-bound termini asymmetrically57. Spo11 removal is impaired in Mre11 nuclease mutants and in cells lacking Sae2, which exhibit nuclease activity in vitro58; however, nuclease activity may not be sufficient for Spo11 cleavage. Certain alleles of Mre11 and Rad50 — termed `S alleles' for separation of meiotic and mitotic function — block Spo11 cleavage but are unlikely to be defective for Mre11 nuclease activity57–59. Studies in S. pombe are also consistent with this; however, in S. pombe, only a single species of DNA oligo was recovered, suggesting that cleavage is symmetric rather than asymmetric60–62.
Based on its role in SPO11 removal and the sensitivity of nuclease-deficient MRE11 alleles to topoisomerase poisons, the nuclease activity of MRE11 has been proposed to affect removal of covalent TOP1–DNA and TOP2–DNA intermediates63. Null alleles, as well as Mre11 nuclease and Rad50S mutants, are sensitive to both Top1 and Top2 inhibitors, but Mre11 nuclease and Rad50S mutants show only mild sensitivity to methyl methanesulphonate (MMS) and IR when compared with null mutants63. Similarly, mouse cells expressing a Rad50S allele exhibited sensitivity to both TOP1 and TOP2 poisons but not other DSB-inducing agents such as IR64.
The 5′–3′ resection of DSB ends underlies the initiation of checkpoint responses and is required for the initiation of HDR10 (fig. 1). The rate of DSB resection was found to be reduced in Mre11- and Rad50-deficient strains, suggesting the possibility that the Mre11 complex is somehow involved in end resection29,65,66. Paradoxically, Mre11 specifies 3′–5′ exonuclease activity — the opposite polarity to that required for resection — and nuclease-deficient alleles of mre11 exhibited milder resection defects than mre11Δ (refs 63,67). However, analysis of DSB resection in vivo in S. cerevisiae reveals that the Mre11 complex and Sae2 catalyse the initiation of resection through the removal of a short tract of ssDNA. This initial resection is followed by the bulk resection of DNA by either the 5′–3′ exonuclease 1 (Exo1) or DNA2 in conjunction with the helicase Sgs1 (refs 68,69). Bulk resection was required for efficient induction of G2 arrest, as well as repair by single-strand annealing (SSA) or HDR repair of lesions and cell survival10.
The available evidence in mammalian cells suggests that the MRE11 complex, in conjunction with CtIP, the mammalian orthologue of Sae2 and Ctp1, mediates analogous functions in DSB resection70. An intriguing possibility is that the initial incision step by MRE11 or CtIP, which produces a 3′ ssDNA overhang of 50–100 bases, discourages engagement of the DSB end by the Ku heterodimer, and thereby inhibits repair of the DSB by NHEJ. An analogous function in blocking NHEJ has been ascribed to components of the Fanconi anaemia (FA) pathway71,72, perhaps consistent with the observation that FA proteins have been shown to physically and functionally interact with the MRE11 complex73–76. Separation of function alleles of S. cerevisiae Sae2 reveal that Spo11 removal and camptothecin resistance can be separated genetically from the opening of hairpins at DSB ends77. These data suggest that Sae2 and Mre11 are not completely redundant in DSB end processing. The ability of the MRE11 complex to promote early steps of nucleolytic resection is clearly important for the efficient induction of meiotic and mitotic recombination but whether resection is catalysed by the enzymatic activities of MRE11, Sae2 or CtIP, or an as yet unidentified factor, remains an important question to resolve.
The molecular requirements for reconstitution of the S. cerevisiae DSB resection process in vitro are generally consistent with the in vivo studies. At the core of the resection machinery is the 3′–5′ helicase Sgs1 that unwinds the DSB end, and the ssDNA thus formed is digested by the nuclease activity of Dna2. The activity of Dna2 is directed to the 5′ strand, and away from the 3′ strand by the ssDNA-binding protein replication protein A (RPA); the net result is resection of the 5′ strand of the DSB end to produce the 3′ ssDNA tail. Sgs1 activity is enhanced by the Mre11 complex and the Rmi1–Top3 complex, although the enzymatic functions of these complexes are not required for resection78,79. These findings in yeast echo those obtained with P. furiosus proteins80. Resection in vitro requires P. furiosus Mre11 and Rad50, as well as HerA, a bidirectional helicase, and NurA, a 5′–3′ exonuclease, all four of which are encoded by the same operon in the P. furiosus genome. The role of RPA in the resection process seen in S. cerevisiae has not been examined in P. furiosus.
Neither the yeast nor the P. furiosus in vitro systems exhibit the two-step mechanism observed in vivo. This would suggest that the first step is dispensable for resection. Presumably, factors present in vivo underlie the requirement for the initial Mre11 complex-dependent step. As noted above, the possibility that this first incision step may regulate the binding of Ku and other factors to the DSB end must be considered.
The MRE11 complex in telomere homeostasis
The ends of linear chromosomes consist of telomeres and are bound by an array of proteins that prevent them from being recognized as DSBs. This protein assembly, called shelterin in mammals, together with the unique DNA sequences at chromosome ends, defines the telomere81,82. Genetic analyses in S. cerevisiae have clearly established that the Mre11 complex regulates telomere length, most likely via its effect on telomerase recruitment81. The MRE11 complex also localizes to mammalian telomeres independently of shelterin or ATM83,84, and recent data offer some clues as to its functions there. first, in contrast to the Mre11 complex in budding yeast, the mammalian MRE11 complex does not appear to exert a strong effect on telomere length homeostasis85. Second, as is the case with interstitial DSB damage, the MRE11 complex is required for activating the ATM-dependent response at dysfunctional telomeres (fig. 3). This induces the rapid assembly of DDR components into telomere-dysfunction-induced foci (TIFs) which can be visualized by immunofluorescence86. Small hairpin RNA-mediated depletion or conditional deletion of key components of the shelterin complex results in its removal from telomeres and TIF formation86. This induction of TIF formation is impaired in most Nbs1- or Mre11-defective cells85,87,88. ATM activation by the MRE11 complex and TIF formation are independent of MRE11 nuclease activity89. However, because the fusion of dysfunctional telomeres completely depends on ATM90, it is unclear whether this phenotype indicates a direct role of the MRE11 complex in the end-joining process, or simply reflects its effects on ATM activity.
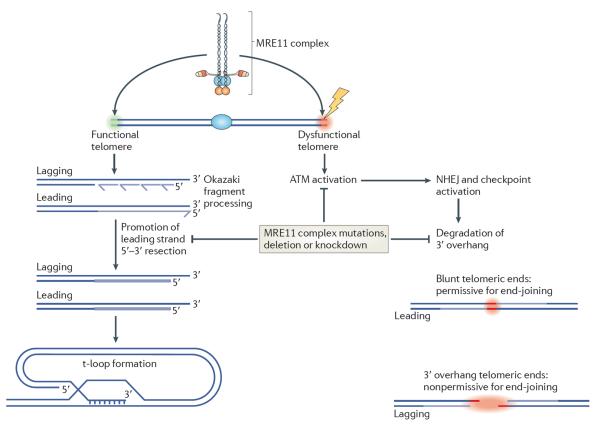
Figure 3. The MRE11 complex controls telomere homeostasis.
At a functional telomere (green area, left), the MRE11 complex recognizes the newly synthesized telomeric ends and promotes their resection to create the 3′ overhang, which is a prerequisite for the formation of the t-loop — a DNA structure resembling the d-loop formed by strand invasion during homology directed repair (HDR). The t-loop is critical for normal telomere protection and maintenance82. The MRE11 complex also recognizes dysfunctional telomeres (red area, right), leading to activation of ataxia-telangiectasia mutated (ATM) and `repair' (that is, fusion) of the telomere through non-homologous end-joining (NHEJ; also known as classical (C)-NHEJ); this ultimately precludes chromosome segregation and causes cell death. The MRE11 complex may also influence the degradation of the 3′ overhang before, or during, the fusion process. MRE11 complex hypomorphism impairs ATM activation, which sharply reduces the frequency of NHEJ-mediated telomere fusion; this also leads to impaired telomeric end processing on both leading and lagging strands, such that residual fusions are restricted to telomeres that have been replicated by the leading strands and are blunt.
Finally, the MRE11 complex seems to promote resection of telomeric DNA to create the single-stranded 3′ overhang that is typically found at the telomere (fig. 3). Although telomere fusions are markedly reduced upon acute telomere dysfunction in MRE11 complex mutants, they are not completely abolished. Also, virtually all of the rare telomere fusions observed in MRE11 complex mutants occur between telomeric ends replicated by the leading strand polymerase88. In contrast to the lagging strand telomere, the leading strand is a blunt end that forms immediately following replication. The lagging strand telomere is not blunt because lagging strand DNA synthesis cannot fully replicate DNA ends; removal of the RNA primer used to initiate Okazaki fragment synthesis leaves behind a stretch of ssDNA. The 3′ overhang inhibits NHEJ91, and if MRE11 complex hypomorphism were to impair resection of the blunt end it would make it a better end-joining substrate, thus accounting for the observed bias toward leading-end fusions; however, this interpretation awaits experimental validation.
The MRE11 complex in human disease
The identification of mutations affecting the MRE11 complex in human genomic instability syndromes provided the first hints of an intimate relationship between the MRE11 complex and ATM-mediated checkpoint signalling. Inherited mutations in MRE11, NBS1 and RAD50 cause ataxia-telangiectasia-like disease (ATLD), NBS and NBS-like disorder (NBSLD), respectively (fig. 4a). The clinical and cellular features of these syndromes underscore the importance of the MRE11 complex in the DDR, and the corresponding animal models have provided tractable systems for genetic analysis4. NBS and ATLD cells exhibit phenotypic similarity to those from patients with ataxia-telangiectasia (A-T), including hypersensitivity to DSB-inducing clastogens, defects in DNA damage-dependent cell-cycle checkpoint arrest, and chromosomal fragility. However, the clinical presentations of patients with NBS and patients with ATLD are distinct. Patients with NBS present with microcephaly and `bird-like' facial features, which are more similar to Seckel syndrome, and are highly predis-posed to cancer. Recently, it was reported that a patient with NBSLD resulting from heteroallelic mutations in RAD50 also exhibited microcephaly92. Morphological abnormalities are not characteristic of patients with A-T or ATLD who exhibit neurodegeneration and ataxia. Although cancer occurs with high frequency in patients with A-T, it has been reported in only two patients with ATLD so far93. However, given the limited number of patients with ATLD that have been identified, it is difficult to exclude the possibility that cancer predisposition is a primary feature of this disease. The implication of all three members of the MRE11 complex in distinct but clinically overlapping syndromes solidifies the concept that MRE11, RAD50 and NBS1 function as a unit, and argues against the possibility that any of the members mediate autonomous functions outside the complex.
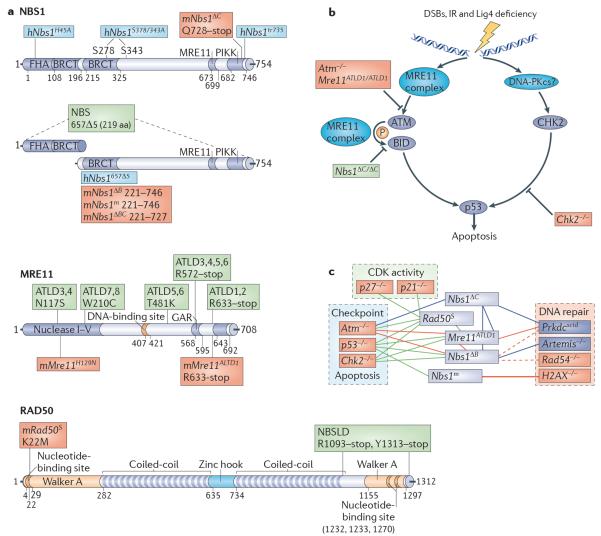
Figure 4. The MRE11 complex in human disease and mouse models.
a | Domain structure of the MRE11 complex. The Nijmegen breakage syndrome 1 (NBS1; also known as nibrin), meiotic recombination 11 (MRE11) and RAD50 components of the human MRE11 complex are illustrated. Domains are indicated by name with the corresponding amino acid numbers shown. Human disease mutations are indicated in green. Mouse alleles are indicated in red and `humanized' mouse alleles in blue. This figure is drawn to scale. b | The MRE11 complex has multiple roles in activating apoptosis after double-strand break (DSB) exposure. The complex activates ataxia-telangiectasia mutated (ATM) and facilitates the phosphorylation of select ATM substrates, including CHK2 and BH3-interacting domain death agonist (BID), to promote p53-dependent apoptosis through the carboxyl terminus of NBS1. CHK2 signals in parallel, and is possibly activated by the DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Mouse alleles affecting various steps in the signalling pathway are indicated. Alleles in red impair apoptosis in both the haematopoietic and nervous system and alleles in green affect the haematopoietic but not the nervous system. c | Genetic interactions between mouse MRE11 complex alleles. MRE11 complex alleles used for genetic analyses are shown in green, components of the non-homologous end-joining (NHEJ; also known as classical (C)-NHEJ) machinery are shown in blue and other DNA damage or cell-cycle regulators are shown in red. Connecting lines indicate that genetic crosses have been analysed. Blue lines indicate that no synthetic interactions were identified, green lines indicate that synthetic interactions were identified, and red lines indicate synthetic lethality. Dashed lines indicate incomplete penetrance of synthetic lethality. Interacting alleles are classified by their major functions of the DNA damage response, although they may affect other aspects of the response. ATLD, ataxia-telangiectasia-like disease; BRCT, BRCA1 C-terminal; FHA, Forkhead-associated; h, humanized; m, mouse; NBSLD, NBS-like disorder; PIKK, PI3K-related protein kinase.
Insights from MRE11 mouse models
Null mouse mutants of Mre11, Rad50 and Nbs1 are not viable89,94,95. This essential nature of MRE11 complex components has necessitated the derivation of conditional hypomorphic alleles and hypermorphic alleles, the design of which has been guided by the corresponding human mutations or in vitro studies (fig. 4a). The phenotypic analyses of mouse models for the human syndromes as well as a hypermorphic allele of Rad50 have been reviewed elsewhere4,96 (table 1). We focus here on recent mouse models that are based on molecular information obtained through structural and biochemical analyses, and on clinical features of the human syndromes that suggest physiological systems in which MRE11 complex functions are particularly significant.
Table 1.
Alleles of the MRE11 complex in mice
| Allele* | Allele type | Phenotypes | Refs |
|---|---|---|---|
| hNbs1657Δ5 | Transgene, N-terminal truncation | S/G2 checkpoint defects, damage sensitivity, chromosomal instability, reduced ATM activity, impaired T cell development, subfertility | 99 |
| hNbs1H45A | Transgene, point mutation | S/G2 checkpoint defects, reduced ATM activity | 104 |
| hNbs1S278/343A | Transgene, point mutations | None described | 104 |
| hNbs1tr735 | Transgene, C-terminal truncation | Apoptosis defect, reduced ATM activity | 104 |
| Mre11Δ (Mre11atm2.1Dof) | Conditional deletion | Embryonic lethality, reduced class switch recombination and DSB repair in B cells (CD19–Cre promoters) | 89,125 |
| Mre11ATLD1 (Mre11atm1Jpt) | C-terminal truncation | S/G2 checkpoint defects, damage sensitivity, chromosomal instability, reduced ATM activation and activity, defective apoptosis, reduced fertility | 126 |
| Mre11H129N (Mre11atm1Dof) | Conditional allele with point mutation | Embryonic lethality, reduced class switch recombination in B cells (CD19–Cre), DNA repair defects and damage sensitivity | 89,125 |
| Nbs1Δ6 (Nbntm1Md) and Nbs1− (Nbntm1Zqw) | Targeted deletion | Early embryonic lethality | 95,170 |
| Nbs1ΔB (Nbntm1Jpt) | N-terminal truncation | S/G2 checkpoint defects, damage sensitivity, chromosomal instability, reduced ATM activity, subfertility | 98 |
| Nbs1ΔBC (Nbntm3Jpt) | N- and C-terminal truncations | S/G2 checkpoint defects, damage sensitivity, chromosomal instability, reduced ATM activity, subfertility, apoptosis defect | 110 |
| Nbs1ΔC (Nbntm2.1Jpt) | C-terminal truncation | Apoptosis defect, S phase checkpoint defect, reduced ATM activity | 105 |
| Nbs1F6 (Nbntm2Zqw), Nbs1Δ6 (Nbntm1.1Md) and Nbs1Δ (Nbntm2Nus) | Conditional deletions | Reduced class switch recombination in B cells (CD19–Cre promoter), microcephaly, neuronal apoptosis, cerebellar defects and ataxia (nestin–Cre promoter), lymphopenia, T cell development defects (Lck–Cre promoter) | 124, 171–173 |
| Nbs1m (Nbntm1Xu) | N-terminal truncation | S/G2 checkpoint defects, damage sensitivity, chromosomal instability, reduced ATM activity, subfertility, cancer | 97 |
| Rad50Δ (Rad50tm1Jpt) | Targeted deletion | Early embryonic lethality | 94 |
| Rad50ind and Rad50− (Rad50tm3Jpt) | Conditional deletion | Chromosomal instability and death in dividing cells (MX1–Cre, PCP2–Cre promoters) | 115 |
| Rad50S (Rad50tm2Jpt) | Knock-in point mutation | Embryonic lethality, bone marrow failure, cancer predisposition, activated DDR, sensitivity to topoisomerase poisons | 64,139,140 |
ATM, ataxia-telangiectasia mutated; C, carboxy; DDR, DNA damage response; DSB, double-strand break; hNbs1, humanized Nbs1; Mre11, meiotic recombination 11; MX1, myxovirus resistance 1; N, amino; Nbn, nibrin; Nbs1, Nijmegen breakage syndrome 1; PCP2, Purkinje cell protein 2.
Allele names are listed from the original publications and are followed by the Mouse Genome Informatics designations, which are hyperlinked to the website.
NBS1 mouse models
Mouse mutants harbouring Nbs1 alleles that affect protein interaction domains of NBS1 have been established. The phenotypes observed are generally consistent with predictions made by the molecular data, but in some of the cases, the data indicate that existing models of NBS1 function might need to be revised.
`Humanized' transgenic mice have been used to carry out structure-function analysis in vivo: in this approach, human bacterial artificial chromosomes (BACs) containing mutant alleles of the Nbs1 locus are used as transgenes to complement Nbs1Δ/Δ mice. For example, mice created with the human Nbs1H45A mutation, which alters a conserved residue in the NBS1 FHA domain, partially phenocopied Nbs1ΔB, Nbs1657Δ5 and Nbs1m mice, which model the common allele found in NBS patients, and encode an N-terminally truncated NBS1 protein lacking the FHA domain altogether97–99. It seems likely that a reduction in the recruitment of CtIP, which interacts with the FHA domain40,41, accounts for many of the cell-cycle checkpoint and repair defects observed in these mutants, as mice lacking MDC1, which also interacts with this domain, showed only subtle defects in checkpoint responses100. However, the fact that Ctp1 deficiency in S. pombe does not affect S phase checkpoint activation may suggest that Nbs1H45A impairs additional protein interactions relevant to checkpoint function47.
The C terminus of NBS1 contains a 24 amino acid conserved motif that interacts with ATM101,102 (fig. 4a). Complementation of cells from patients with NBS with a cDNA lacking this domain failed to rescue phosphorylation of some ATM substrates and intra-S and G2/M checkpoint defects, although ATM activation was normal101. Whereas NBS1 was dispensable for ATM activation in vitro using purified human MRE11 and RAD50 (ref. 103), the nbs1 C-terminal domain was required for DNA-dependent ATM activation in Xenopus laevis extracts102. Moreover, in mice that lack the NBS1 C-terminal domain, produced using either the BAC transgenic approach (to create the Nbs1tr735 allele) or conventional targeted mutation (the Nbs1ΔC allele)104,105, ATM activation was unaffected, MRE11 complex protein levels and subcellular localization were unchanged, and no impairment of MRE11 complex association with DNA damage was evident. Accordingly, checkpoint functions were also largely unaffected save for a mild defect in the intra-S phase checkpoint105.
Strikingly, thymocytes from Nbs1ΔC and Nbs1tr735 mice were defective in ATM-dependent IR-induced apoptosis. This defect correlated with defects in the ability of ATM to phosphorylate SMC1 and BH3-interacting domain death agonist (BID), which are effectors of the intra-S phase checkpoint and ATM-dependent apoptosis, respectively106–108. The discrepancies between findings in the mouse versus in vitro systems and overexpression in human cells may in part reflect species-specific differences. It is also likely that the presence of partially redundant activities109 and the preservation of stoichiometry and intracellular localization provided by the in vivo setting may provide a more nuanced assessment of function. We favour the view that the NBS1 C terminus is dispensable for ATM activation but is required to permit ATM access to certain substrates (fig. 4b). The limited phenotypic outcomes observed in Nbs1ΔC and Nbs1tr735 are consistent with this. In this regard, it is notable that the C terminus of NBS1 is sufficient to promote apoptosis in Nbs1ΔB/ΔB mice, despite a marked reduction in levels of the NBS1ΔB protein110.
NBS1 is phosphorylated by ATM in response to damage, and this has been proposed to be a prerequisite for checkpoint activation by the MRE11 complex111,112. However, mice expressing a humanized allele lacking both of the prominent ATM phosphorylation sites at Ser278 and Ser343 showed normal checkpoint responses, suggesting that these sites are not essential for checkpoint activation in mice104. As a result, the role of ATM phosphorylation in regulating MRE11 complex function remains unclear. Additional phosphorylation sites exist and it is possible that compound mutations inactivating them en masse are required to see an effect.
RAD50 mouse models
The MRE11 complex is required for the completion of DNA replication, presumably reflecting the importance of HDR during this process113,114. Data obtained from a conditional knockout of Rad50 (Rad50Δlox) lend strong support for this idea. As is the case with Mre11 and Nbs1 genes, inactivation of the Rad50 gene in cultured cells or proliferative tissue leads to precipitous cell death associated with dramatic genome instability. Telomere dysfunction is not seen, indicating that RAD50 function at telomeres is not acutely required for viability8,115. By contrast, deletion of Rad50 in postmitotic Purkinje cells of the Rad50Δlox mouse had no effect, even for 1-year-old mice. Similarly, Rad50 deletion in quiescent liver cells was completely innocuous. However, partial resection of the liver to induce division of RAD50-deficient hepatocytes was associated with widespread DNA damage, indicating that RAD50 deficiency had a profound effect even in this single round of replication. Collectively, these and other data indicate that the essential function of the MRE11 complex is in the HDR-dependent resolution of DNA replication-associated DSBs115 (box 1). By extension, these data suggest that HDR itself is dispensable in non-dividing cells.
MRE11 mouse models
The nuclease domain of MRE11 is among the most highly conserved components of DDR factors, with easily recognizable orthologues from bacterio phage T4 to humans4. However, the consequences of nuclease deficiency differ widely according to phylo-genetic context, which may be due to redundant activities in some settings or species-specific substrates for MRE11 in others. for example, in S. cerevisiae, nuclease-dead mre11 mutants have a relatively mild phenotype in vegetatively growing cells. Conversely, nuclease-dead S. pombe rad32 mutants (Rad32 is the S. pombe orthologue of Mre11) nearly phenocopy the clastogen sensitivity of rad32Δ mutants, although nuclease deficiency blocks the initiation of meiotic recombination in both yeasts17,60–62,116. In mammalian systems, the MRE11 nuclease activity has been implicated in the activation of ATM through several approaches and phenotypic outcomes, including effects on the stability of replication forks and the initiation of checkpoints and repair processes54,117–120.
A nuclease-dead allele of Mre11, Mre11H129N, leads to embryonic lethality when homozygous in mice, indicating that the nuclease activity is essential during development89. Mre11H129N cells rapidly senesced and showed a similar spectrum of spontaneous and damage-induced chromosomal aberrations as seen in cells deficient for RAD50, NBS1 or MRE11, suggesting a severe defect in the repair of spontaneous DNA lesions8. These defects in DNA repair correlated with defects in the accumulation of RPA and RAD51 foci, suggesting that the resection of DSB ends is impaired. Notably, the presumptive effect on DSB resection was not associated with defects in cell-cycle checkpoint activation; ATM activation and downstream effects on targets such as CHK2 were normal, as was G2/M arrest following radiation treatment. Similarly, the Rad50S mouse, which may also exhibit impaired MRE11 nuclease function, did not display checkpoint deficiencies121. Together, these data suggest that the role of MRE11 nuclease activity in DSB resection is not strictly required for the activation of ATM or many aspects of the DDR.
Roles in immune system development
A primary feature of human genetic instability diseases is variable immune deficiency. In patients with A-T and patients with NBS, this includes aberrant immunoglobulin isotype profiles in serum, reduced numbers of mature T cells and increased sinopulmonary infections. Although the underlying cause of many of these defects remains unclear, mouse models have provided an excellent system for analysing the roles of the MRE11 complex in DNA repair and the consequences of an impaired DDR for immunological development.
There are mixed reports for whether the MRE11 complex affects class switch recombination (CSR). In ATM-deficient mice, there is a substantial defect in T cell development and CSR122,123. furthermore, conditional NBS1 or MRE11 deletion in lymphocytes, which leads to cell death within several cell passages, results in defects in CSR, raising the possibility that the MRE11 complex has a role in CSR124,125. The nuclease-deficient Mre11H129N allele also resulted in deficient CSR, and it has been suggested that defects in resection may underlie this phenotype125. This is at odds with the observation that mice expressing the Nbs1ΔB and Mre11ATLD1 alleles, with substantial defects in MRE11 complex formation and function, showed normal thymocyte development as well as normal CSR98,126. However, trans-rearrangements caused by aberrant V(D)J recombination and increased levels of unrepaired DNA were detected in these and similar animal models, suggesting a more subtle defect in the fidelity of end-joining126,127. future identification of alleles that affect CSR without severely affecting cell viability or other functions of the complex may shed light on these contrasting results.
The MRE11 complex also functions in A-NHEJ6,125,128–132 (fig. 1). In mice lacking the DNA-dependent protein kinase catalytic subunit (DNA-PKcs, encoded by the Prkdc gene) or the Artemis (ART) nuclease, hairpin-capped coding joint ends generated by the RAG recombinases, which initiate V(D)J recombination, are unresolved, leading to immunodeficiency133. Prkdcscid mice, as well as DNA-PKcs knockouts, are synthetically lethal with Atm−/− or Mre11ATLD1 (refs 134–136). These genetic interactions are not simply due to deficiencies in the ability of DNA-PKcs to regulate the ART nuclease, as Art knockouts do not show a synthetic interaction with ATM or MRE11 complex alleles136,137. Prkdcscid/scid Nbs1ΔBΔB mice are nearly inviable, but a limited number of double-mutant mice and cells have allowed the role of the MRE11 complex in cells lacking a primary component of NHEJ to be investigated. using a hyperactive RAG protein to initiate DNA breaks, it was demonstrated that an A-NHEJ pathway could be activated in cells lacking DNA-PKcs138. This system, together with analysis of the endo genous TCRtm loci in Nbs1ΔB/ΔBArt−/− double-mutant mice, revealed that the MRE11 complex is required for A-NHEJ-mediated joining of V(D)J substrates128. This A-NHEJ activity of the MRE11 complex was independent of the nuclease activities of MRE11 (ref. 128). Consistent with this, only mild defects in NHEJ were observed in B cells from mice expressing the nuclease-defective allele of MRE11 despite substantially impaired CSR125. Additional MRE11 complex alleles that separate the diverse functions of the complex will be essential for elucidating its precise roles in V(D)J recombination and CSR in vivo.
Checkpoints, apoptosis and malignancy
Mouse mutants for the MRE11 complex have been invaluable for yielding novel insights into how MRE11 signalling is integrated with the DDR during apoptosis and tumour suppression (fig. 4; table 1; see Supplementary information S1 (table)).
Intercrosses of Rad50S/S mice have provided insight into how the MRE11 complex affects ATM activation and DNA damage signalling. Rad50S/S mice exhibit precipitous loss of haematopoietic stem cells and die of anaemia by 4 months of age139. The severity of this outcome was reduced by creating Rad50S/Δ mice, indicating that the Rad50S allele is hypermorphic140; and deleting ATM in Rad50S/S mice completely rescued the Rad50S/S pheno-type. Surprisingly, lymphomas, radiation sensitivity and chromosome instability normally associated with ATM deficiency were reduced in Rad50S/SAtm−/− mice64,140. This effect of the Rad50S allele was attributed to hyperactivation of ATR pathways and thereby partially compensates for ATM deficiency (for a review of Rad50S function in mice and yeast, see ref. 121).
Crosses of mutants of the MRE11 complex with mouse models of ATLD and NBS (Mre11ATLD1/ATLD1 and Nbs1ΔB/ΔB, respectively) have revealed that ATM and CHK2 function in parallel pathways to activate apoptosis141 (fig. 4b). The apoptotic defects of ATM mutants have been attributed in part to impaired phosphorylation of CHK2 and p53. Mre11ATLD1/ATLD1 and Atm−/− mice exhibit impaired DNA damage-induced CHK2 hyper-phosphorylation and intermediate defects in thymocyte apoptosis, similar to cells lacking CHK2. Intercrosses of Atm−/− or Mre11ATLD1/ATLD1 mice with CHK2-deficient mice resulted in a complete apoptotic defect, approximating that seen in p53-deficient mice105,141. This indicates that CHK2-dependent apoptosis operates in the absence of ATM activity and that ATM phosphorylation of CHK2 is not essential for its induction of apoptosis. Supporting this, Nbs1ΔC mice that show normal ATM-dependent phosphorylation of CHK2 (ref. 105) also synergize with CHK2 deficiency and exhibit profound apoptotic defects (T.H.S. and J.H.J.P., unpublished observations). As thymocytes predominantly reside in G0/G1 phase, we favour the possibility that DNA-PKcs, which can phosphorylate CHK2 in vitro and affect apoptosis independently of ATM, is an activator of CHK2 apoptotic activity142,143.
Apoptosis occurs in the developing brain in response to radiation or defects in DNA repair144. Mice lacking DNA ligase 4 (Lig4−/−), which catalyses the final ligation step during NHEJ (fig. 1), exhibit apoptosis in postmitotic neurons and die in late embryogenesis145,146. Using a conditional knockout of Lig4 under the control of the nestin promoter that is active in the brain, a similar disparity was observed between the apoptotic responses of Nbs1ΔB and Mre11ATLD1. Radiation or Lig4 deletion in the brains of Nbs1ΔB/ΔB mice led to apoptosis similar to that observed in wild-type cells, whereas either Atm−/− or Mre11ATLD1/ATLD1 mice exhibited reduced apoptosis110. We proposed that these differences in apoptotic signalling could account for the striking difference in neuronal pathology of NBS compared with ATLD or A-T. It is thought that in a background competent for ATM-dependent apoptosis, microcephaly would predominate, whereas, when apoptosis is impaired, neurodegeneration would arise. However, this idea is questioned by the recent identification of heteroallelic RAD50 alleles in a patient with NBSLD who presented with microcephaly, but also showed defects in p53 signalling, suggestive of defects in apoptosis92. Resolving this issue will require the generation of an animal model of NBSLD for in-depth analysis of apoptosis.
Loss of CHK2 in either Nbs1ΔB/ΔB or Mre11ATLD1/ATLD1 mice leads to increased predisposition to a wide variety of tumour types after a long latency period, in contrast to the rapid lymphomas observed in ATM. This is observed with MRE11 complex alleles that affect the CHK1-dependent S and G2/M transitions or HDR, suggesting that the functions of the complex in monitoring replication-associated DNA damage may affect tumour suppression141. Supporting this possibility, increased tumour predisposition is observed in CHK2-null mice that lack functional breast cancer associated 1 (BRCA1) or are heterozygous for CHK1 (refs 147, 148). A more detailed understanding of how the MRE11 complex suppresses replicative damage should provide further insight into its functions in tumour suppression.
Conclusions and perspectives
As our understanding of how the MRE11 complex functions in the DDR has increased, issues such as the precise role of MRE11 nuclease activity, the mechanism by which the eukaryotic MRE11 complex binds DNA, the importance of the RAD50 coiled-coil domains and the mechanisms underlying the effects of the complex on HDR and NHEJ have grown richer and more complicated. The ongoing merger of information from genetic analyses with biochemical and structural analysis of the eukaryotic MRE11 complex will undoubtedly continue to illuminate these issues. As this occurs, it is likely that species-specific and cell-type-specific differences in how the complex influences the DDR will be revealed. Even with these gaps, the importance of the MRE11 complex in driving the successful execution of S phase has been firmly established, as has its role in preserving genomic integrity by activating DNA repair and signal transduction in the DDR. Through these functions, the MRE11 complex sustains the viability of proliferating cells, and suppresses the oncogenic potential of DNA replication-associated DNA damage.
Supplementary Material
Box 1 | The MRE11 complex promotes HDR and replication fork stability.
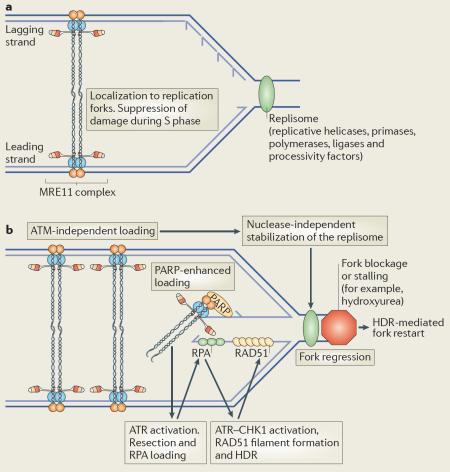
Yeast lacking components of the Mre11 complex are highly sensitive to DNA damage in S/G2 phase, and show profound defects in homology-directed repair (HDR)7. Cells from mouse models expressing hypomorphic alleles of MRE11 complex members exhibit checkpoint defects in S and G2 phases of the cell cycle and display increased chromatid breaks and fragments, consistent with damage occurring in regions of replicating DNA98,126. In vivo, the MRE11 complex is required for the viability of cycling but not post-mitotic cells115, and colocalizes with proliferating cell nuclear antigen (PCNA) at replication foci during S phase, when levels of the MRE11 complex on chromatin are most abundant149,150. Depletion of the MRE11 complex during DNA replication results in enhanced DNA breakage151 (see the figure, panel a). The MRE11 complex is also recruited to stalled replication forks following hydroxyurea treatment150,152, a process that is enhanced by poly(ADP-ribose) polymerase (PARP) activity153 but does not require ataxia-telangiectasia mutated (ATM)150 (see the figure, panel b). DNA double-strand breaks (DSBs) can arise at stalled forks owing to single-strand breaks or as a result of replication fork regression. The MRE11 complex and ATM promote resection at DSBs, allowing replication protein A (RPA) binding and activation of the ATR and CHK1 kinases that potentiate checkpoint responses, promote RAD51 filament formation and HDR, and allow replication to resume (see the figure, panel b)154–157. Independently of MRE11 nuclease activity, the MRE11 complex stabilizes components of the replisome152, a complex that includes the replicative helicase, polymerases and processivity factors, and promotes fork restart through HDR pathways in conjunction with ATM or ATR158 and RAD51 (ref. 120) (see the figure, panel b). Together, these data are consistent with the MRE11 complex having a primary role in the maintenance of the replication fork during DNA replication. The precise roles of the MRE11 complex's structural and enzymatic activities in these processes remain to be clarified.
Acknowledgements
J.H.J.P. is supported by grants from the US National Institutes of Health, the Geoffrey Beene Foundation and the Goodwin Foundation. T.H.S. is a Ramon y Cajal investigator and is supported by grants from the Ministerio de Ciencia e Innovación, Spain. We are grateful to M. Hohl for providing data and help with figures, members of our laboratories for insightful discussions, and F. Moreno-Herrero, C. Wyman and R. Kanaar for scanning force microscopy images. We apologize to our many colleagues whose important work could not be described owing to space limitations and the focus of the Review.
Glossary
- Cytotoxic response
A cellular response to stimuli leading to cell death.
- Cytostatic response
A cellular response to stimuli leading to a suppression of cell growth.
- Topoisomerase poison
A class of drugs used in cancer therapy that trap covalent intermediates of topoisomerases, causing DNA damage that is exacerbated by DNA replication.
- Clastogenic cancer therapy
A type of cancer therapy that relies on a clastogen, or DNA break-inducing agent, to target proliferating cells in tumours.
- Ataxia
A neurological condition characterized by loss of motor control. This often results from defects in the development or degeneration of the cerebellum.
- Homology directed repair
A major double-strand break repair pathway that is template-mediated and therefore considered to be highly accurate. Particularly important for sister chromatid regulation in S/G2 phase.
- Non-homologous end-joining
A major double-strand break repair pathway that involves the ligation of free ends, sometimes after processing that leads to the loss or gain of sequence. This pathway is particularly well studied in the context of V(D)J recombination.
- Alternative non-homologous end-joining
A poorly characterized end-joining pathway (or pathways) that is not dependent on the core non-homologous end-joining components. This pathway frequently uses short microhomologies (5–25 nucleotides) and is thought to be resection dependent.
- Sister chromatids
Identical chromatids that are joined by a centromere and generated during S phase DNA replication.
- Resection
The process of converting double-stranded DNA to single-stranded DNA by the exonucleolytic removal of one strand. Resection is often performed in conjunction with the action of a helicase and is implicated in both the checkpoint activation and multiple repair pathways.
- Scanning force microscopy
A type of microscopy that uses a physical probe to scan the surfaces of a specimen and provide high-resolution images at a nanoscale level. Also known as atomic force microscopy.
- Endonuclease
An enzyme that cleaves the phosphodiester bond of DNA within a polynucleotide chain.
- Exonuclease
An enzyme that cleaves the phosphodiester bond of DNA from the end of a polynucleotide chain.
- Zinc finger domain
A protein structural motif that coordinates zinc ions via Cys and His residues to stabilize folds involved in nucleic acid or protein binding.
- FKBP domain
A domain originally found in the FK506-binding protein (FKBP) that mediates its interactions with the immunosuppressant FK506. Binding of FK506 or analogues leads to dimerization and is used as an inducible artificial dimerization domain in fusion proteins.
- Isosteric mutant
An amino acid substitution that approximates the spatial and chemical properties of the residue that it replaces.
- BRCA1 C-terminal domain
A phosphopeptide-binding domain first identified in the carboxyl terminus of the breast cancer associated 1 (BRCA1) protein. These domains are usually found in tandem.
- Methyl methanesulphonate
A carcinogenic alkylating agent that generates strand breaks and is used in cancer therapy.
- Microcephaly
A neurodevelopmental disorder characterized by reduced head circumference and often accompanied by neurological problems including mental retardation and delayed development of motor functions.
- Hypomorphic allele
An allele that results in a partial loss of function.
- Hypermorphic allele
An allele that results in a partial gain of function or increased activity.
- Class switch recombination
The process by which B cells change the production of antibody from one class (or isotype) to another. This involves the exchange of constant and variable regions and involves the induction and repair of DNA double-strand breaks. Also known as isotype switching.
- V(D)J recombination
A process that assembles diverse immunoglobulin and T-cell receptor genes from existing variable (V), diversity (D) and joining (J) gene segments. V(D)J recombination is initiated by the RAG1–RAG2 recombinase in a sequence-specific manner. Also known as antigen receptor gene rearrangement.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 2.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 3.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stracker TH, Theunissen JW, Morales M, Petrini JH. The Mre11 complex and the metabolism of chromosome breaks: the importance of communicating and holding things together. DNA Repair (Amst.) 2004;3:845–854. doi: 10.1016/j.dnarep.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Lamarche BJ, Orazio NI, Weitzman MD. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 2010;584:3682–3695. doi: 10.1016/j.febslet.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McVey M, Lee SE. MMEJ repair of double-strand breaks (director's cut): deleted sequences and alternative endings. Trends Genet. 2008;24:529–538. doi: 10.1016/j.tig.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bressan DA, Baxter BK, Petrini JH. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adelman CA, Petrini JH. Division of labor: DNA repair and the cell cycle specific functions of the Mre11 complex. Cell Cycle. 2009;8:1510–1514. doi: 10.4161/cc.8.10.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherry SM, et al. The Mre11 complex influences DNA repair, synapsis, and crossing over in murine meiosis. Curr. Biol. 2007;17:373–378. doi: 10.1016/j.cub.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mimitou EP, Symington LS. DNA end resection: many nucleases make light work. DNA Repair (Amst.) 2009;8:983–995. doi: 10.1016/j.dnarep.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopfner KP, et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature. 2002;418:562–566. doi: 10.1038/nature00922. [DOI] [PubMed] [Google Scholar]
- 12.Hopfner KP, Tainer JA. Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr. Opin. Struct. Biol. 2003;13:249–255. doi: 10.1016/s0959-440x(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 13.van Noort J, et al. The coiled-coil of the human Rad50 DNA repair protein contains specific segments of increased flexibility. Proc. Natl Acad. Sci. USA. 2003;100:7581–7586. doi: 10.1073/pnas.1330706100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jager M, Wyman C, van Gent DC, Kanaar R. DNA end-binding specificity of human Rad50/Mre11 is influenced by ATP. Nucleic Acids Res. 2002;30:4425–4431. doi: 10.1093/nar/gkf574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jager M, et al. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell. 2001;8:1129–1135. doi: 10.1016/s1097-2765(01)00381-1. [DOI] [PubMed] [Google Scholar]
- 16.de Jager M, et al. Differential arrangements of conserved building blocks among homologs of the Rad50/Mre11 DNA repair protein complex. J. Mol. Biol. 2004;339:937–949. doi: 10.1016/j.jmb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Williams RS, et al. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell. 2008;135:97–109. doi: 10.1016/j.cell.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson DE, Trujillo KM, Sung P, Erickson HP. Structure of the Rad50·Mre11 DNA repair complex from Saccharomyces cerevisiae by electron microscopy. J. Biol. Chem. 2001;276:37027–37033. doi: 10.1074/jbc.M106179200. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, et al. Regulation of Mre11/Rad50 by Nbs1: effects on nucleotide-dependent DNA binding and association with Ataxia-telangiectasia-like disorder mutant complexes. J. Biol. Chem. 2003;278:45171–45181. doi: 10.1074/jbc.M308705200. [DOI] [PubMed] [Google Scholar]
- 20.Hopfner KP, et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 21.Trujillo KM, et al. Yeast Xrs2 binds DNA and helps target Rad50 and Mre11 to DNA ends. J. Biol. Chem. 2003;278:48957–48964. doi: 10.1074/jbc.M309877200. [DOI] [PubMed] [Google Scholar]
- 22.Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond WE, Kleckner N. RAD50 protein of S. cerevisiae exhibits ATP-dependent DNA binding. Nucleic Acids Res. 1993;21:3851–3856. doi: 10.1093/nar/21.16.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trujillo KM, Yuan SS, Lee EY, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J. Biol. Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- 25.Paull TT, Gellert M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol. Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- 26.Trujillo KM, Sung P. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50·Mre11 complex. J. Biol. Chem. 2001;276:35458–35464. doi: 10.1074/jbc.M105482200. [DOI] [PubMed] [Google Scholar]
- 27.Evans RM, Hollenberg SM. Zinc fingers: gilt by association. Cell. 1988;52:1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- 28.Hopfner KP, Putnam CD, Tainer JA. DNA double-strand break repair from head to tail. Curr. Opin. Struct. Biol. 2002;12:115–122. doi: 10.1016/s0959-440x(02)00297-x. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov EL, Korolev VG, Fabre F. XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics. 1992;132:651–664. doi: 10.1093/genetics/132.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartsuiker E, Vaessen E, Carr AM, Kohli J. Fission yeast Rad50 stimulates sister chromatid recombination and links cohesion with repair. EMBO J. 2001;20:6660–6671. doi: 10.1093/emboj/20.23.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiltzius JJ, Hohl M, Fleming JC, Petrini JH. The Rad50 hook domain is a critical determinant of Mre11 complex functions. Nature Struct. Mol. Biol. 2005;12:403–407. doi: 10.1038/nsmb928. [DOI] [PubMed] [Google Scholar]
- 32.Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 33.Ercan S, Lieb JD. C. elegans dosage compensation: a window into mechanisms of domain-scale gene regulation. Chromosome Res. 2009;17:215–227. doi: 10.1007/s10577-008-9011-0. [DOI] [PubMed] [Google Scholar]
- 34.Graumann PL, Knust T. Dynamics of the bacterial SMC complex and SMC-like proteins involved in DNA repair. Chromosome Res. 2009;17:265–275. doi: 10.1007/s10577-008-9014-x. [DOI] [PubMed] [Google Scholar]
- 35.Hudson DF, Marshall KM, Earnshaw WC. Condensin: architect of mitotic chromosomes. Chromosome Res. 2009;17:131–144. doi: 10.1007/s10577-008-9009-7. [DOI] [PubMed] [Google Scholar]
- 36.Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE. Sister chromatid cohesion: a simple concept with a complex reality. Annu. Rev. Cell Dev. Biol. 2008;24:105–129. doi: 10.1146/annurev.cellbio.24.110707.175350. [DOI] [PubMed] [Google Scholar]
- 37.Moreno-Herrero F, et al. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature. 2005;437:440–443. doi: 10.1038/nature03927. [DOI] [PubMed] [Google Scholar]
- 38.Becker E, Meyer V, Madaoui H, Guerois R. Detection of a tandem BRCT in Nbs1 and Xrs2 with functional implications in the DNA damage response. Bioinformatics. 2006;22:1289–1292. doi: 10.1093/bioinformatics/btl075. [DOI] [PubMed] [Google Scholar]
- 39.Xu C, et al. Structure of a second BRCT domain identified in the nijmegen breakage syndrome protein Nbs1 and its function in an MDC1-dependent localization of Nbs1 to DNA damage sites. J. Mol. Biol. 2008;381:361–372. doi: 10.1016/j.jmb.2008.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams RS, et al. Nbs1 flexibly tethers Ctp1 and Mre11-Rad50 to coordinate DNA double-strand break processing and repair. Cell. 2009;139:87–99. doi: 10.1016/j.cell.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloyd J, et al. A supramodular FHA/BRCT-repeat architecture mediates Nbs1 adaptor function in response to DNA damage. Cell. 2009;139:100–111. doi: 10.1016/j.cell.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu X, Chini CC, He M, Mer G, Chen J. The BRCT domain is a phospho-protein binding domain. Science. 2003;302:639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- 43.Manke IA, Lowery DM, Nguyen A, Yaffe MB. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- 44.Durocher D, et al. The molecular basis of FHA domain:phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol. Cell. 2000;6:1169–1182. doi: 10.1016/s1097-2765(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 45.Limbo O, et al. Ctp1 is a cell-cycle-regulated protein that functions with Mre11 complex to control double-strand break repair by homologous recombination. Mol. Cell. 2007;28:134–146. doi: 10.1016/j.molcel.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akamatsu Y, et al. Molecular characterization of the role of the Schizosaccharomyces pombe nip1+/ctp1+ gene in DNA double-strand break repair in association with the Mre11-Rad50-Nbs1 complex. Mol. Cell Biol. 2008;28:3639–3651. doi: 10.1128/MCB.01828-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter-Goff ME, Rhind N. The role of MRN in the S-phase DNA damage checkpoint is independent of its Ctp1-dependent roles in double-strand break repair and checkpoint signaling. Mol. Biol. Cell. 2009;20:2096–2107. doi: 10.1091/mbc.E08-09-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapman JR, Jackson SP. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9:795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melander F, et al. Phosphorylation of SDT repeats in the MDC1 N terminus triggers retention of NBS1 at the DNA damage-modified chromatin. J. Cell Biol. 2008;181:213–226. doi: 10.1083/jcb.200708210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spycher C, et al. Constitutive phosphorylation of MDC1 physically links the MRE11–RAD50–NBS1 complex to damaged chromatin. J. Cell Biol. 2008;181:227–240. doi: 10.1083/jcb.200709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu L, Luo K, Lou Z, Chen J. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc. Natl Acad. Sci. USA. 2008;105:11200–11205. doi: 10.1073/pnas.0802885105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams RS, Williams JS, Tainer JA. Mre11–Rad50–Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem. Cell Biol. 2007;85:509–520. doi: 10.1139/O07-069. [DOI] [PubMed] [Google Scholar]
- 53.Connelly JC, de Leau ES, Leach DR. Nucleolytic processing of a protein-bound DNA end by the E. coli SbcCD (MR) complex. DNA Repair (Amst.) 2003;2:795–807. doi: 10.1016/s1568-7864(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 54.Stracker TH, Carson CT, Weitzman MD. Adenovirus oncoproteins inactivate the Mre11–Rad50–NBS1 DNA repair complex. Nature. 2002;418:348–352. doi: 10.1038/nature00863. [DOI] [PubMed] [Google Scholar]
- 55.Keeney S, Kleckner N. Covalent protein-DNA complexes at the 5′ strand termini of meiosis-specific double-strand breaks in yeast. Proc. Natl Acad. Sci. USA. 1995;92:11274–11278. doi: 10.1073/pnas.92.24.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keeney S. Mechanism and control of meiotic recombination initiation. Curr. Top. Dev. Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- 57.Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lengsfeld BM, Rattray AJ, Bhaskara V, Ghirlando R, Paull TT. Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol. Cell. 2007;28:638–651. doi: 10.1016/j.molcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nairz K, Klein F. mre11S—a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes Dev. 1997;11:2272–2290. doi: 10.1101/gad.11.17.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartsuiker E, et al. Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, but Rec12Spo11 removal is dispensable for other MRN-dependent meiotic functions. Mol. Cell Biol. 2009;29:1671–1681. doi: 10.1128/MCB.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothenberg M, Kohli J, Ludin K. Ctp1 and the MRN-complex are required for endonucleolytic Rec12 removal with release of a single class of oligonucleotides in fission yeast. PLoS Genet. 2009;5:e1000722. doi: 10.1371/journal.pgen.1000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milman N, Higuchi E, Smith GR. Meiotic DNA double-strand break repair requires two nucleases, MRN and Ctp1, to produce a single size class of Rec12 (Spo11)-oligonucleotide complexes. Mol. Cell Biol. 2009;29:5998–6005. doi: 10.1128/MCB.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llorente B, Symington LS. The Mre11 nuclease is not required for 5′ to 3′ resection at multiple HO-induced double-strand breaks. Mol. Cell Biol. 2004;24:9682–9694. doi: 10.1128/MCB.24.21.9682-9694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morales M, et al. DNA damage signaling in hematopoietic cells: a role for Mre11 complex repair of topoisomerase lesions. Cancer Res. 2008;68:2186–2193. doi: 10.1158/0008-5472.CAN-07-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsubouchi H, Ogawa H. A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis. Mol. Cell Biol. 1998;18:260–268. doi: 10.1128/mcb.18.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SE, et al. Saccharomyces Ku70, Mre11/Rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell. 1998;94:399–409. doi: 10.1016/s0092-8674(00)81482-8. [DOI] [PubMed] [Google Scholar]
- 67.Lee SE, Bressan DA, Petrini JH, Haber JE. Complementation between N-terminal Saccharomyces cerevisiae mre11 alleles in DNA repair and telomere length maintenance. DNA Repair (Amst.) 2002;1:27–40. doi: 10.1016/s1568-7864(01)00003-9. [DOI] [PubMed] [Google Scholar]
- 68.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sartori AA, et al. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adamo A, et al. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol. Cell. 2010;39:25–35. doi: 10.1016/j.molcel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 72.Pace P, et al. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science. 2010;329:219–223. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- 73.Roques C, et al. MRE11–RAD50–NBS1 is a critical regulator of FANCD2 stability and function during DNA double-strand break repair. EMBO J. 2009;28:2400–2413. doi: 10.1038/emboj.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107:4223–4233. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 75.Yang YG, et al. The Fanconi anemia group A protein modulates homologous repair of DNA double-strand breaks in mammalian cells. Carcinogenesis. 2005;26:1731–1740. doi: 10.1093/carcin/bgi134. [DOI] [PubMed] [Google Scholar]
- 76.Donahue SL, Campbell C. A Rad50-dependent pathway of DNA repair is deficient in Fanconi anemia fibroblasts. Nucleic Acids Res. 2004;32:3248–3257. doi: 10.1093/nar/gkh649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim HS, et al. Functional interactions between Sae2 and the Mre11 complex. Genetics. 2008;178:711–723. doi: 10.1534/genetics.107.081331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Niu H, et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature. 2010;467:108–111. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cejka P, et al. DNA end resection by Dna2–Sgs1–RPA and its stimulation by Top3–Rmi1 and Mre11–Rad50–Xrs2. Nature. 2010;467:112–116. doi: 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hopkins BB, Paull TT. The P. furiosus mre11/rad50 complex promotes 5′ strand resection at a DNA double-strand break. Cell. 2008;135:250–260. doi: 10.1016/j.cell.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sabourin M, Zakian VA. ATM-like kinases and regulation of telomerase: lessons from yeast and mammals. Trends Cell Biol. 2008;18:337–346. doi: 10.1016/j.tcb.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 83.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 84.Zhu XD, Kuster B, Mann M, Petrini JH, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nature Genet. 2000;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
- 85.Attwooll CL, Akpinar M, Petrini JH. The Mre11 complex and the response to dysfunctional telomeres. Mol. Cell Biol. 2009;29:5540–5551. doi: 10.1128/MCB.00479-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 87.Deng Y, Guo X, Ferguson DO, Chang S. Multiple roles for MRE11 at uncapped telomeres. Nature. 2009;460:914–918. doi: 10.1038/nature08196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dimitrova N, de Lange T. Cell cycle-dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of nonhomologous end joining (NHEJ) in G1 and resection-mediated inhibition of NHEJ in G2. Mol. Cell Biol. 2009;29:5552–5563. doi: 10.1128/MCB.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buis J, et al. Mre11 nuclease activity has essential roles in DNA repair and genomic stability distinct from ATM activation. Cell. 2008;135:85–96. doi: 10.1016/j.cell.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 91.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nature Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 92.Waltes R, et al. Human RAD50 deficiency in a Nijmegen breakage syndrome-like disorder. Am. J. Hum. Genet. 2009;84:605–616. doi: 10.1016/j.ajhg.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uchisaka N, et al. Two brothers with Ataxia-telangiectasia-like disorder with lung adenocarcinoma. J. Pediatr. 2009;155:435–438. doi: 10.1016/j.jpeds.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 94.Luo G, et al. Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development, and sensitivity to ionizing radiation. Proc. Natl Acad. Sci. USA. 1999;96:7376–7381. doi: 10.1073/pnas.96.13.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu J, Petersen S, Tessarollo L, Nussenzweig A. Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice. Curr. Biol. 2001;11:105–109. doi: 10.1016/s0960-9822(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 96.Adelman CA, Petrini JH, Attwooll CL. Modeling disease in the mouse: lessons from DNA damage response and cell cycle control genes. J. Cell. Biochem. 2006;97:459–473. doi: 10.1002/jcb.20701. [DOI] [PubMed] [Google Scholar]
- 97.Kang J, Bronson RT, Xu Y. Targeted disruption of NBS1 reveals its roles in mouse development and DNA repair. EMBO J. 2002;21:1447–1455. doi: 10.1093/emboj/21.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams BR, et al. A murine model of Nijmegen breakage syndrome. Curr. Biol. 2002;12:648–653. doi: 10.1016/s0960-9822(02)00763-7. [DOI] [PubMed] [Google Scholar]
- 99.Difilippantonio S, et al. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nature Cell Biol. 2005;7:675–685. doi: 10.1038/ncb1270. [DOI] [PubMed] [Google Scholar]
- 100.Lou Z, et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 101.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 102.You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol. Cell Biol. 2005;25:5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 104.Difilippantonio S, et al. Distinct domains in Nbs1 regulate irradiation-induced checkpoints and apoptosis. J. Exp. Med. 2007;204:1003–1011. doi: 10.1084/jem.20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stracker TH, Morales M, Couto SS, Hussein H, Petrini JH. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature. 2007;447:218–221. doi: 10.1038/nature05740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kitagawa R, Bakkenist CJ, McKinnon PJ, Kastan MB. Phosphorylation of SMC1 is a critical downstream event in the ATM–NBS1–BRCA1 pathway. Genes Dev. 2004;18:1423–1438. doi: 10.1101/gad.1200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zinkel SS, et al. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 108.Kamer I, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 109.Lee JH, Goodarzi AA, Jeggo PA, Paull TT. 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 2010;29:574–585. doi: 10.1038/emboj.2009.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shull ER, et al. Differential DNA damage signaling accounts for distinct neural apoptotic responses in ATLD and NBS. Genes Dev. 2009;23:171–180. doi: 10.1101/gad.1746609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao S, et al. Functional link between Ataxiatelangiectasia and Nijmegen breakage syndrome gene products. Nature. 2000;405:473–477. doi: 10.1038/35013083. [DOI] [PubMed] [Google Scholar]
- 112.Lim DS, et al. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature. 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- 113.Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Delacote F, Lopez BS. Importance of the cell cycle phase for the choice of the appropriate DSB repair pathway, for genome stability maintenance: the trans-S double-strand break repair model. Cell Cycle. 2008;7:33–38. doi: 10.4161/cc.7.1.5149. [DOI] [PubMed] [Google Scholar]
- 115.Adelman CA, De S, Petrini JH. Rad50 is dispensable for the maintenance and viability of postmitotic tissues. Mol. Cell Biol. 2009;29:483–492. doi: 10.1128/MCB.01525-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hartsuiker E, Neale MJ, Carr AM. Distinct requirements for the Rad32Mre11 nuclease and Ctp1CtIP in the removal of covalently bound topoisomerase I and II from DNA. Mol. Cell. 2009;33:117–123. doi: 10.1016/j.molcel.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uziel T, et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jazayeri A, Balestrini A, Garner E, Haber JE, Costanzo V. Mre11–Rad50–Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J. 2008;27:1953–1962. doi: 10.1038/emboj.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dupre A, et al. A forward chemical genetic screen reveals an inhibitor of the Mre11–Rad50–Nbs1 complex. Nature Chem. Biol. 2008;4:119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hashimoto Y, Chaudhuri AR, Lopes M, Costanzo V. Rad51 protects nascent DNA from Mre11-dependent degradation and promotes continuous DNA synthesis. Nature Struct. Mol. Biol. 2010;17:1305–1311. doi: 10.1038/nsmb.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Usui T, Petrini JH, Morales M. Rad50S alleles of the Mre11 complex: questions answered and questions raised. Exp. Cell Res. 2006;312:2694–2699. doi: 10.1016/j.yexcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 122.Barlow C, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 123.Reina-San-Martin B, Chen HT, Nussenzweig A, Nussenzweig MC. ATM is required for efficient recombination between immunoglobulin switch regions. J. Exp. Med. 2004;200:1103–1110. doi: 10.1084/jem.20041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reina-San-Martin B, Nussenzweig MC, Nussenzweig A, Difilippantonio S. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc. Natl Acad. Sci. USA. 2005;102:1590–1595. doi: 10.1073/pnas.0406289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dinkelmann M, et al. Multiple functions of MRN in end-joining pathways during isotype class switching. Nature Struct. Mol. Biol. 2009;16:808–813. doi: 10.1038/nsmb.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Theunissen JW, et al. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11ATLD1/ATLD1 mice. Mol. Cell. 2003;12:1511–1523. doi: 10.1016/s1097-2765(03)00455-6. [DOI] [PubMed] [Google Scholar]
- 127.Helmink BA, et al. MRN complex function in the repair of chromosomal Rag-mediated DNA double-strand breaks. J. Exp. Med. 2009;206:669–679. doi: 10.1084/jem.20081326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deriano L, Stracker TH, Baker A, Petrini JH, Roth DB. Roles for NBS1 in alternative nonhomologous end-joining of V(D)J recombination intermediates. Mol. Cell. 2009;34:13–25. doi: 10.1016/j.molcel.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taylor EM, et al. The Mre11/Rad50/Nbs1 complex functions in resection-based DNA end joining in Xenopus laevis. Nucleic Acids Res. 2010;38:441–454. doi: 10.1093/nar/gkp905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rass E, et al. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nature Struct. Mol. Biol. 2009;16:819–824. doi: 10.1038/nsmb.1641. [DOI] [PubMed] [Google Scholar]
- 131.Xie A, Kwok A, Scully R. Role of mammalian Mre11 in classical and alternative nonhomologous end joining. Nature Struct. Mol. Biol. 2009;16:814–818. doi: 10.1038/nsmb.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rahal EA, et al. ATM regulates Mre11-dependent DNA end-degradation and microhomology-mediated end joining. Cell Cycle. 2010;9:2866–2877. doi: 10.4161/cc.9.14.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Revy P, Buck D, le Deist F, de Villartay JP. The repair of DNA damages/modifications during the maturation of the immune system: lessons from human primary immunodeficiency disorders and animal models. Adv. Immunol. 2005;87:237–295. doi: 10.1016/S0065-2776(05)87007-5. [DOI] [PubMed] [Google Scholar]
- 134.Sekiguchi J, et al. Genetic interactions between ATM and the nonhomologous end-joining factors in genomic stability and development. Proc. Natl Acad. Sci. USA. 2001;98:3243–3248. doi: 10.1073/pnas.051632098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gurley KE, Kemp CJ. Synthetic lethality between mutation in Atm and DNA-PKcs during murine embryogenesis. Curr. Biol. 2001;11:191–194. doi: 10.1016/s0960-9822(01)00048-3. [DOI] [PubMed] [Google Scholar]
- 136.Stracker TH, et al. Artemis and nonhomologous end joining-independent influence of DNA-dependent protein kinase catalytic subunit on chromosome stability. Mol. Cell Biol. 2009;29:503–514. doi: 10.1128/MCB.01354-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rooney S, Alt FW, Sekiguchi J, Manis JP. Artemis-independent functions of DNA-dependent protein kinase in Ig heavy chain class switch recombination and development. Proc. Natl Acad. Sci. USA. 2005;102:2471–2475. doi: 10.1073/pnas.0409857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Corneo B, et al. Rag mutations reveal robust alternative end joining. Nature. 2007;449:483–486. doi: 10.1038/nature06168. [DOI] [PubMed] [Google Scholar]
- 139.Bender CF, et al. Cancer predisposition and hematopoietic failure in Rad50S/S mice. Genes Dev. 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morales M, et al. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 2005;19:3043–3054. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stracker TH, Couto SS, Cordon-Cardo C, Matos T, Petrini JH. Chk2 suppresses the oncogenic potential of DNA replication-associated DNA damage. Mol. Cell. 2008;31:21–32. doi: 10.1016/j.molcel.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J, Stern DF. Regulation of CHK2 by DNA-dependent protein kinase. J. Biol. Chem. 2005;280:12041–12050. doi: 10.1074/jbc.M412445200. [DOI] [PubMed] [Google Scholar]
- 143.Callen E, et al. Essential role for DNA-PKcs in DNA double-strand break repair and apoptosis in ATM-deficient lymphocytes. Mol. Cell. 2009;34:285–297. doi: 10.1016/j.molcel.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee Y, McKinnon PJ. Responding to DNA double strand breaks in the nervous system. Neuroscience. 2007;145:1365–1374. doi: 10.1016/j.neuroscience.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 145.Frank KM, et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol. Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 146.Orii KE, Lee Y, Kondo N, McKinnon PJ. Selective utilization of nonhomologous end-joining and homologous recombination DNA repair pathways during nervous system development. Proc. Natl Acad. Sci. USA. 2006;103:10017–10022. doi: 10.1073/pnas.0602436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao L, et al. ATM–Chk2–p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J. 2006;25:2167–2177. doi: 10.1038/sj.emboj.7601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Niida H, et al. Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo. EMBO J. 2010;29:3558–3570. doi: 10.1038/emboj.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maser RS, et al. Mre11 complex and DNA replication: linkage to E2F and sites of DNA synthesis. Mol. Cell Biol. 2001;21:6006–6016. doi: 10.1128/MCB.21.17.6006-6016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mirzoeva OK, Petrini JH. DNA replication-dependent nuclear dynamics of the Mre11 complex. Mol. Cancer Res. 2003;1:207–218. [PubMed] [Google Scholar]
- 151.Costanzo V, et al. Mre11 protein complex prevents double-strand break accumulation during chromosomal DNA replication. Mol. Cell. 2001;8:137–147. doi: 10.1016/s1097-2765(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 152.Tittel-Elmer M, Alabert C, Pasero P, Cobb JA. The MRX complex stabilizes the replisome independently of the S phase checkpoint during replication stress. EMBO J. 2009;28:1142–1156. doi: 10.1038/emboj.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bryant HE, et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J. 2009;28:2601–2615. doi: 10.1038/emboj.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jazayeri A, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nature Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 155.Garcia-Muse T, Boulton SJ. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 2005;24:4345–4355. doi: 10.1038/sj.emboj.7600896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Myers JS, Cortez D. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J. Biol. Chem. 2006;281:9346–9350. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee AY, Liu E, Wu X. The Mre11/Rad50/Nbs1 complex plays an important role in the prevention of DNA rereplication in mammalian cells. J. Biol. Chem. 2007;282:32243–32255. doi: 10.1074/jbc.M705486200. [DOI] [PubMed] [Google Scholar]
- 158.Trenz K, Smith E, Smith S, Costanzo V. ATM and ATR promote Mre11 dependent restart of collapsed replication forks and prevent accumulation of DNA breaks. EMBO J. 2006;25:1764–1774. doi: 10.1038/sj.emboj.7601045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl Acad. Sci. USA. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mochan TA, Venere M, DiTullio RA, Jr, Halazonetis TD. 53BP1 and NFBD1/MDC1-Nbs1 function in parallel interacting pathways activating Ataxia-telangiectasia mutated (ATM) in response to DNA damage. Cancer Res. 2003;63:8586–8591. [PubMed] [Google Scholar]
- 161.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc. Natl Acad. Sci. USA. 2008;105:16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liao S, Toczylowski T, Yan H. Identification of the Xenopus DNA2 protein as a major nuclease for the 5′→3′ strand-specific processing of DNA ends. Nucleic Acids Res. 2008;36:6091–6100. doi: 10.1093/nar/gkn616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nature Rev. Mol. Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kabotyanski EB, Gomelsky L, Han JO, Stamato TD, Roth DB. Double-strand break repair in Ku86- and XRCC4-deficient cells. Nucleic Acids Res. 1998;26:5333–5342. doi: 10.1093/nar/26.23.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee-Theilen M, Matthews AJ, Kelly D, Zheng S, Chaudhuri J. CtIP promotes microhomology-mediated alternative end joining during class-switch recombination. Nature Struct. Mol. Biol. 2010 Dec;5 doi: 10.1038/nsmb.1942. (doi:10.1038/nsmb.1942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Y, Jasin M. An essential role for CtIP in chromosomal translocation formation through an alternative end-joining pathway. Nature Struct. Mol. Biol. 2011;18:75–79. doi: 10.1038/nsmb.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Demuth I, et al. An inducible null mutant murine model of Nijmegen breakage syndrome proves the essential function of NBS1 in chromosomal stability and cell viability. Hum. Mol. Genet. 2004;13:2385–2397. doi: 10.1093/hmg/ddh278. [DOI] [PubMed] [Google Scholar]
- 171.Frappart PO, et al. An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nature Med. 2005;11:538–544. doi: 10.1038/nm1228. [DOI] [PubMed] [Google Scholar]
- 172.Kracker S, et al. Nibrin functions in Ig class-switch recombination. Proc. Natl Acad. Sci. USA. 2005;102:1584–1589. doi: 10.1073/pnas.0409191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saidi A, Li T, Weih F, Concannon P, Wang ZQ. Dual functions of Nbs1 in the repair of DNA breaks and proliferation ensure proper V(D)J. recombination and T-cell development. Mol. Cell Biol. 2010;30:5572–5581. doi: 10.1128/MCB.00917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.