Abstract
Transforming growth factor–β (TGFβ) is a multifunctional cytokine that plays diverse roles in physiologic processes as well as human disease, including cancer, heart disease, and fibrotic disorders. In the immune system, TGFβ regulates regulatory T cell (Treg) maturation and immune homeostasis. Although genetic manipulation of the TGFβ pathway modulates immune tolerance in mouse models, the contribution of this pathway to human allergic phenotypes is not well understood. We demonstrate that patients with Loeys-Dietz syndrome (LDS), an autosomal dominant disorder caused by mutations in the genes encoding receptor subunits for TGFβ, TGFBR1 and TGFBR2, are strongly predisposed to develop allergic disease, including asthma, food allergy, eczema, allergic rhinitis, and eosinophilic gastrointestinal disease. LDS patients exhibited elevated immunoglobulin E levels, eosinophil counts, and T helper 2 (TH2) cytokines in their plasma. They had an increased frequency of CD4+ T cells that expressed both Foxp3 and interleukin-13, but retained the ability to suppress effector T cell proliferation. TH2 cytokine–producing cells accumulated in cultures of naïve CD4+ T cells from LDS subjects, but not controls, after stimulation with TGFβ, suggesting that LDS mutations support TH2 skewing in naïve lymphocytes in a cell-autonomous manner. The monogenic nature of LDS demonstrates that altered TGFβ signaling can predispose to allergic phenotypes in humans and underscores a prominent role for TGFβ in directing immune responses to antigens present in the environment and foods. This paradigm may be relevant to nonsyndromic presentations of allergic disease and highlights the potential therapeutic benefit of strategies that inhibit TGFβ signaling.
INTRODUCTION
Allergic diseases, including asthma, eczema, allergic rhinitis, and food allergy, are causes of tremendous morbidity and appear to be rising in prevalence in most of the developed world. Food and aeroallergens have also been increasingly recognized as prominent factors in the pathogenesis of eosinophilic gastrointestinal diseases (EGIDs), especially eosinophilic esophagitis (EoE) (1, 2). Although all these disorders demonstrate strong familial associations, their complex inheritance has frustrated efforts to elucidate their genetic basis. The inflammation associated with allergic conditions is promoted by CD4+ T helper 2 (TH2) cells that secrete interleukin-4 (IL-4), IL-5, and IL-13; however, the molecular pathways regulating these responses remain to be fully elucidated. Both epidemiologic and genetic studies have purported an important role for transforming growth factor–β (TGFβ) in allergic disorders, although how this multipotential cytokine with both potent pro- and anti-inflammatory activities contributes to allergic disease is not clear (3-6). Although T cell–specific deletion of TGFβR2 in mice was found to result in lethal inflammation as a result of uncontrolled T cell activation and differentiation, TGFβ can also promote the differentiation of proinflammatory TH17 and TH9 cells (7-12). Most information regarding the role of TGFβ in the immune system has been derived from engineered mouse models where the function of TGFβ ligands, receptor subunits, or signaling effectors has been disrupted. Few human correlates of these models exist, leaving in question the physiologic relevance of the observed phenotypes and inferred mechanisms in people with allergic disease. Here, we evaluate the immunophenotype of patients with Loeys-Dietz syndrome (LDS), who harbor naturally occurring mutations in the receptor for TGFβ and therefore provide an opportunity to examine the immunologic consequences of altered TGFβ signaling in human disease (13).
LDS has previously been associated with altered cardiovascular, craniofacial, and skeletal development, consistent with the known importance of TGFβ in these systems. Here, we demonstrate that affected individuals have a high prevalence of multiple immunologic phenotypes, including asthma, food allergy, eczema, allergic rhinitis, and EGID. The monogenic nature of LDS suggests that dysregulated TGFβ signaling is sufficient to predispose to allergic phenotypes in humans, and underscores the prominent role TGFβ plays in directing immune responses to mucosal antigens, including those present in the environment and foods.
RESULTS
Immunologic phenotype of LDS
Among 58 LDS patients, the median age was 13.3 years [interquartile range (IQR), 12.8], and 27 of 58 (47%) were male (Table 1). Of the 58 patients, 14 (24%) and 44 (76%) had a heterozygous mutation in TGFBR1 and TGFBR2, respectively (Table 1). Specific mutations are listed in table S1. Thirty-one of the 58 (53%) participants reported an adverse reaction to food, and 23 of the 43 patients (53%) who had food allergen–specific testing were positive to ≥1 of the most common food allergens (median, 2; IQR, 0 to 5). Eighteen of 58 had a convincing history of an immediate reaction to a food (14 had a positive allergen-specific test to the same food; the other 4 never had testing to the suspect food), providing a conservative estimate of 31% for the prevalence of food allergy in LDS patients, compared to 6% of children and 2 to 4% of adults in the general population (Table 1 and table S2) (14, 15). The remaining 13 LDS patients who reported adverse food reactions did not meet our strict criteria for diagnosing food allergy. The most common food antigens were egg, milk, soy, peanut, and tree nuts (table S2). Eleven of 58 (19%) participants had been prescribed self-injectable epinephrine before their diagnosis with LDS.
Table 1.
Characteristics of LDS patients with mutations in TβR1 and TβR2. Number of subjects (%) for each category is reported, except for age, where median age in years (25th to 75th percentile) is listed. EGID, eosinophilic gastrointestinal disease, diagnosed by biopsy; EoE, eosinophilic infiltrate in the esophagus; EoG, eosinophilic infiltrate in the gastric mucosa; EoC, eosinophilic infiltrate in the colon.
| TβR1 | TβR2 | All | |
|---|---|---|---|
| Number of subjects | 14 (24) | 44 (76) | 58 |
| Male | 8 (57) | 19 (43) | 27 (47) |
| Age at enrollment (years) | 12.9 (9.8–22.2) | 13.6 (7.2–19.2) | 13.3 (7.3–20.1) |
| Food allergy | 4 (29) | 14 (32) | 18 (31) |
| Asthma | 7 (50) | 19 (43) | 26 (45) |
| Allergic rhinitis | 6 (43) | 22 (50) | 28 (48) |
| Eczema | 7 (50) | 15 (34) | 22 (38) |
| EGID | 2 (14) | 4 (9) | 6 (10) |
| EoE | 2 (14) | 4 (9) | 6 (10) |
| EoG | 2 (14) | 3 (7) | 5 (9) |
| EoC | 2 (14) | 2 (5) | 4 (7) |
Twenty-six of 58 (45%) respondents reported physician-diagnosed asthma compared to 8% of adults and 10 to 13% of children in the general population (Table 1) (16-18). Sixteen of 58 (28%) currently required asthma medication (12 of 58 daily inhaled corticosteroid alone or in combination with a long-acting β-agonist, 11 of 58 daily leukotriene antagonist, and 16 of 58 intermittent short-acting β-agonist). Twenty-eight of 58 (48%) had been diagnosed with allergic rhinitis, which affects between 10 and 40% of children and between 8 and 30% of adults in the United States (19-22). Eczema was diagnosed in 22 of 58 (38%) LDS subjects (Table 1) compared to 8 to 17% of children and 8 to 11% of adults in the general population (23-25). Thirty of 41 (64%) were sensitized to ≥1 of the seven aeroallergens tested (median, 2; IQR, 0.75 to 5). Thirty-eight of 58 (66%) reported gastrointestinal complaints (29 of 58 with poor growth, 6 of 58 with repetitive vomiting, 15 of 58 with chronic abdominal pain, 6 of 58 with dysphagia) that were potentially consistent with EGID. Ten of these patients had gastrointestinal biopsies performed, and of these, 6 (60%) showed overt histologic evidence of EoE (Fig. 1A and Table 1). The prevalence of EoE in the general population is about 0.05% (26, 27). Of the six LDS patients with biopsy-confirmed EoE, five were found to have eosinophilic gastritis (EoG) and four had eosinophilic colitis (EoC) (Fig. 1, B and C, and Table 1). Five of six individuals with EGID demonstrated clinical improvement with food avoidance diets. Children with LDS had body mass index (BMI) z scores significantly below normal, and the BMI z scores of LDS children with food allergy were significantly lower than those of LDS children without food allergy (fig. S1).
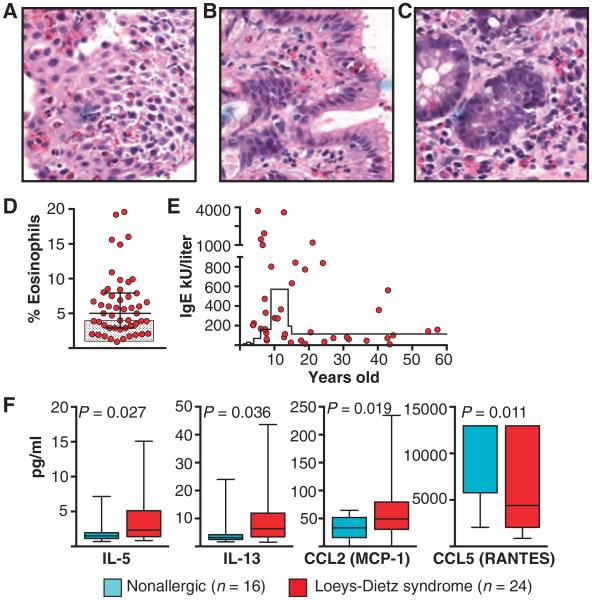
Fig. 1. Evidence for allergic predilection in LDS patients.
(A to C) Biopsies stained with hematoxylin and eosin demonstrating an eosinophilic infiltrate in the esophagus (A), stomach (B), and colon (C) of a child with LDS. Magnification, ×40. (D) Percentage of eosinophils in the peripheral blood of LDS patients (n = 50). Levels were significantly increased (P = 0.009) compared to the norm (shaded box) by Wilcoxon test. Line and whiskers indicate mean and SD, respectively. (E) Total serum levels of IgE (kU/liter) from LDS patients versus age (n = 41). Levels were elevated (P = 0.016; Student’s t test, two-tailed) above the 95% confidence interval for age as indicated by the solid line. Each point represents an individual patient in (D) and (E). (F) Levels of IL-5, IL-13, CCL2 (MCP-1), and CCL5 (RANTES; pg/ml) in plasma from patients with LDS (n = 24) and age-matched nonallergic controls (n = 16). Significant P values are indicated; comparisons were done by Wilcoxon test.
LDS patients also had significantly elevated peripheral eosinophil counts and total immunoglobulin E (IgE) levels (Fig. 1, D and E). Levels of IgG, IgA, and IgM were within the normal range, although IgG levels clustered at the upper end of normal and IgM levels at the lower limit (fig. S2). Total white blood cell counts were normal (7087 ± 2589/mm3). We found statistically higher levels of the TH2 cytokines IL-5 and IL-13 in plasma from LDS patients compared to unaffected controls, as well as CCL2 (MCP-1), a chemokine important in recruiting inflammatory cells and promoting degranulation of mast cells and basophils (Fig. 1F). Serum levels of CCL5 (RANTES), a chemokine known to be down-regulated by TGFβ, were lower (Fig. 1F) (28). Cytokine profiles from LDS subjects were specific for a TH2-dominated disorder because no differences in expression levels of 21 other cytokines were detected (table S3).
Regulatory T cell development in LDS
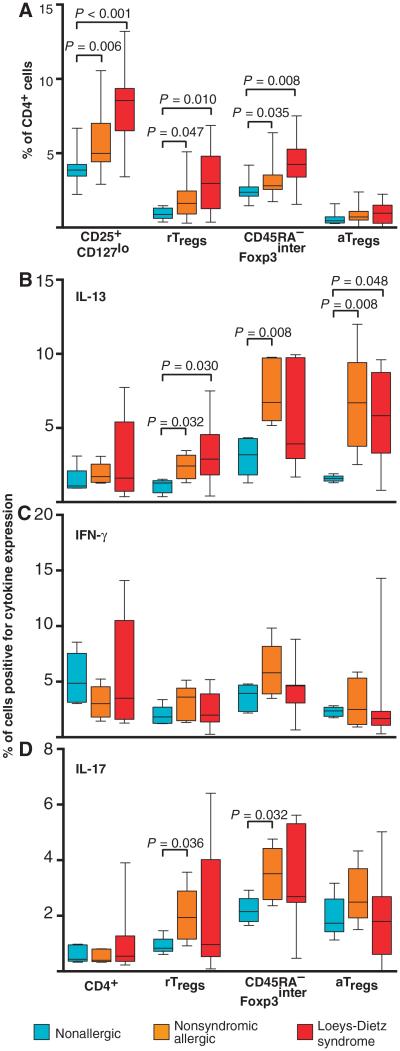
The tolerogenic functions of TGFβ are thought to be executed, at least in part, through its ability to promote the development and function of regulatory T cells (Tregs). Human Tregs were recently reported to consist of phenotypically and functionally distinct subpopulations, on the basis of their expression of CD45RA and the level of expression of CD25/Foxp3 (29). The three subpopulations that comprise the total Treg population (CD4+CD25+CD127lo cells) include resting Tregs (rTregs) (CD45RA+CD25interFoxp3inter), activated Tregs (aTregs) (CD45RA−CD25highFoxp3high), and a CD45RA−CD25interFoxp3inter group. The number of total Tregs in the peripheral blood of LDS patients was significantly elevated compared to unaffected controls (8.2 ± 1.6% in LDS and 5.8 ± 2.0% in controls; Fig. 2A), whereas no difference in the frequency of total CD4+ lymphocytes was evident (41.5 ± 9.0% in LDS and 40.2 ± 6.1% in controls). Further analysis revealed increased Tregs expressing intermediate levels of Foxp3 (Foxp3inter), but no difference in the frequency of aTregs (Fig. 2A) (29). Surprisingly, a significantly increased percentage of LDS rTregs and aTregs, which have previously been shown to secrete little cytokine (29), produced the TH2 cytokine IL-13 compared to nonallergic controls (Fig. 2B). No difference in expression of IL-17 or interferon-γ (IFN-γ) was evident (Fig. 2, C and D), but IL-10 levels were higher in LDS rTregs compared to nonallergic controls (fig. S3). Children with nonsyndromic allergic disease also demonstrated an increased frequency of rTregs and CD45RA−Foxp3inter Tregs, as well as Foxp3+ cells that produced IL-13 (Fig. 2, A and B). A greater frequency of Foxp3inter Tregs in allergic children also produced IL-17, but not IFN-γ (Fig. 2, C and D). Despite their propensity to produce TH2 cytokines, Tregs from LDS patients expressed normal levels of GATA3 and Foxp3, both of which can regulate effector cytokine expression by Foxp3+ cells (fig. S4) (30, 31).
Fig. 2. Frequency and function of Foxp3+ cells in patients with LDS and nonsyndromic allergic disease.
(A) Number of total Tregs (CD4+CD25+CD127lo) in individuals with LDS (n = 16) and nonsyndromic allergic disease (n = 26) compared to age-matched nonallergic controls (n = 8). Further analysis revealed increases in rTregs and CD45RA−Foxp3inter cells, but no difference in the frequency of aTregs, in LDS (n = 16) and nonsyndromic allergic subjects (n = 26) compared to nonallergic controls (n = 8). (B to D) Percentage of CD4+ lymphocytes as well as each Treg subset that expresses the cytokines IL-13 (B), IFN-γ (C), and IL-17 (D) in LDS patients (n = 7), children with non-syndromic allergic disease (n = 5), and nonallergic controls (n = 5). Significant P values are indicated; all comparisons were done by Wilcoxon test.
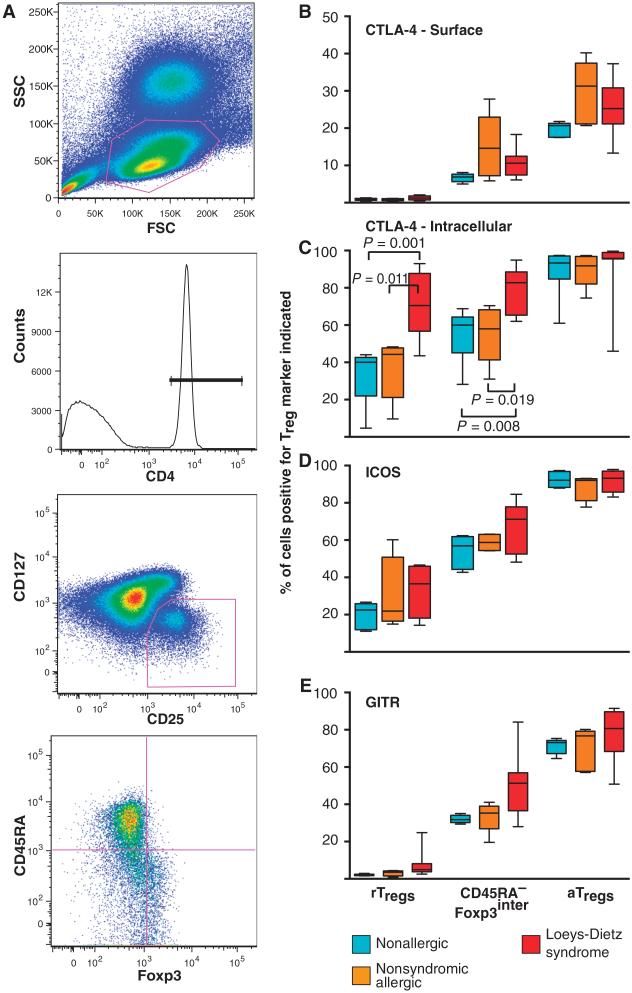
Fig. 4. Expression of Treg markers by Foxp3+ cells from patients with LDS, nonsyndromic allergic disease, and nonallergic controls.
(A) Gating scheme used to identify the three distinct subsets of Foxp3+ cells. Peripheral blood mononuclear cells (PBMCs) from LDS patients (n = 8), age-matched subjects with nonsyndromic allergic disease (n = 5), and nonallergic controls (n = 5) were stained with CD4, CD25, and CD127 antibodies. Tregs were defined as CD25+CD127lo cells after first gating on lymphocytes [based on forward scatter (FSC) and side scatter (SSC)] that were CD4+. CD4+CD25+CD127lo cells were then divided into rTregs, CD45RA−Foxp3inter Tregs, and aTregs based on their expression of CD45RA and Foxp3. (B to E) Percentage of each Treg subset that expresses the indicated Treg marker (CTLA-4, ICOS, and GITR). Boxes define the 25 and 75% quartiles, divisions within the boxes the medians, and whiskers the range. No difference in expression of any Treg marker was found except that a significantly greater percentage of rTregs and CD45RA−Foxp3inter Tregs from LDS patients expressed intracellular CTLA-4 compared to individuals with nonsyndromic allergic disease and nonallergic controls. Significant P values are indicated; comparison by Wilcoxon test.
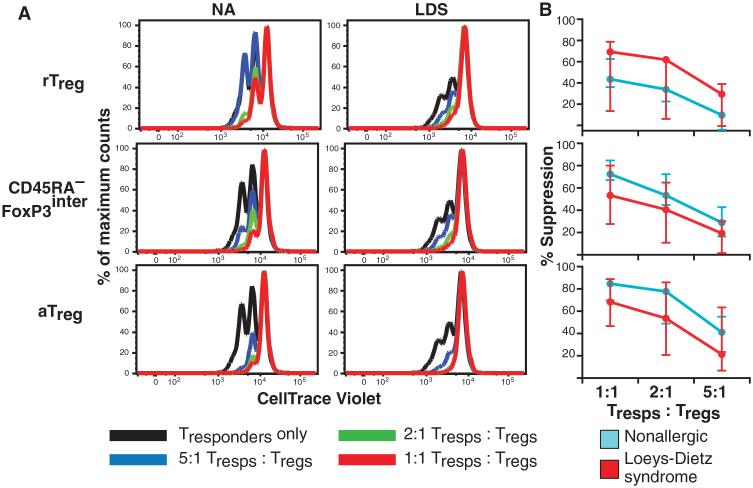
To ascertain whether Tregs from LDS patients retain the ability to suppress effector T cell proliferation, purified Tregs were cultured at various ratios with responder T cells in the presence of a T cell receptor cross-linking stimulus. All three populations of Tregs from LDS patients effectively suppressed effector T cell proliferation (Fig. 3). We found no difference in expression of Helios, which has been reported to mark a population of Tregs with increased regulatory potential, by LDS Tregs compared to controls (fig. S5) (32).
Fig. 3. Suppressive activity of LDS Tregs.
(A and B) The three subsets of Tregs (rTregs, CD45RA−Foxp3inter Tregs, and aTregs) were purified and cocultured at various ratios (1:1, 2:1, and 5:1) with CD4+ effector T cells that had been labeled with CellTrace Violet. Cultures were stimulated with anti-CD3 and irradiated antigen-presenting cells, and dilution of the dye, a marker of proliferation, was assessed 4 days later. (A) Results from a representative experiment (n = 3). (B) Percent suppression of effector T cell proliferation at the different ratios (median and range are indicated). LDS (n = 3) and nonallergic (NA) control (n = 3) Tregs effectively suppress effector T cell proliferation.
The three subclasses of Tregs were also evaluated for expression of several Treg markers, including CTLA-4 (cytotoxic T lymphocyte antigen–4), GITR [glucocorticoid-induced tumor necrosis factor receptor (TNFR)–related protein], and ICOS (inducible T cell costimulator), which may contribute to the immunosuppressive capacities of these cells. Increased levels of intracellular CTLA-4 were seen in rTregs and CD45RA−Foxp3inter Tregs from LDS patients compared to nonallergic and nonsyndromic allergic controls (Fig. 4). No differences were evident between allergic patients without LDS and controls (Fig. 4). Expression of all these markers was highest in aTregs and lowest in rTregs, as previously demonstrated (Fig. 4) (29).
Skewing potential of naïve CD4+ lymphocytes in LDS
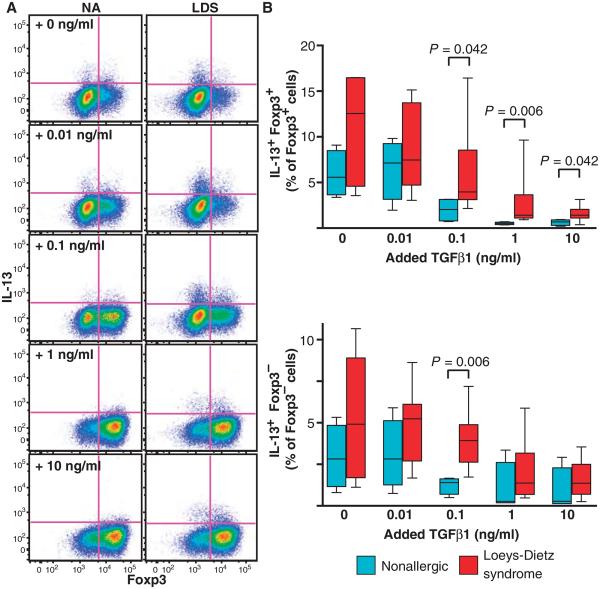
Tregs have been reported to exhibit phenotypic and functional plasticity under inflammatory conditions; therefore, it remained unclear whether the changes in Treg function we observed in LDS were an indirect consequence of the general allergic milieu in these patients, or whether mutations in the TGFβ receptor directly affect the differentiation of naïve T cells. To assess whether LDS mutations influence the propensity for naïve lymphocytes to acquire T helper effector functions in response to TGFβ, we purified and cultured CD4+CD45RA+CD45RO−CD25− naïve T cells in the presence of various doses of recombinant TGFβ1 for 4 days. Naïve T cells from both LDS patients and controls demonstrated an equal propensity to up-regulate expression of Foxp3 in response to TGFβ in a dose-dependent manner (fig. S6). Although the frequency of both IL-13+ Foxp3+ and IL-13+ Foxp3− T lymphocytes decreased in both control and LDS cultures as the dose of added TGFβ1 increased, there was a higher percentage of IL-13+ cells in LDS samples exposed to TGFβ (Fig. 5). No difference in IL-17 or IFN-γ expression was evident (fig. S7). The addition of a TGFβ-neutralizing antibody or a TGFβ receptor kinase inhibitor to the cultures suppressed the differentiation of Foxp3+ IL-13+ cells from naïve T lymphocytes in both LDS patients and controls, suggesting that TGFβ contributes to their development (fig. S8). No difference in IL-13 expression by naïve lymphocytes from LDS patients and controls was observed before culture (fig. S9).
Fig. 5. Skewing potential of naïve CD4+ lymphocytes from LDS subjects and nonallergic controls after TGFβ stimulation.
(A) Representative dot plot depicting expression of Foxp3 and IL-13 in LDS (n = 7) and nonallergic (NA; n = 4) CD4+ lymphocytes 4 days after naïve CD4+ T cells were cultured with increasing doses of recombinant TGFβ1 as indicated. (B) Percentage of IL-13–expressing Foxp3+ and Foxp3− cells in cultures from LDS patients (n = 7) compared to nonallergic controls (n = 4) after treatment with the indicated doses of recombinant TGFβ1. Significant P values are indicated; comparisons by Mann-Whitney test.
Dysregulated TGFβ signaling in LDS
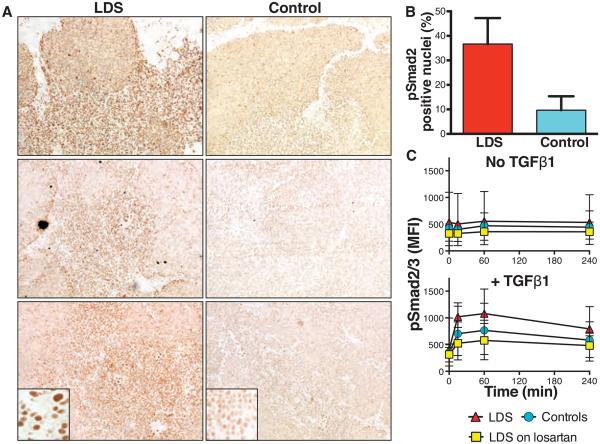
The best-characterized TGFβ signaling pathway involves phosphorylation and heterodimerization of type I and II TGFβ receptors, resulting in phosphorylation and nuclear translocation of Smad2 and Smad3 proteins. LDS patients showed excessive nuclear accumulation of phosphorylated Smad2 (pSmad2) in thymic tissue, most prominent in the medulla, when compared to age-matched controls (Fig. 6, A and B). Additionally, CD4+ lymphocytes in the peripheral blood of LDS patients demonstrated increased expression of pSmad2/3 after stimulation with TGFβ1 when compared to unaffected controls (Fig. 6C). However, no significant difference in expression of pSmad2/3 was found between LDS patients treated with the angiotensin II receptor blocker losartan, which has been shown to attenuate TGFβ signaling in other disorders (33-35), compared to controls (Fig. 6C). These results point to enhanced, rather than repressed, TGFβ signaling in the immune system of LDS patients.
Fig. 6. Status of TGFβ signaling in the immune system of LDS patients.
(A) Immunostaining for pSmad2 in thymic tissue from three patients with LDS and age-matched controls (n = 3) with increased intensity of nuclear pSmad2 in LDS patients. (B) Percentage of nuclei that stained positively for pSmad2 in LDS versus control thymi from (A) as evaluated by a blinded observer. P < 0.001, Fisher’s exact test. (C) Expression of pSmad2/3 before (No TGFβ1) and at various time points after (+ TGFβ1) treatment of whole blood with recombinant TGFβ1 from patients with LDS (n = 4) and unaffected relatives (n = 4; Controls), and LDS patients receiving therapeutic doses of losartan (n = 4; LDS on losartan). Mean fluorescence intensities (MFI) of pSmad2/3 staining in gated CD4+ lymphocytes are plotted. Levels of pSmad2/3 were increased (P = 0.050; comparison by longitudinal analysis) after stimulation in LDS versus controls and LDS on losartan. Medians and IQRs (whiskers) are shown.
DISCUSSION
Here, we have demonstrated that mutations in either gene encoding TGFβ receptor subunits are sufficient to predispose to allergic phenotypes in humans. Although allergic diseases are known to have strong familial associations, our understanding of how genetic variants relate to specific dysfunction at the cellular level is often lacking (36-38). The alterations in TGFβ signaling that lead to TH2 disease in LDS are complex. Most mutations in LDS patients are missense mutations that are predicted to disrupt the kinase activity of the receptors, and, in some cases, mutant receptors have been shown to lack the ability to propagate canonical TGFβ signaling (through pSmad2/3) when expressed in cells naïve for the corresponding receptor subunit (39-41). However, we found paradoxically enhanced TGFβ signaling in thymic tissue and CD4+ lymphocytes from LDS patients, similar to what has been seen in the aorta (13). Decreased serum levels of CCL5 (RANTES) and increased expression of CTLA-4 by Tregs are consistent with excessive TGFβ signaling in LDS, because expression of these molecules is down- and up-regulated, respectively, by TGFβ (28, 42). Collectively, these data suggest that an increase in TGFβ signaling contributes to LDS immunologic pheno-types, but we cannot exclude a role for impaired TGFβ signaling in a critical developmental stage and/or cell type–specific manner. These issues will be best addressed using mouse models of LDS.
The increased frequency of peripheral Foxp3+ Tregs in LDS patients is also an expected consequence of increased TGFβ signaling, but counterintuitive given the loss of tolerance. Although LDS Tregs expressed normal levels of typical Treg markers including Helios and IL-10 and retained their ability to suppress effector T cell proliferation, they produced the TH2 cytokine IL-13, a finding neither described nor observed in nonallergic controls but recapitulated by Tregs from children with nonsyndromic allergic disease (29). No alterations in TH1 or TH17 cytokine production were observed in LDS Tregs. Previous studies have suggested that Tregs may have functional and phenotypic plasticity in certain disease states, including multiple sclerosis where Tregs from affected individuals have been found to express TH1 cytokines (43). Although additional studies are necessary to evaluate the role for TH2 cytokine–producing Foxp3+ cells in the pathogenesis of allergic disease, our data provide evidence for Treg plasticity in these disorders.
The primary defect in TGFβ signaling caused by LDS mutations appears to confer lymphocytes with an intrinsic propensity to acquire and/or maintain TH2 effector functions when stimulated with TGFβ. Whether this relates to loss of a suppressive effect of TGFβ on naïve T lymphocytes in LDS, or an off-setting positive influence in a subset of cells, remains to be determined. No difference in acquisition of TH1 or TH17 effector function was observed, suggesting that LDS mutations specifically influence TH2 development. Although LDS mutations may promote TH2 immunity through multiple mechanisms, potentially including effector cytokine production by cell types other than T cells, these data suggest that cell-autonomous changes in lymphocyte responses to TGFβ contribute to this phenotype.
Although other Mendelian disorders with a predisposition for allergic disease have been described, these disorders prominently feature overt evidence of immunodeficiency that is not seen in LDS (44, 45). The prevalence of allergic disease in LDS patients is significantly greater than that observed in the general population (14-17, 26, 27, 46, 47). Although our study was limited by our inability to directly challenge all patients with suspected food allergy or to do pulmonary function testing to confirm asthma diagnoses, the preponderance of evidence, including increased levels of total and allergen-specific IgE, serum and Treg-produced TH2 cytokines, peripheral eosinophilia, and propensity for lymphocyte skewing to TH2 effector phenotypes in LDS patients, strongly suggests that this disease is dominated by a TH2 immune response. Although EoE can be difficult to discern from gastroesophageal reflux disease both clinically and pathologically, the esophageal eosinophilic inflammation in most LDS patients failed to respond to treatment with proton pump inhibitors but markedly improved with food avoidance diets. Furthermore, nearly all LDS patients had eosinophilic inflammation in other parts of the gastrointestinal tract beyond the esophagus, suggesting that EGID is a bona fide feature of this disease. The pathogenesis of EGIDs is not completely understood, but growing evidence indicates that aeroallergens and food allergens play a central role (48). Recent studies have also emphasized a prominent role for TH2 cytokines in the development of EGID, and children and adults with EoE often have other atopic diseases as well (49-51). The increased prevalence of EGID in LDS patients is therefore consistent with their propensity to develop other TH2-mediated phenotypes.
The immunologic phenotype in LDS provides valuable insight into TGFβ’s intricate role in directing immune responses to mucosal antigens and suggests that alterations in TGFβ signaling are sufficient to promote TH2 immunity and allergic disease in humans. Our findings in LDS further demonstrate that mutations in a single gene can predispose to the complex phenotypes associated with allergic disorders, and therefore would suggest that genes involved in the TGFβ signaling pathway should be prioritized when evaluating data from large genome-wide association studies aimed at elucidating the genetic contributions to allergic disease. Finally, identification of a single pathway that can strongly predispose to allergic disease may have tremendous therapeutic implications. Although excessive TGFβ signaling is already known to be pathogenic in remodeling of the lung in asthmatics, the esophagus of patients with EoE, and the skin of patients with eczema, LDS provides the first direct evidence that altered TGFβ signaling is sufficient to incite these disorders (52-55). This observation may facilitate the development of therapeutic strategies for these common conditions. Losartan is a U.S. Food and Drug Administration–approved drug, which has known efficacy in reducing excessive TGFβ signaling in several disorders (33, 34). Studies in mice with Marfan syndrome, an aortic aneurysm syndrome closely related mechanistically and phenotypically to LDS, have revealed a remarkable ability of this drug to prevent the major cardiovascular complications associated with this disease (35). Whether losartan can modify allergic disease remains to be tested, but our finding that losartan mitigates TGFβ signaling alterations in lymphocytes of LDS patients suggests that this or related treatment approaches may hold promise.
MATERIALS AND METHODS
Study design and patients
All patients with confirmed mutations in TβRI or TβRII, encoded by TGFBR1 or TGFBR2, respectively, who were seen in the Johns Hopkins Connective Tissue Disorders clinic through 31 December 2008, living in the United States, not deceased, and able to be contacted were asked to participate in the study. Seventy-one patients met criteria, and 65 agreed to participate. Detailed questionnaires regarding allergic and gastrointestinal disease, including biopsy reports, skin and/or allergen-specific in vitro IgE testing, types of food reactions, medication use, and type and duration of gastrointestinal and allergic symptoms, were sent to each participant. Questionnaires were returned from 58 of 71 subjects (82%). Information was also confirmed and/or retrieved by review of the patients’ medical records. Cases of asthma, eczema, and allergic rhinitis were identified by self-report of doctor-diagnosed disease, review of medical records, and/or clinical examination by the authors. The diagnosis of food allergy was based on a convincing history of acute reaction after exposure to the implicated food. In all but four cases (where no testing to the suspected food had been performed), subjects also had an allergen-specific IgE level to the implicated food of >0.35 kUA/liter (ImmunoCAP, Phadia) or positive skin prick test administered by a board-certified allergist. Nonallergic controls in this study had no clinical features of LDS and no history of symptoms suggestive of allergic disease. Children with nonsyndromic food allergy were recruited from the Johns Hopkins Pediatric Allergy Clinic. Nine of these 26 children had also been diagnosed with EoE based on esophageal biopsy findings, 16 with asthma, 18 with eczema, and 18 with allergic rhinitis. This study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Laboratory data
Complete blood counts and quantitative Ig levels were performed for 50 of 58 (86%) subjects by a commercial laboratory. Allergen-specific IgE levels to the seven most common food (egg white, milk, peanut, soybean, sesame, wheat, and codfish) and aeroallergens (alternaria, Dermatophagoides farinae, timothy grass, ragweed, dog, cat, and white oak) were measured in 41 of 58 (71%).
Measurement of plasma cytokines
Plasma cytokines were measured with multiplex bead immunoassay (human 27-plex panel; Bio-Plex, Bio-Rad) according to the manufacturer’s directions. Limits of detection for the Bio-Rad assay are as follows: IL-1β, 0.6 pg/ml;IL-1Ra, 5.5 pg/ml;IL-2, 1.6 pg/ml;IL-4, 0.7 pg/ml; IL-5, 0.6 pg/ml; IL-6, 2.6 pg/ml; IL-7, 1.1 pg/ml; IL-8, 1.0 pg/ml; IL-9, 2.5 pg/ml; IL-10, 0.3 pg/ml; IL-12 (p70), 3.5 pg/ml; IL-13, 0.7 pg/ml; IL-15, 2.4 pg/ml; IL-17, 3.3 pg/ml; CCL11 (eotaxin), 2.5 pg/ml; bFGF (basic fibroblast growth factor), 1.9 pg/ml; G-CSF (granulocyte colony-stimulating factor), 1.7 pg/ml; GM-CSF (granulocyte-macrophage colony-stimulating factor), 2.2 pg/ml; IFN-γ, 6.4 pg/ml; CXCL10 (IP-10), 6.1 pg/ml; CCL2 (MCP-1), 1.1 pg/ml; CCL3 (MIP-1α), 1.6 pg/ml; CCL4 (MIP-1β), 2.4 pg/ml; PDGF-BB (platelet-derived growth factor–BB), 2.9 pg/ml; CCL5 (RANTES), 1.8 pg/ml;TNF-α, 6.0 pg/ml; and VEGF (vascular endothelial growth factor), 3.1 pg/ml. Concentrations of CCL3 (MIP-1α) and CCL4 (MIP-1β) for both LDS and nonallergic subjects were above the upper limit of the assay and therefore were not included in our analysis.
Isolation of PBMCs
Blood was collected in EDTA tubes. Blood for analysis of Treg subsets was subjected to double Percoll (density, 61 and 55%; GE Healthcare) centrifugation and fixed in 4% paraformaldehyde and frozen at −80°C (56). Blood for analysis of surface marker expression, cytokine production, and isolation of naïve T cells was processed by Ficoll (GE Healthcare) or single Percoll gradients.
Flow cytometry
To determine the percentage of CD4+ cells present as Treg subsets, fixed cells were stained with CD4-PerCP, CD25-APC (allophycocyanin), CD127-FITC (fluorescein isothiocyanate), and CD45RA–PE (phycoerythrin)–Cy7 (all BD Biosciences), and subsets were identified as previously described (29). Expression of Treg markers was determined by surface staining of fresh PBMCs using the cocktail of antibodies described above to define Tregs along with GITR-PE, ICOS-PE, CTLA-4–PE (all from BD Biosciences), or Helios-PE (BioLegend), followed by intracellular staining with Foxp3 (BioLegend, clone 259D) and/or GATA3 (eBioscience) using the Foxp3 Fixation/Permeabilization kit (eBioscience) according to the manufacturer’s directions. Treg subsets were identified by first gating on CD4+CD25highCD127lo cells. rTregs were defined as CD45RA+ with intermediate expression of Foxp3, CD45RA−Foxp3inter Tregs as CD45RA− with intermediate expression of Foxp3, and aTregs as CD45RA− with high expression of Foxp3. Intracellular cytokine production was detected by culturing PBMCs in AIM V medium (Invitrogen) with phorbol 12-myristate 13-acetate (25 ng/ml) (Sigma-Aldrich) and ionomycin (250 ng/ml) (Sigma-Aldrich) in the presence of brefeldin (3 μg/ml) (eBioscience) for 4 to 4.5 hours. Cells were then treated with IC fixation buffer (eBioscience) according to the manufacturer’s instructions and then stained with CD4-PerCP, CD25-APC, CD127-PE, CD45RA-PE-Cy7, and either IL-13–FITC (eBioscience), IFN-γ–FITC (BD Biosciences), IL-10–FITC (Caltag), or IL-17–FITC (BD Biosciences).
For Treg suppression assays, PBMCs were isolated from LDS patients or nonallergic controls and enriched for CD4+ T cells (Miltenyi) using negative selection, and then stained with CD4-APC, CD25-PE, CD45RA-PE-Cy7 (all from BD Pharmingen), and CD127-FITC (eBioscience). Cells retained on the column were retrieved and irradiated at 32 Gy for use as antigen-presenting cells. Tregs were first identified as being CD4+CD25high and CD127lo. The three subpopulations of Tregs were then sorted on the basis of their expression of CD45RA and CD25. T responder cells were identified as CD4+CD45RA+CD25−CD127+ and were labeled with CellTrace Violet according to the manufacturer’s instructions (Life Technologies). Labeled T responder cells (5 × 104) were cultured with 5 × 104 (1:1), 2.5 × 104 (2:1), or 1 × 104 (5:1) of each Treg subset in duplicate in 96-well round-bottom plates in the presence of 1 × 105 irradiated antigen-presenting cells and soluble anti-human CD3 (0.7 mg/ml) (eBioscience) for 4 days. Cells were subsequently stained with 7-aminoactinomycin D (BD Biosciences) and CD4 APC to assess for dilution of the CellTrace Violet dye in viable T responder cells.
To assess the skewing potential of naïve CD4+ T cells in LDS patients and nonallergic controls, enriched CD4+ T cells were prepared as described above. These cells were stained with CD4-APC, CD25-PE, CD45RA-PE-Cy7 (all from BD Biosciences), and CD45ROFITC (eBioscience) and then flow-sorted; naïve cells, defined as CD4+CD25−CD45RA+CD45RO−, were collected. The naïve cells were cultured at about 1 × 105 cells/ml in 200 ml of Iscove’s modified Dulbecco’s medium (Life Technologies) containing 5% fetal bovine serum in 96-well flat-bottom plates that had been coated with anti-human CD3ε (10 μg/ml) (eBioscience). Cultures were stimulated with anti-human CD28 monoclonal antibody (1 μg/ml), along with recombinant TGFβ1 (0, 0.01, 0.1, 1, or 10 ng/ml) (R&D Systems) for 4 days. In some experiments, TGFβ-neutralizing antibody (100 μg/ml) (Genzyme) or 5 μM SD-208 kinase inhibitor (Tocris) was added to the cultures. Cultures were then stimulated to induce cytokine expression with 1× Stimulation Cocktail (eBioscience) for 4.25 hours, and subsequently stained with CD4-APC (BD Biosciences), Foxp3–Pacific Blue (BioLegend), IL-13–PE (BD Biosciences), IFN-γ–AF700 (BioLegend), and IL-17–PerCP–Cy5.5 (eBioscience) using the Transcription Factor Buffer kit (BD Biosciences) according to the manufacturer’s instructions. Lymphocytes were analyzed by first gating on CD4+ cells and then evaluating expression of Foxp3 and each cytokine listed above on the CD4+ population.
For phosflow experiments, whole blood was collected into EDTA tubes from patients with LDS and unaffected relatives. Blood was aliquoted and incubated in the presence or absence of recombinant human TGFβ1 (5 ng/ml) (R&D Systems) at 37°C for 0, 15, 60, and 240 min. Blood was then processed with the Perm Buffer III Phosflow kit (BD Biosciences) per the manufacturer’s instructions. Cells were stained with CD4-PerCP (BD Biosciences) and unlabeled pSmad2/3 (Cell Signaling) followed by staining with anti-rabbit IgG AF488 (Cell Signaling).
Data were acquired with an LSRII (BD Biosciences) and analyzed with FACSDiva (6.5, BD Biosciences) and FlowJo (9.3.1, Tree Star Inc.) software. Cells were sorted with MoFlo (Beckman Coulter). Appropriate isotype and/or secondary antibody controls were included in all experiments.
Immunohistochemistry
Thymic tissue from LDS patients obtained at the time of aortic surgery was stained with antibodies directed against pSmad2 (13). Control samples were obtained from age-matched individuals without LDS, another connective tissue disorder, or primary syndrome whose thymus had been removed during cardiac surgery. The number of positively stained nuclei in three high-powered fields per slide was scored by a blinded observer. Gastrointestinal biopsies were obtained for clinically indicated reasons and processed per clinical standards of the institution at which they were obtained.
Statistics
Statistics were performed with Prism 5.0 (GraphPad Software) or Stata 12.1 (Stata Corp.). Evaluation of pSmad2/3 was by longitudinal analysis fitting to a linear plus quadratic term. For analysis of Ig levels, the 95th percentile for Ig concentration for age was subtracted from the patient’s Ig concentration (57, 58). Student’s t test was then performed and compared to a value of zero. Statistical analysis was similarly performed to evaluate for significantly low levels of Ig, where the 5th percentile for age was used as the reference. The proportion of nuclei that stained positively for pSmad by immunohistochemistry was compared by Fisher’s exact test. All other comparisons were by Mann-Whitney/Wilcoxon test. The box in box plots defines the 25 and 75% quartiles, the division within the box defines the median, and the whiskers define the range.
Supplementary Material
Acknowledgments
We thank G. Dhillon for her assistance with data entry, as well as R. L. Blosser, A. Tam, T. L. Niles, and H. Zhang for their assistance with cell sorting and flow cytometry. We are also indebted to all the patients and their families for their contributions to this research.
Funding: This work was supported by an NIH K23 Mentored Research Development Award (K23AI091869), an ARTrust Faculty Development Award, a Johns Hopkins University Clinician Scientist Award (to P.A.F.-G.), an NIH Training Grant (T32 DK07632), and a George Ferry Young Investigator Development Award (North American Society for Pediatric Gastroenterology, Hepatology and Nutrition; A.L.G.), as well as research grants from the NIH, the National Marfan Foundation, and the Howard Hughes Medical Institute (to H.C.D.).
Footnotes
Author contributions: P.A.F.-G. and A.L.G. designed the experiments, performed the experiments, analyzed and interpreted the data, clinically evaluated the patients, and wrote the paper. G.O. and M.O.-H. clinically evaluated the patients. K.C., L.M., and M.K.H. performed the experiments. R.A.W. interpreted the data, wrote the paper, and clinically evaluated the patients. H.C.D. designed the experiments, interpreted the data, clinically evaluated the patients, and wrote the paper.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am. J. Gastroenterol. 2003;98:777–782. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 2.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J. Allergy Clin. Immunol. 2002;109:363–368. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 3.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO. GABRIEL Consortium, A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, Romieu I, Shi M, Hancock DB, Li H, Sienra-Monge JJ, Chiu GY, Xu H, del Rio-Navarro BE, London SJ. Evaluation of candidate genes in a genome-wide association study of childhood asthma in Mexicans. J. Allergy Clin. Immunol. 2010;125:321–327.e13. doi: 10.1016/j.jaci.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, Bastian JF, Broide DH. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response totopical corticosteroids. Allergy. 2010;65:109–116. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oddy WH, Halonen M, Martinez FD, Lohman IC, Stern DA, Kurzius-Spencer M, Guerra S, Wright AL. TGF-β in human milk is associated with wheeze in infancy. J. Allergy Clin. Immunol. 2003;112:723–728. doi: 10.1016/s0091-6749(03)01941-9. [DOI] [PubMed] [Google Scholar]
- 7.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 9.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 10.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-β-induced Foxp3+T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MO, Flavell RA. TGF-β: A master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 14.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987;79:683–688. [PubMed] [Google Scholar]
- 15.Sampson HA. Update on food allergy. J. Allergy Clin. Immunol. 2004;113:805–819. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC. National prevalence and risk factors for food allergy and relationship to asthma: Results from the National Health and Nutrition Examination Survey 2005-2006. J. Allergy Clin. Immunol. 2010;126:798–806.e14. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trends in Asthma Morbidity and Mortality. (American Lung Association, Epidemiology and Statistics Unit, Research and Program Services; Washington, DC: 2011. [Google Scholar]
- 18.Vital Statistics. (Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 19.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2010. Vital Health Stat. 2011;10(250):1–80. [PubMed] [Google Scholar]
- 20.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat. 2010;10(249):1–207. [PubMed] [Google Scholar]
- 21.Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J. Allergy Clin. Immunol. 2010;126:778–783.e776. doi: 10.1016/j.jaci.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 22.Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, Simmons AL, Wingertzahn MA, Boyle JM. Burden of allergic rhinitis: Results from the Pediatric Allergies in America survey. J. Allergy Clin. Immunol. 2009;124:S43–S70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: Data from the 2003 National Survey of Children’s Health. J. Invest. Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanifin JM, Reed ML. Eczema Prevalence and Impact Working Group, A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82–91. doi: 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- 25.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J. Am. Acad. Dermatol. 2000;43:649–655. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 26.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N. Engl. J. Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 27.Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, Bonis PA. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J. Pediatr. Gastroenterol. Nutr. 2011;52:300–306. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang WC, Yen FC, Shie FS, Pan CM, Shiao YJ, Yang CN, Huang FL, Sung YJ, Tsay HJ. TGF-β1 blockade of microglial chemotaxis toward Aβ aggregates involves SMAD signaling and down-regulation of CCL5. J. Neuroinflammation. 2010;7(28) doi: 10.1186/1742-2094-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Souabni A, Flavell RA, Wan YY. An intrinsic mechanism predisposes Foxp3-expressing regulatory T cells to Th2 conversion in vivo. J. Immunol. 2010;185:5983–5992. doi: 10.4049/jimmunol.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oestreich KJ, Weinmann AS. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nat. Rev. Immunol. 2012;12:799–804. doi: 10.1038/nri3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC, Tam AJ, Getnet D, Drake CG. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavoie P, Robitaille G, Agharazii M, Ledbetter S, Lebel M, Larivière R. Neutralization of transforming growth factor-β attenuates hypertension and prevents renal injury in uremic rats. J. Hypertens. 2005;23:1895–1903. doi: 10.1097/01.hjh.0000182521.44440.c5. [DOI] [PubMed] [Google Scholar]
- 35.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: A twin study. J. Allergy Clin. Immunol. 2000;106:53–56. doi: 10.1067/mai.2000.108105. [DOI] [PubMed] [Google Scholar]
- 37.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, Gober L, Kim C, Glessner J, Frackelton E, Thomas K, Blanchard C, Liacouras C, Verma R, Aceves S, Collins MH, Brown-Whitehorn T, Putnam PE, Franciosi JP, Chiavacci RM, Grant SF, Abonia JP, Sleiman PM, Hakonarson H. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat. Genet. 2010;42:289–291. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ober C, Yao TC. The genetics of asthma and allergic disease: A 21st century perspective. Immunol. Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, Neumann A, Brattain MG, Chang J, Kim SJ, Kinzler KW, Vogelstein B, Willson JK, Markowitz S. Mutational inactivation of transforming growth factor β receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 40.Mizuguchi T, Collod-Beroud, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, Ihara M, Kinoshita A, Yoshiura K, Junien C, Kajii T, Jondeau G, Ohta T, Kishino T, Furukawa Y, Nakamura Y, Niikawa N, Boileau C, Matsumoto N. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat. Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai C, Wen X, He W, Liu Y. Inhibition of proinflammatory RANTES expression by TGF-β1 is mediated by glycogen synthase kinase-3β-dependent β-catenin signaling. J. Biol. Chem. 2011;286:7052–7059. doi: 10.1074/jbc.M110.174821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1–like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, Holland SM, Su HC. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, Takada H, Hara T, Kawamura N, Ariga T, Kaneko H, Kondo N, Tsuge I, Yachie A, Sakiyama Y, Iwata T, Bessho F, Ohishi T, Joh K, Imai K, Kogawa K, Shinohara M, Fujieda M, Wakiguchi H, Pasic S, Abinun M, Ochs HD, Renner ED, Jansson A, Belohradsky BH, Metin A, Shimizu N, Mizutani S, Miyawaki T, Nonoyama S, Karasuyama H. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CC, Schuller D, Spector SL, Tilles SA. Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology, The diagnosis and management of rhinitis: An updated practice parameter. J. Allergy Clin. Immunol. 2008;122:S1–S84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Leung DY, Nicklas RA, Li JT, Bernstein IL, Blessing-Moore J, Boguniewicz M, Chapman JA, Khan DA, Lang D, Lee RE, Portnoy JM, Schuller DE, Spector SL, Tilles SA. Disease management of atopic dermatitis: An updated practice parameter. Joint Task Force on Practice Parameters. Ann. Allergy Asthma Immunol. 2004;93:S1–S21. doi: 10.1016/s1081-1206(10)61385-3. [DOI] [PubMed] [Google Scholar]
- 48.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2008;6:531–535. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 49.Blanchard C, Rothenberg ME. Basic pathogenesis of eosinophilic esophagitis. Gastrointest. Endosc. Clin. N. Am. 2008;18:133–143. doi: 10.1016/j.giec.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown-Whitehorn TF, Spergel JM. The link between allergies and eosinophilic esophagitis: Implications for management strategies. Expert Rev. Clin. Immunol. 2010;6:101–109. doi: 10.1586/eci.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J. Immunol. 2002;168:2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 52.Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, Fukuda T, Elias JA, Hamid QA. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J. Allergy Clin. Immunol. 2003;111:875–881. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 53.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor–β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 54.Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM. Overexpression of Smad2 drives house dust mite–mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am. J. Respir. Crit. Care Med. 2010;182:143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo L, Fulkerson PC, Finkelman FD, Mingler M, Fischetti CA, Blanchard C, Rothenberg ME. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13Rα2–inhibited pathway. J. Immunol. 2010;185:660–669. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frischmeyer-Guerrerio PA, Guerrerio AL, Chichester KL, Bieneman AP, Hamilton RA, Wood RA, Schroeder JT. Dendritic cell and T cell responses in children with food allergy. Clin. Exp. Allergy. 2011;41:61–71. doi: 10.1111/j.1365-2222.2010.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kjellman NM, Johansson SG, Roth A. Serum IgE levels in healthy children quantified by a sandwich technique (PRIST) Clin. Allergy. 1976;6:51–59. doi: 10.1111/j.1365-2222.1976.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 58.Jolliff CR, Cost KM, Stivrins PC, Grossman PP, Nolte CR, Franco SM, Fijan KJ, Fletcher LL, Shriner HC. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin. Chem. 1982;28:126–128. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.