Abstract
Over the past two decades, adiponectin has been studied in more than eleven thousand publications. A classical adipokine, adiponectin was among the first factors secreted from adipose tissue that were found to promote metabolic function. Circulating levels of adiponectin consistently decline with increasing body mass index. Clinical and basic science studies have identified adiponectin’s cardiovascular-protective actions, providing a mechanistic link to the increased incidence of cardiovascular disease in obese individuals. While progress has been made in identifying receptors essential for the metabolic actions of adiponectin (AdipoR1 and AdipoR2), few studies have examined the receptor-mediated signaling pathways in cardiovascular tissues. T-cadherin, a GPI-anchored adiponectin-binding protein, was recently identified as critical for the cardiac-protective and revascularization actions of adiponectin. Adiponectin is abundantly present on the surfaces of vascular and muscle tissues through a direct interaction with T-cadherin. Consistent with this observation, adiponectin is absent from T-cadherin-deficient tissues. Since T-cadherin lacks an intracellular domain, additional studies would further our understanding of this signaling pathway. Here, we review the diverse cardiometabolic actions of adiponectin.
Keywords: Adiponectin, Cardiovascular, T-cadherin, AdipoR1, AdipoR2
II. Obesity increases the risk of cardiovascular disease
It is well-appreciated that elevated body mass index (BMI) is associated with an increased risk of cardiovascular disease and overall mortality. Severe obesity (BMI >40 kg/m2) can shorten lifespan by up to 10 years (1). While vascular disease is the main cause of mortality in obese individuals, the mechanisms underlying obesity-associated mortality are incompletely understood. Here, we discuss recent findings that have elucidated the role of the adipocyte-secreted protein adiponectin in vascular function and disease.
III. Adipokines in action
Besides energy storage, adipose tissue has an important endocrine function. Factors secreted from this tissue are termed adipokines. Many adipokines are pro-inflammatory but a subset has anti-inflammatory functions (2). With increasing adiposity, expression of pro-inflammatory adipokines is increased while expression of anti-inflammatory adipokines is reduced. Classical examples of pro- and anti-inflammatory adipokines are tumor necrosis factor α (TNFα) and adiponectin, respectively. With increasing BMI, serum levels of TNFα are elevated while circulating adiponectin levels are reduced. These changes in adipokine profile contribute to the state of low-grade inflammation observed in obese individuals.
The adiponectin monomer has a collectin protein-like structure with a globular head and a collagenous tail. These monomeric subunits coalesce to form large oligomeric structures. Adiponectin circulates as three distinct fractions including trimers, hexamers and high molecular weight isoforms that are composed of 12 to 18 monomers (30kDa per monomer). The high molecular weight isoform is considered to be the most biologically active (3). The trimeric form undergoes proteolytic cleavage, at least in vitro, to form an 18-25kDa globular fragment (4). Similar cleavage may also occur in vivo by leukocyte elastase secreted by tissue-resident inflammatory cells (5). However, circulating levels of globular adiponectin are barely, if at all, detectable (6, 7).
Adiponectin has diverse effects throughout the body including cardiovascular-protection and metabolic regulation. Many of these protective actions can be attributed to its anti-inflammatory properties. Ouchi, et al. reported that adiponectin inhibits nuclear factor κB (NF-κB) activation in endothelial cells following treatment with pro-inflammatory factors such as TNFα (8). Conversely, TNFα inhibits the expression of adiponectin. Adiponectin has been shown to promote macrophage clearance of apoptotic cells via interaction with cell surface proteins calreticulin (CRT) and low density lipoprotein related receptor (LRP1) (9). We and others have recently extended observations of adiponectin’s anti-inflammatory effects by identifying that this adipokine promotes M2 macrophage polarization (10-12).
Numerous studies to date have examined the insulin sensitizing actions of adiponectin. Yamauchi et al. have demonstrated that adiponectin modulates glucose uptake, gluconeogenesis and fatty acid oxidation in skeletal muscle and liver (13). Mouse genetic evidence suggests that overexpression of globular or full-length adiponectin is protective in mouse models of obesity, such as the ob/ob leptin-deficient mouse (14, 15). Strikingly, despite having an elevated body weight, adiponectin transgenic mice on an ob/ob background have preserved metabolic function (14).
Cardiovascular and metabolic dysfunction are strongly associated. Increased adipose tissue vascularity is associated with improved metabolic function and reduced accumulation of inflammatory cells (16). Serum adiponectin levels are positively associated with capillary density in adipose tissue in mice (17). Pro-angiogenic and anti-inflammatory actions of adiponectin are presumed to contribute to metabolic adaptation during the development of obesity including adipocyte proliferation (14) and vascular cell migration and proliferation (18). These actions are thought to maintain adequate nutrient supply during tissue expansion.
IV. Clinical studies implicating adiponectin in cardiovascular disease
Epidemiological evidence supports a protective role for adiponectin in cardiovascular disease. Serum levels of adiponectin in normal, healthy individuals can exceed 40μg/mL. Low levels of adiponectin are associated with cardiovascular risk factors including smoking (19), diabetes (20) and dyslipidemia (21). Reduced circulating levels of adiponectin are also linked to a higher prevalence of ischemic heart disease in both men and women (22, 23) whereas individuals with high adiponectin have a lower risk of myocardial infarction independent of other variables (24). Low serum adiponectin is also predictive of a future coronary artery ischemic event (25, 26), and the development of hypertension (27) and the levels of this adipokine correlate with left ventricular hypertrophy (28, 29). High serum adiponectin is associated with better recovery of cardiac function post-injury (30). On the contrary, hyperadiponectinemia is associated with mortality in patients with heart or respiratory failure (31, 32).
Peripheral artery disease is characterized by reduced blood flow in the lower extremities usually due to atherosclerotic plaque accumulation. Clinically, levels of total and high molecular weight adiponectin are inversely associated with development of this condition (33, 34). In those affected by peripheral artery disease, ankle-brachial pressure index and declining limb function are proportionally associated with hypoadiponectinemia (35, 36). In patients requiring a bypass surgery for this condition, serum adiponectin is a positive predictor of surgical recovery (37). Collectively, these data highlight the important clinical connection between adiponectin and cardiovascular function.
V. Angiogenic actions of adiponectin
Previous experimental studies by our laboratory and others have shown that adiponectin promotes revascularization in a mouse model of peripheral artery disease. In the murine hind limb ischemia model, blood flow is unilaterally restricted and recovery monitored over the course of 2-4 weeks (38, 39). Mice deficient in adiponectin have impaired recovery compared with wild-type mice (40-42). In this model, administration of exogenous adiponectin promotes the revascularization response and rescues the impairment observed in adiponectin-deficient mice (40, 41). Intracellular signaling molecules that are important for this effect include 5′ adenosine monophosphate-activated protein kinase (AMPK) (40), endothelial nitric oxide synthase (eNOS) and cyclooxygenase-2 (COX-2) (43). Treatment with an adenovirus-expressing dominant-negative AMPK (dnAMPK) attenuates blood flow recovery in wild-type mice. In addition, exogenous adiponectin treatment is ineffective at improving blood flow in the dnAMPK-treated mice. A similar study subjected endothelial cell-specific COX-2-deficient mice to hind limb ischemia. Again, these mice had impaired revascularization that was not improved by adiponectin treatment (43).
In situations where vessel expansion is pathological, such as retinal neovascularization, adiponectin inhibits detrimental vessel expansion (44). This study showed that the action of adiponectin to inhibit dysregulated angiogenesis was mediated by its ability to suppress TNFα expression. Accordingly, when treated with a pharmacological TNFα inhibitor, the pathological vascularization is reduced in the retina (44). Rosiglitazone, a PPARγ-agonist known to increase serum adiponectin (45), also ameliorates pathological neovascularization in this model (46). Similarly, in a murine mammary tumor model, loss or reduced expression of adiponectin results in decreased tumor angiogenesis (47-49). The poorly vascularized tumors in adiponectin-deficient mice display reduced growth; however, the rate of metastases is significantly increased. Collectively, these data suggest that adiponectin does not act as a classical angiogenic factor. Rather, it is likely that it functions to promote physiological and inhibit pathological angiogenesis as a consequence of its ability to promote endothelial cell function.
VI. Cardiac actions of adiponectin
In multiple experimental models of cardiac dysfunction, overexpression of adiponectin preserves function, whereas its deficiency exacerbates damage due to cardiac stress. In a model of ischemia reperfusion injury, adiponectin-deficiency is marked by a larger infarct size (50). Exogenous adiponectin preserves cardiac cell survival at least in part, through activation of both AMPK- and COX-2-dependent signaling mechanisms. Immediately after ischemic injury, serum adiponectin is localized to injured cardiac tissue (51). It was noted that a striking decrease in serum levels of the high molecular weight isoform of adiponectin was observed in parallel with the cardiac localization of adiponectin suggesting that this isoform predominantly accumulates in damaged cardiac tissue. The protective actions of acutely-administered exogenous adiponectin protein were subsequently identified in a preclinical porcine ischemia reperfusion model (52). In other studies, it has been shown that following myocardial infarction, mice lacking adiponectin are more susceptible to systolic dysfunction and capillary loss (53). Similarly, in mice subjected to pressure overload by transverse aortic constriction, poor ventricular remodeling and reduced cardiac angiogenesis propelled the transition from hypertrophy to heart failure in the absence of adiponectin (54-56).
VII. The “AdipoR” adiponectin receptors
A small number of recent in vivo studies have provided insight on the receptor(s) responsible for mediating the numerous cardiovascular actions of adiponectin. Adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2, respectively) have the structure of seven transmembrane-spanning domains with an extracellular C-terminus (57). Ubiquitously expressed, the receptors can be found on the plasma membrane as homomers or heteromers (58, 59). AdipoR1 promotes cell surface-localization of AdipoR2 though direct interactions via their N-terminus domains (60).
Although several groups have generated AdipoR1- or AdipoR2-deficient mice (13, 61, 62), they have never been tested in cardiac or vascular in vivo models, and the majority of the AdipoR1 and AdipoR2 research to date has focused on their metabolic functions. Two groups independently reported mouse models of AdipoR1 gene ablation. In these studies AdipoR1-deficiency is associated with metabolic dysfunction and excess adipose tissue accumulation (13, 61). Similarly it has been shown that AdipoR1 muscle-specific ablation or overexpression in mice is marked by metabolic perturbation and protection, respectively (63, 64). In murine genetic and diet-induced models of metabolic syndrome, overexpression of AdipoR1 in macrophages improves glucose tolerance and insulin sensitivity (65, 66). However, controversy exists regarding whether AdipoR2-deficiency is protective or detrimental to systemic metabolism (13, 61, 62). Mechanistically, the diverse actions of adiponectin may be mediated by its ability to modulate sphingolipid metabolism via AdipoR1 and AdipoR2 (67).
One study has described a possible functional role for caveolin 3 in the cardioprotective actions of adiponectin using the ischemia-reperfusion model. The authors proposed a mechanism involving protein-protein interactions among caveolin 3, AdipoR1 and APPL1, which comprise an adiponectin “signalsome” (68). Finally, recent studies suggest that the actions of adiponectin may be mediated through additional receptors including PAQR3 (renamed AdipoR3) (69) and AdipoRX (70). AdipoR1 and AdipoR2 are members of the PAQR family (69)_ENREF_141 and it is possible that other members of this poorly characterized family may confer actions of adiponectin or other C1q/TNF-related proteins (CTRPs) that share homology with adiponectin.
VIII. AdipoR1 & AdipoR2 in cardiovascular cells
AdipoR1 and AdipoR2 are expressed on many cardiovascular-relevant cells including endothelial cells, vascular smooth muscle cells, and monocytes. Recent in vitro studies implicate AdipoR1 and AdipoR2 in mediating the diverse actions of adiponectin. For example, treatment of 293 cells with siRNA-targeting both receptors inhibits adiponectin-stimulated ERK1/2 activation. However, reducing expression of either AdipoR1 or AdipoR2 individually does not inhibit the stimulatory actions of adiponectin (71). Similarly, the ability of adiponectin to induce eNOS phosphorylation in human umbilical vein endothelial cells (HUVECs) was attenuated only with downregulation of both AdipoR1 and AdipoR2, while individual receptor siRNA knockdowns had no effect (72). Additional siRNA knockdown experiments suggest an in vitro pro-angiogenic role for AdipoR1 in the AMPK signaling pathway (43). Also in HUVECs, decreased expression or inhibition of AdipoR1 or AdipoR2 impairs the angiogenic actions of adiponectin (41, 73). Accordingly, it is reported that overexpression of AdipoR1 and AdipoR2 enhances the anti-inflammatory effects of adiponectin in endothelial cells (74). Therefore, AdipoR1 and AdipoR2 appear to mediate multiple in vitro cardiovascular actions of recombinant adiponectin protein in cell culture models. However, few if any studies have examined the in vivo activities of AdipoR1 and AdipoR2 in murine genetic models.
IX. T-cadherin in the heart and vasculature
Another membrane-associated, adiponectin-binding protein is T-cadherin. T-cadherin is a GPI-anchored protein that lacks an intracellular domain (75). In contrast to AdipoR1 and AdipoR2, it is highly expressed in the plasma membrane of heart, skeletal muscle and vascular tissue (76). Adiponectin and T-cadherin co-localize on the surfaces of murine cardiovascular tissues (77). A potential in vivo functional relationship between these two proteins has been assessed using genetic models of adiponectin and/or T-cadherin deficiency (41, 77). In these studies, cardiac or vascular stress were induced to determine whether ligand deficiency phenocopied receptor deficiency. Furthermore, the ability of T-cadherin-deficient mice to respond to exogenous adiponectin was evaluated.
Neither adiponectin-deficient nor T-cadherin-deficient mice display a cardiovascular phenotype under non-stress conditions. However, Denzel, et al. demonstrated in vivo that T-cadherin-deficiency mimics the phenotype of adiponectin-deficiency in both chronic and acute mouse models of cardiac injury (77). In the transverse aortic constriction model of pressure overload, mice lacking T-cadherin developed increased hypertrophy and reduced cardiac capillary density. In the acute model, infarct size following ischemia-reperfusion was larger in mice lacking T-cadherin. Adiponectin-deficient mice, analyzed in parallel to T-cadherin-deficient mice, had a similar degree of cardiac impairment in these models (50, 54, 77). While exogenous adiponectin rescues the stress-induced cardiac injuries in adiponectin-deficient mice, it does not improve recovery in T-cadherin-deficient mice (77). The conclusion from these studies is that T-cadherin is essential for the cardiac-protective actions of adiponectin.
T-cadherin is also highly expressed throughout the vasculature including endothelial cells, smooth muscle cells and pericytes (76). In skeletal muscle tissue, T-cadherin and adiponectin are abundant and highly co-localized (41). Given these observations, T-cadherin-deficient mice were evaluated in a model of chronic limb ischemia. Similar to the previously described cardiac study (77), mice deficient in T-cadherin were phenotypically similar to adiponectin-deficient mice such that both had impaired blood flow recovery and limb function (41). Furthermore, systemic administration of adiponectin did not promote revascularization in mice lacking T-cadherin. The conclusion from these studies is that T-cadherin is essential for the vascular-protective actions of adiponectin (41).
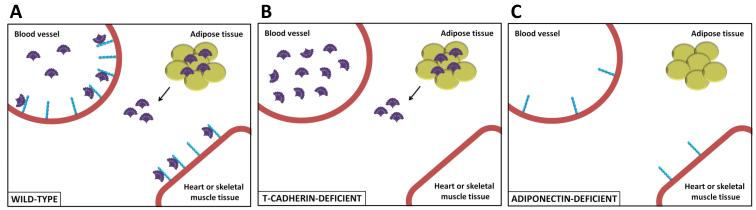
One mechanism for these effects is that T-cadherin is responsible for tissue-localization of adiponectin (78). In vitro, several laboratories have identified a direct interaction between T-cadherin and adiponectin by co-immunoprecipitation experiments (41, 77, 79). T-cadherin selectively binds the hexameric and high molecular weight isoforms of adiponectin (79). While adiponectin is present on the cell surfaces of the vascular endothelium and myocytes in wild-type mice, adiponectin is absent from these tissues in T-cadherin-deficient mice (41, 77, 78). In contrast, expression of either AdipoR1 or AdipoR2 is not sufficient for adiponectin localization to T-cadherin-deficient skeletal muscle, heart or vasculature. Along with a lack of cardiovascular tissue-resident adiponectin, T-cadherin-deficient mice have significantly elevated serum levels of hexameric and high molecular weight adiponectin (Figure 1). Thus, it appears that a majority of the whole body adiponectin is localized to cardiovascular tissues by T-cadherin (41). Strikingly, in the setting of adiponectin-deficiency, tissue expression of T-cadherin is also reduced suggesting a regulatory axis between these proteins (41, 77).
Figure 1. T-cadherin is essential for localization of adiponectin to cardiovascular tissues.
A) In wild-type mice, high molecular weight adiponectin (shown in purple) is tethered to vascular and muscle tissue through binding T-cadherin (shown in blue). B) In the absence of T-cadherin, adiponectin is liberated leading to increased serum adiponectin levels while the adipokine is absent from cardiac and skeletal muscle tissue. C) In the setting of adiponectin-deficiency, T-cadherin tissue expression is reduced suggesting a feedback regulatory axis between these proteins.
With regard to adiponectin ligand-receptor interactions it should be noted that aspects of adiponectin biology are unusual. For example, traditional signaling molecules are 1,000-fold less abundant than adiponectin in serum. Adiponectin monomers are also found as oligomeric units in serum. High molecular weight adiponectin that may exceed 500kDa in size is structurally similar to C1q and the collectin family of proteins. Given the high abundance and complex structure of adiponectin, it is reasonable to expect that its receptor interactions will be atypical. In this regard, T-cadherin, also referred to as truncated-cadherin, lacks the typical cadherin family transmembrane and intracellular domains. Thus, T-cadherin may function as a co-receptor to localize adiponectin to the tissue of interest for presentation to a receptor (e.g. AdipoR1 or AdipoR2) with intracellular signaling capabilities (41, 77). Furthermore, one might predict that T-cadherin has the ability to facilitate interactions between adiponectin and multiple other proteins as it tethers adiponectin to the surfaces of cardiovascular tissues (80-82). Finally, adiponectin is capable of activating intracellular signaling molecules such as AMPK (50). Given the critical role of T-cadherin in mediating the cardiovascular protective actions of adiponectin, future studies of the potential signaling capabilities of this complex would greatly advance this field.
X. Genomic associations with cardiovascular disease
Genomic associations between adiponectin (Adipoq) and cardiovascular disease have been well documented (83-88). Few studies have identified a significant cardiovascular association for AdipoR1 or AdipoR2 polymorphisms (89-92) with other studies reporting negative results (93-96). In contrast, the gene encoding T-cadherin (CDH13) is associated with blood pressure (97), serum lipids (98, 99), myocardial infarction (99), and ischemic stroke (100). Furthermore, numerous studies (Table 1) have identified polymorphisms in CDH13 that affect circulating levels of adiponectin (101-106).
Table 1. Genetic associations between adiponectin receptors (AdipoRI, AdipoR2 and CDH13), circulating adiponectin levels, and cardiovascular disease.
| Gene(s) | Population | Results |
|---|---|---|
| ADIPOR1 | Individuals with type II diabetes (USA and Italy) |
Association with coronary artery disease (89) |
| ADIPOR2 | Individuals with impaired glucose tolerance (Finland) |
Association with cardiovascular disease and type 2 diabetes (90) |
| ADIPOR2 | Individuals with coronary artery disease (Greece) |
Association with coronary artery disease (91) |
| ADIPOR2 | Overweight individuals with insulin resistance (Germany) |
Association with circulating adiponectin levels and blood lipid levels (92) |
|
ADIPOR1, ADIPOR2 |
Male physicians with prostate cancer (USA) |
No association with circulating adiponectin levels (93) |
|
ADIPOR1, ADIPOR2 |
Females aged 40-79 (USA) | No association with circulating adiponectin levels (94) |
|
ADIPOR1, ADIPOR2 |
General population and type II diabetes cohorts (Australia) |
No association with circulating adiponectin levels (95) |
| ADIPOR1 | Individuals with coronary heart diseases (China) |
No association with coronary heart diseases (96) |
| CDH13 | General population (Germany, Estonia, and UK) |
Association with blood pressure (97) |
| CDH13 | Predominantly Hispanic populations (USA, Dominican Republic) |
Association with blood lipid levels (98) |
| CDH13 | Individuals with coronary artery disease and hyperlipidemia (Taiwan) |
Association with hyperlipidemia and myocardial infarction (99) |
| CDH13 | Young-onset hypertensive subjects (Taiwan) |
Association with metabolic syndrome, type 2 diabetes and ischemic stroke (100) |
| CDH13 | General population and overweight individuals or individuals with dyslipidemia (Europe) |
Association with circulating adiponectin levels(101) |
| CDH13 | General population of mothers and young adult offspring (Phillipines) |
Association with circulating adiponectin levels (102) |
| CDH13 | General population (Korea) | Association with circulating adiponectin levels (103) |
| CDH13 | Males aged 40-69 years (Korea) | Association with circulating adiponectin levels (104) |
| CDH13 | General population (Japan) | Association with circulating adiponectin levels (105) |
| CDH13 | Individuals treated with fenofibrate (USA) | Association with circulating adiponectin levels (106) |
XI. Summary
The anti-inflammatory adipokine adiponectin has important metabolic and cardiovascular-protective actions. Clinically, hypoadiponectinemia is associated with both obesity and cardiovascular disease, whereas high serum adiponectin levels correlate with improved cardiovascular function. Administration of exogenous adiponectin is protective in mouse models of cardiac or vascular stress such as ischemia reperfusion, cardiac pressure overload and peripheral artery disease. In the state of adiponectin-deficiency, cardiovascular injury is exacerbated. Adiponectin promotes cardiac cell survival and endothelial cell function through AMPK-dependent signaling mechanisms. However, the receptor-mediated signaling pathways are incompletely understood. The candidate adiponectin receptors AdipoR1 and AdipoR2 have received the most attention. These receptors appear to mediate the metabolic actions of adiponectin, but their cardiovascular actions are relatively unexplored. Recent studies identified T-cadherin as essential for localizing adiponectin to cardiovascular tissues. T-cadherin tethers the high molecular weight isoforms of adiponectin to the heart, skeletal muscle and vasculature. In the setting of T-cadherin-deficiency, tissue-localized adiponectin is absent and serum adiponectin is elevated. Consistent with this observation is the fact that multiple clinical studies have found an association between serum adiponectin and the T-cadherin gene, CDH13. In experimental disease models, the protective actions of exogenous adiponectin in ischemia-reperfusion injury, pressure overload and peripheral artery disease are lost in the state of T-cadherin-deficiency. However, since T-cadherin lacks a transmembrane and intracellular domain, other proteins are likely to be required for intracellular signaling. The atypical receptor-interactions of adiponectin are consistent with the atypical features of this ligand including its complex quaternary structure and high abundance in serum. While much progress has been made, additional studies are needed to clarify the cardiovascular-protective mechanisms of adiponectin in both experimental models and the clinical population.
Research agenda.
Since GPI-anchored T-cadherin is essential for cardiovascular-protective actions of adiponectin, studies should be completed to determine how intracellular signaling is transduced.
Determine whether AdipoR1 or AdipoR2 are important for the cardiovascular actions of adiponectin in vivo.
Identify and validate strategies to mimic the actions of adiponectin, taking into account its abundance and structural complexity, to develop candidates for cardiovascular therapy.
Acknowledgments
This work was supported by National Institutes of Health grants AG34972, HL81587 and HL68758 to KW and by pre-doctoral training grants in Cardiovascular Biology HL007969 and Biomolecular Pharmacology GM008541 to JPD.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prospective Studies C. Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–59. [PubMed] [Google Scholar]
- 4.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waki H, Yamauchi T, Kamon J, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–6. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 6.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 9.Takemura Y, Ouchi N, Shibata R, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–86. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–60. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovren F, Pan Y, Quan A, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–63. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–9. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 16.Sung HK, Doh KO, Son JE, et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Aprahamian T. Elevated adiponectin expression promotes adipose tissue vascularity under conditions of diet-induced obesity. Metabolism In Press. 2013 doi: 10.1016/j.metabol.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–9. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sull JW, Kim HJ, Yun JE, Park EJ, Kim G, Jee SH. Serum adiponectin is associated with smoking status in healthy Korean men. Endocr J. 2009;56:73–8. doi: 10.1507/endocrj.k08e-231. [DOI] [PubMed] [Google Scholar]
- 20.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–9. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 22.Pischon T, Hu FB, Girman CJ, et al. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis. 2011;219:322–9. doi: 10.1016/j.atherosclerosis.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 24.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 25.Kojima S, Funahashi T, Sakamoto T, et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima S, Funahashi T, Otsuka F, et al. Future adverse cardiac events can be predicted by persistently low plasma adiponectin concentrations in men and marked reductions of adiponectin in women after acute myocardial infarction. Atherosclerosis. 2007;194:204–13. doi: 10.1016/j.atherosclerosis.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Iwashima Y, Katsuya T, Ishikawa K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–23. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 28.Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004;13:236–42. doi: 10.1080/08037050410021397. [DOI] [PubMed] [Google Scholar]
- 29.Mitsuhashi H, Yatsuya H, Tamakoshi K, et al. Adiponectin level and left ventricular hypertrophy in Japanese men. Hypertension. 2007;49:1448–54. doi: 10.1161/HYPERTENSIONAHA.106.079509. [DOI] [PubMed] [Google Scholar]
- 30.Shibata R, Numaguchi Y, Matsushita K, et al. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol. 2008;101:1712–5. doi: 10.1016/j.amjcard.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 31.Walkey AJ, Rice TW, Konter J, et al. Plasma adiponectin and mortality in critically ill subjects with acute respiratory failure. Crit Care Med. 2010;38:2329–34. doi: 10.1097/CCM.0b013e3181fa0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 33.Ho DY, Cook NR, Britton KA, et al. High-molecular-weight and total adiponectin levels and incident symptomatic peripheral artery disease in women: a prospective investigation. Circulation. 2011;124:2303–11. doi: 10.1161/CIRCULATIONAHA.111.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joosten MM, Joshipura KJ, Pai JK, et al. Total adiponectin and risk of symptomatic lower extremity peripheral artery disease in men. Arterioscler Thromb Vasc Biol. 2013;33:1092–7. doi: 10.1161/ATVBAHA.112.301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45:40–6. doi: 10.1016/j.jvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Iwashima Y, Horio T, Suzuki Y, et al. Adiponectin and inflammatory markers in peripheral arterial occlusive disease. Atherosclerosis. 2006;188:384–90. doi: 10.1016/j.atherosclerosis.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 37.Komai H, Shibata R, Juri M, Matsushita K, Ouchi N, Murohara T. Plasma adiponectin as a predictive factor of survival after a bypass operation for peripheral arterial disease. J Vasc Surg. 2009;50:95–9. doi: 10.1016/j.jvs.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 38.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–79. [PMC free article] [PubMed] [Google Scholar]
- 39.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–46. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 40.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–4. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 41.Parker-Duffen JL, Nakamura K, Silver M, et al. T-cadherin Is Essential for Adiponectin-mediated Revascularization. J Biol Chem. 2013;288:24886–97. doi: 10.1074/jbc.M113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo M, Shibata R, Miura R, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284:1718–24. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohashi K, Ouchi N, Sato K, et al. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29:3487–99. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression. Circ Res. 2009;104:1058–65. doi: 10.1161/CIRCRESAHA.109.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang WS, Jeng CY, Wu TJ, et al. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25:376–80. doi: 10.2337/diacare.25.2.376. [DOI] [PubMed] [Google Scholar]
- 46.Higuchi A, Ohashi K, Shibata R, Sono-Romanelli S, Walsh K, Ouchi N. Thiazolidinediones reduce pathological neovascularization in ischemic retina via an adiponectin-dependent mechanism. Arterioscler Thromb Vasc Biol. 2010;30:46–53. doi: 10.1161/ATVBAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam JB, Chow KH, Xu A, et al. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One. 2009;4:e4968. doi: 10.1371/journal.pone.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15:3256–64. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landskroner-Eiger S, Qian B, Muise ES, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15:3265–76. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibata R, Sato K, Kumada M, et al. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc Res. 2007;74:471–9. doi: 10.1016/j.cardiores.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Kondo K, Shibata R, Unno K, et al. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3:166–73. doi: 10.1161/CIRCINTERVENTIONS.109.872044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibata R, Izumiya Y, Sato K, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–74. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimano M, Ouchi N, Shibata R, et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210–20. doi: 10.1016/j.yjmcc.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Shea KM, Chess DJ, Khairallah RJ, et al. Effects of adiponectin deficiency on structural and metabolic remodeling in mice subjected to pressure overload. Am J Physiol Heart Circ Physiol. 2010;298:H1639–45. doi: 10.1152/ajpheart.00957.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Y, Takashima S, Maeda N, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–13. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 58.Kosel D, Heiker JT, Juhl C, et al. Dimerization of adiponectin receptor 1 is inhibited by adiponectin. J Cell Sci. 2010;123:1320–8. doi: 10.1242/jcs.057919. [DOI] [PubMed] [Google Scholar]
- 59.Almabouada F, Diaz-Ruiz A, Rabanal-Ruiz Y, Peinado JR, Vazquez-Martinez R, Malagon MM. Adiponectin receptors form homomers and heteromers exhibiting distinct ligand binding and intracellular signaling properties. J Biol Chem. 2013;288:3112–25. doi: 10.1074/jbc.M112.404624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keshvari S, Rose FJ, Charlton HK, et al. Characterisation of the adiponectin receptors: The non-conserved N-terminal region of AdipoR2 prevents its expression at the cell-surface. Biochem Biophys Res Commun. 2013;432:28–33. doi: 10.1016/j.bbrc.2013.01.092. [DOI] [PubMed] [Google Scholar]
- 61.Bjursell M, Ahnmark A, Bohlooly YM, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–93. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Michael MD, Kash S, et al. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683–92. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- 63.Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 64.Patel SA, Hoehn KL, Lawrence RT, et al. Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology. 2012;153:5231–46. doi: 10.1210/en.2012-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo N, Chung BH, Wang X, et al. Enhanced adiponectin actions by overexpression of adiponectin receptor 1 in macrophages. Atherosclerosis. 2013;228:124–35. doi: 10.1016/j.atherosclerosis.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo N, Wang X, Zhang W, Garvey WT, Fu Y. AdR1-TG/TALLYHO mice have improved lipid accumulation and insulin sensitivity. Biochem Biophys Res Commun. 2013;433:567–72. doi: 10.1016/j.bbrc.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Wang X, Jasmin JF, et al. Essential role of caveolin-3 in adiponectin signalsome formation and adiponectin cardioprotection. Arterioscler Thromb Vasc Biol. 2012;32:934–42. doi: 10.1161/ATVBAHA.111.242164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garitaonandia I, Smith JL, Kupchak BR, Lyons TJ. Adiponectin identified as an agonist for PAQR3/RKTG using a yeast-based assay system. J Recept Signal Transduct Res. 2009;29:67–73. doi: 10.1080/10799890902729456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–96. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Lee MH, Klein RL, El-Shewy HM, Luttrell DK, Luttrell LM. The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry. 2008;47:11682–92. doi: 10.1021/bi801451f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng KK, Lam KS, Wang Y, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–94. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 73.Adya R, Tan BK, Chen J, Randeva HS. Protective actions of globular and full-length adiponectin on human endothelial cells: novel insights into adiponectin-induced angiogenesis. J Vasc Res. 2012;49:534–43. doi: 10.1159/000338279. [DOI] [PubMed] [Google Scholar]
- 74.Zhang P, Wang Y, Fan Y, Tang Z, Wang N. Overexpression of adiponectin receptors potentiates the antiinflammatory action of subeffective dose of globular adiponectin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:67–74. doi: 10.1161/ATVBAHA.108.178061. [DOI] [PubMed] [Google Scholar]
- 75.Philippova M, Joshi MB, Kyriakakis E, Pfaff D, Erne P, Resink TJ. A guide and guard: the many faces of T-cadherin. Cell Signal. 2009;21:1035–44. doi: 10.1016/j.cellsig.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 76.Ivanov D, Philippova M, Antropova J, et al. Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochem Cell Biol. 2001;115:231–42. doi: 10.1007/s004180100252. [DOI] [PubMed] [Google Scholar]
- 77.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–52. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res. 2008;68:1407–16. doi: 10.1158/0008-5472.CAN-07-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peake PW, Shen Y, Campbell LV, Charlesworth JA. Human adiponectin binds to bacterial lipopolysaccharide. Biochem Biophys Res Commun. 2006;341:108–15. doi: 10.1016/j.bbrc.2005.12.162. [DOI] [PubMed] [Google Scholar]
- 81.Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–8. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 82.Peake PW, Shen Y, Walther A, Charlesworth JA. Adiponectin binds C1q and activates the classical pathway of complement. Biochem Biophys Res Commun. 2008;367:560–5. doi: 10.1016/j.bbrc.2007.12.161. [DOI] [PubMed] [Google Scholar]
- 83.Arregui M, Fisher E, Knuppel S, et al. Significant associations of the rs2943634 (2q36.3) genetic polymorphism with adiponectin, high density lipoprotein cholesterol and ischemic stroke. Gene. 2012;494:190–5. doi: 10.1016/j.gene.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 84.Chiodini BD, Specchia C, Gori F, et al. Adiponectin gene polymorphisms and their effect on the risk of myocardial infarction and type 2 diabetes: an association study in an Italian population. Ther Adv Cardiovasc Dis. 2010;4:223–30. doi: 10.1177/1753944710371483. [DOI] [PubMed] [Google Scholar]
- 85.Katakami N, Kaneto H, Matsuoka TA, et al. Adiponectin G276T gene polymorphism is associated with cardiovascular disease in Japanese patients with type 2 diabetes. Atherosclerosis. 2012;220:437–42. doi: 10.1016/j.atherosclerosis.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 86.Liu F, He Z, Deng S, Zhang H, Li N, Xu J. Association of adiponectin gene polymorphisms with the risk of ischemic stroke in a Chinese Han population. Mol Biol Rep. 2011;38:1983–8. doi: 10.1007/s11033-010-0320-y. [DOI] [PubMed] [Google Scholar]
- 87.Oliveira CS, Saddi-Rosa P, Crispim F, et al. Association of ADIPOQ variants, total and high molecular weight adiponectin levels with coronary artery disease in diabetic and non-diabetic Brazilian subjects. J Diabetes Complications. 2012;26:94–8. doi: 10.1016/j.jdiacomp.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Zhou L, Xi B, Wei Y, et al. Association between adiponectin gene polymorphisms and coronary artery disease across different populations. Thromb Res. 2012;130:52–7. doi: 10.1016/j.thromres.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 89.Soccio T, Zhang YY, Bacci S, et al. Common haplotypes at the adiponectin receptor 1 (ADIPOR1) locus are associated with increased risk of coronary artery disease in type 2 diabetes. Diabetes. 2006;55:2763–70. doi: 10.2337/db06-0613. [DOI] [PubMed] [Google Scholar]
- 90.Siitonen N, Pulkkinen L, Lindstrom J, et al. Association of ADIPOR2 gene variants with cardiovascular disease and type 2 diabetes risk in individuals with impaired glucose tolerance: the Finnish Diabetes Prevention Study. Cardiovasc Diabetol. 2011;10:83. doi: 10.1186/1475-2840-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halvatsiotis I, Tsiotra PC, Ikonomidis I, et al. Genetic variation in the adiponectin receptor 2 (ADIPOR2) gene is associated with coronary artery disease and increased ADIPOR2 expression in peripheral monocytes. Cardiovasc Diabetol. 2010;9:10. doi: 10.1186/1475-2840-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Broedl UC, Lehrke M, Fleischer-Brielmaier E, et al. Genetic variants of adiponectin receptor 2 are associated with increased adiponectin levels and decreased triglyceride/VLDL levels in patients with metabolic syndrome. Cardiovasc Diabetol. 2006;5:11. doi: 10.1186/1475-2840-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dhillon PK, Penney KL, Schumacher F, et al. Common polymorphisms in the adiponectin and its receptor genes, adiponectin levels and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2618–27. doi: 10.1158/1055-9965.EPI-11-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohen SS, Gammon MD, North KE, et al. ADIPOQ, ADIPOR1, and ADIPOR2 polymorphisms in relation to serum adiponectin levels and BMI in black and white women. Obesity (Silver Spring) 2011;19:2053–62. doi: 10.1038/oby.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peters KE, Beilby J, Cadby G, et al. A comprehensive investigation of variants in genes encoding adiponectin (ADIPOQ) and its receptors (ADIPOR1/R2), and their association with serum adiponectin, type 2 diabetes, insulin resistance and the metabolic syndrome. BMC Med Genet. 2013;14:15. doi: 10.1186/1471-2350-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alobeidy BF, Li C, Alzobair AA, et al. The Association Study between Twenty One Polymorphisms in Seven Candidate Genes and Coronary Heart Diseases in Chinese Han Population. PLoS One. 2013;8:e66976. doi: 10.1371/journal.pone.0066976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Org E, Eyheramendy S, Juhanson P, et al. Genome-wide scan identifies CDH13 as a novel susceptibility locus contributing to blood pressure determination in two European populations. Hum. Mol. Genet. 2009;18:2288–96. doi: 10.1093/hmg/ddp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong C, Beecham A, Wang L, et al. Genetic loci for blood lipid levels identified by linkage and association analyses in Caribbean Hispanics. J. Lipid Res. 2011;52:1411–9. doi: 10.1194/jlr.P013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shia WC, Ku TH, Tsao YM, et al. Genetic copy number variants in myocardial infarction patients with hyperlipidemia. BMC Genomics. 2011;12(Suppl 3):S23. doi: 10.1186/1471-2164-12-S3-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chung CM, Lin TH, Chen JW, et al. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes. 2011;60:2417–23. doi: 10.2337/db10-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ling H, Waterworth DM, Stirnadel HA, et al. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS Study. Obesity (Silver Spring) 2009;17:737–44. doi: 10.1038/oby.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu Y, Li Y, Lange EM, et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum Mol Genet. 2010;19:4955–64. doi: 10.1093/hmg/ddq423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jee SH, Sull JW, Lee JE, et al. Adiponectin concentrations: a genome-wide association study. Am J Hum Genet. 2010;87:545–52. doi: 10.1016/j.ajhg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jo J, Sull JW, Park EJ, Jee SH. Effects of smoking and obesity on the association between CDH13 (rs3865188) and adiponectin among Korean men: the KARE study. Obesity (Silver Spring) 2012;20:1683–7. doi: 10.1038/oby.2011.128. [DOI] [PubMed] [Google Scholar]
- 105.Morisaki H, Yamanaka I, Iwai N, et al. CDH13 gene coding T-cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum Mutat. 2012;33:402–10. doi: 10.1002/humu.21652. [DOI] [PubMed] [Google Scholar]
- 106.Aslibekyan S, An P, Frazier-Wood AC, et al. Preliminary evidence of genetic determinants of adiponectin response to fenofibrate in the Genetics of Lipid Lowering Drugs and Diet Network. Nutr Metab Cardiovasc Dis. 2012 doi: 10.1016/j.numecd.2012.07.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]