Abstract
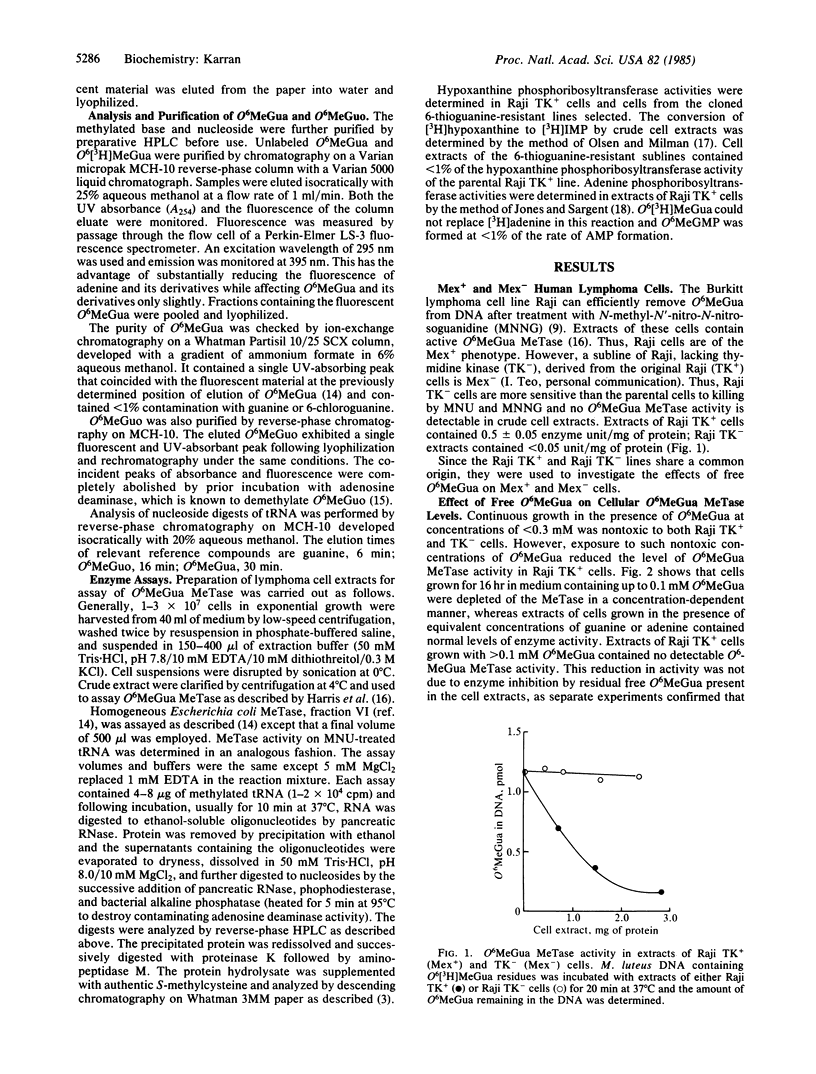
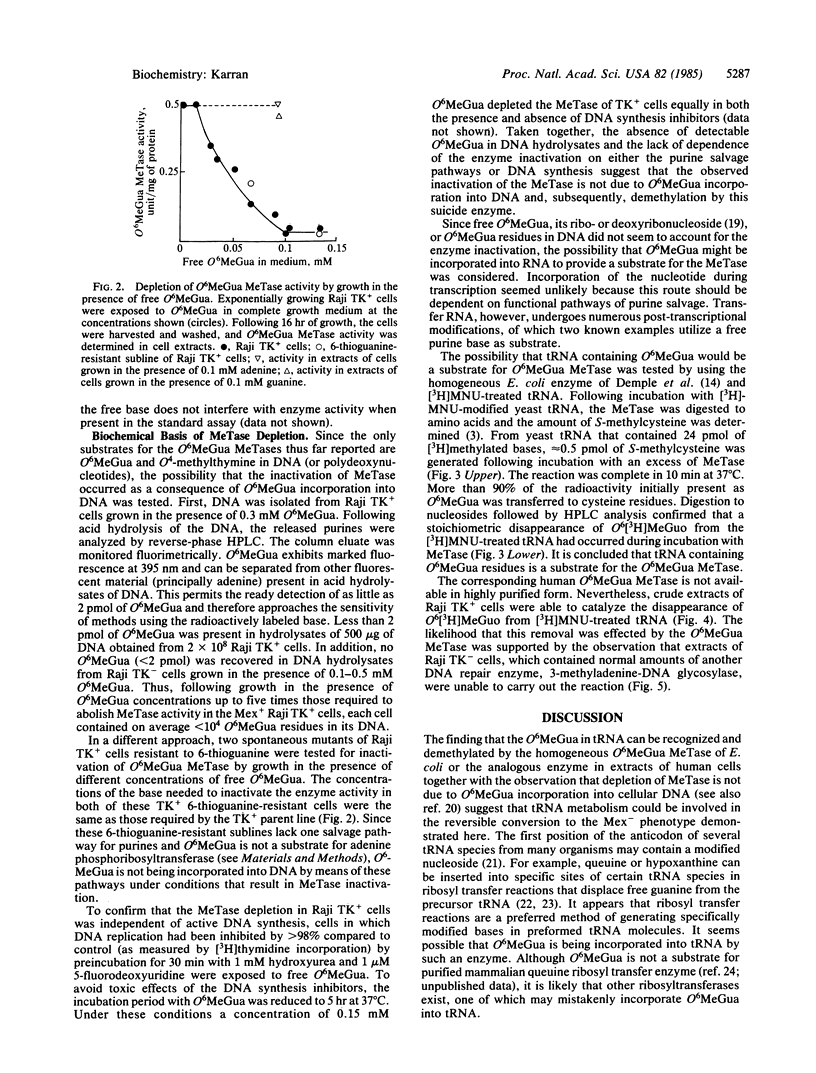
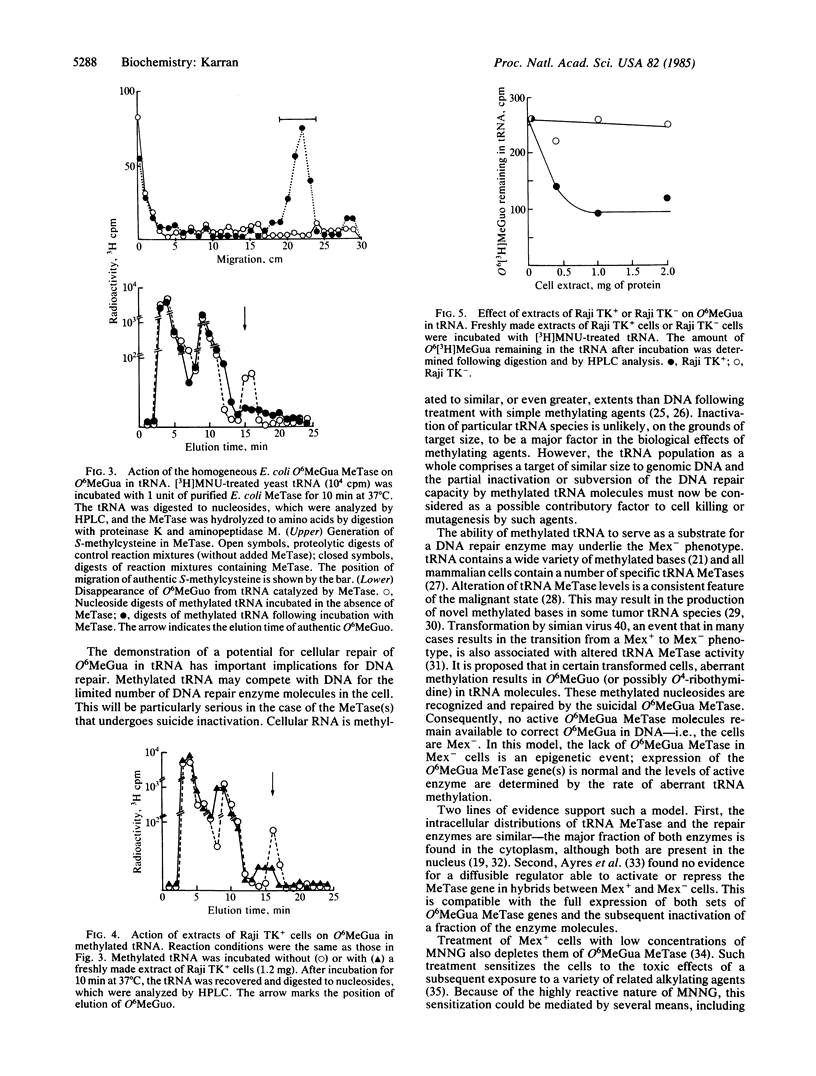
Mex+ human lymphoma cell lines contain O6-methylguanine-DNA methyltransferase, a DNA repair enzyme that undergoes suicide inactivation on interaction with its substrate. The cells are therefore competent to remove the alkylation lesion O6-methylguanine from their DNA. However, several repair-deficient lymphoma cell lines (Mex-) are also known. It is shown here that Mex+ cells can be converted temporarily to a Mex- phenotype by growth in nontoxic concentrations of free O6-methylguanine. The depletion of methyltransferase activity is not a result of O6-methylguanine incorporation into DNA and subsequent demethylation by the enzyme. It is proposed that O6-methylguanine is mistakenly incorporated into tRNA molecules by means of a post-transcriptional ribosyl transfer reaction. The demethylation of such bases in tRNA has been demonstrated by using bacterial and human DNA repair enzymes. The existence of such a subversive repair of a methylated base in tRNA raises the possibility of competition between DNA and RNA for cellular DNA repair enzymes. Furthermore, it is proposed that the known aberrant methylation of tRNA in certain transformed cells, together with subversive tRNA repair, could account for the Mex- phenotype.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres K., Sklar R., Larson K., Lindgren V., Strauss B. Regulation of the capacity for O6-methylguanine removal from DNA in human lymphoblastoid cells studied by cell hybridization. Mol Cell Biol. 1982 Aug;2(8):904–913. doi: 10.1128/mcb.2.8.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogden J. M., Eastman A., Bresnick E. A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res. 1981 Jul 10;9(13):3089–3103. doi: 10.1093/nar/9.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Elliott M. S., Trewyn R. W. Inosine biosynthesis in transfer RNA by an enzymatic insertion of hypoxanthine. J Biol Chem. 1984 Feb 25;259(4):2407–2410. [PubMed] [Google Scholar]
- Farkas W. R., Jacobson K. B., Katze J. R. Substrate and inhibitor specificity of tRNA-guanine ribosyltransferase. Biochim Biophys Acta. 1984 Feb 24;781(1-2):64–75. doi: 10.1016/0167-4781(84)90124-6. [DOI] [PubMed] [Google Scholar]
- Hall J. A., Saffhill R. The incorporation of O6-methyldeoxyguanosine and O4-methyldeoxythymidine monophosphates into DNA by DNA polymerases I and alpha. Nucleic Acids Res. 1983 Jun 25;11(12):4185–4193. doi: 10.1093/nar/11.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Karran P., Lindahl T. O6-Methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983 Jul;43(7):3247–3252. [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979 Jul 5;280(5717):76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- Karran P., Williams S. A. The cytotoxic and mutagenic effects of alkylating agents on human lymphoid cells are caused by different DNA lesions. Carcinogenesis. 1985 May;6(5):789–792. doi: 10.1093/carcin/6.5.789. [DOI] [PubMed] [Google Scholar]
- Kerr S. J. tRNA methyltransferases. Methods Enzymol. 1974;29:716–726. [PubMed] [Google Scholar]
- Kuchino Y., Borek E. Tumour-specific phenylalanine tRNA contains two supernumerary methylated bases. Nature. 1978 Jan 12;271(5641):126–129. doi: 10.1038/271126a0. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Kasai H., Yamaizumi Z., Nishimura S., Borek E. Under-modified Y base in a tRHAPhe isoacceptor observed in tumor cells. Biochim Biophys Acta. 1979 Nov 22;565(1):215–218. doi: 10.1016/0005-2787(79)90098-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Karran P. Enzymatic removal of mutagenic and lethal lesions from alkylated DNA. Prog Clin Biol Res. 1983;132B:241–250. [PubMed] [Google Scholar]
- McCarthy T. V., Karran P., Lindahl T. Inducible repair of O-alkylated DNA pyrimidines in Escherichia coli. EMBO J. 1984 Mar;3(3):545–550. doi: 10.1002/j.1460-2075.1984.tb01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. T., Lawley P. D., Shah S. A. Cellular reactions of O6-methylguanine, a product of some alkylating carcinogens. Biochem J. 1973 Oct;136(2):387–393. doi: 10.1042/bj1360387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman A., Hall R. H., Yohn D. S., Grace J. T., Jr The in vitro soluble RNA methylase activity of SV40-induced hamster tumors. Cancer Res. 1967 Aug;27(8):1409–1414. [PubMed] [Google Scholar]
- Nishimura S. Structure, biosynthesis, and function of queuosine in transfer RNA. Prog Nucleic Acid Res Mol Biol. 1983;28:49–73. doi: 10.1016/s0079-6603(08)60082-3. [DOI] [PubMed] [Google Scholar]
- Olsen A. S., Milman G. Chinese hamster hypoxanthine-guanine phosphoribosyltransferase. Purification, structural, and catalytic properties. J Biol Chem. 1974 Jul 10;249(13):4030–4037. [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E., Jackson A. Alkylation of meseenger RNA by dimethylnitrosamine. Chem Biol Interact. 1976 Mar;12(3-4):279–284. doi: 10.1016/0009-2797(76)90044-2. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Foote R. S., Mitra S., Perry W. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983 Feb 25;258(4):2327–2333. [PubMed] [Google Scholar]
- Penman B. W., Crespi C. L., Komives E. A., Liber H. L., Thilly W. G. Mutation of human lymphoblasts exposed to low concentrations of chemical mutagens for long periods of time. Mutat Res. 1983 Mar;108(1-3):417–436. doi: 10.1016/0027-5107(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M., Plant J. E., Sturrock J. E., Crathorn A. R. Quantitative aspects of the repair of alkylated DNA in cultured mammalian cells. I. The effect on HeLa and Chinese hamster cell survival of alkylation of cellular macromolecules. Chem Biol Interact. 1971 Feb;3(1):29–47. doi: 10.1016/0009-2797(71)90024-x. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Becker Y. Kinetics of O6-methylguanine repair in human normal and ataxia telangiectasia cell lines and correlation of repair capacity with cellular sensitivity to methylating agents. Cancer Res. 1981 Dec;41(12 Pt 1):5114–5120. [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. V., Kirsch J. F. Enzymatic displacement of oxygen and sulfur from purines. J Am Chem Soc. 1968 Nov 20;90(24):6849–6850. doi: 10.1021/ja01026a054. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B., Rice M., Day R. S., 3rd, Foote R. S., Mitra S. O6-Methylguanine-DNA methyltransferase in human cells. Mutat Res. 1984 Jan;131(1):27–36. doi: 10.1016/0167-8817(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of human colon tumor cells with DNA methylating agents inhibits their ability to repair chloroethyl monoadducts. Carcinogenesis. 1984 Jan;5(1):83–87. doi: 10.1093/carcin/5.1.83. [DOI] [PubMed] [Google Scholar]


