Abstract
The cannabinoid receptor agonist, WIN 55,212-2, increases extracellular norepinephrine levels in the rat frontal cortex under basal conditions, likely via desensitization of inhibitory α2-adrenergic receptors located on norepinephrine terminals. Here, the effect of WIN 55,212-2 on stress-induced norepinephrine release was assessed in the medial prefrontal cortex (mPFC), in adult male Sprague-Dawley rats using in vivo microdialysis. Systemic administration of WIN 55,212-2 thirty minutes prior to stressor exposure prevented stress-induced cortical norepinephrine release induced by a single exposure to swim when compared to vehicle. To further probe cortical cannabinoid-adrenergic interactions, postsynaptic α2-adrenergic receptor (AR)-mediated responses were assessed in mPFC pyramidal neurons using electrophysiological analysis in an in vitro cortical slice preparation. We confirm prior studies showing that clonidine increases cortical pyramidal cell excitability and that this was unaffected by exposure to acute stress. WIN 55,212-2, via bath application, blocked postsynaptic α2-AR mediated responses in cortical neurons irrespective of exposure to stress. Interestingly, stress exposure prevented the desensitization of α2-AR mediated responses produced by a history of cannabinoid exposure. Together, these data indicate the stress-dependent nature of cannabinoid interactions via both pre- and postsynaptic ARs. In summary, microdialysis data indicate that cannabinoids restrain stress-induced cortical NE efflux. Electrophysiology data indicate that cannabinoids also restrain cortical cell excitability under basal conditions; however, stress interferes with these CB1-α2 AR interactions, potentially contributing to over-activation of pyramidal neurons in mPFC. Overall, cannabinoids are protective of the NE system and cortical excitability but stress can derail this protective effect, potentially contributing to stress-related psychopathology. These data add to the growing evidence of complex, stress-dependent modulation of monoaminergic systems by cannabinoids and support the potential use of cannabinoids in the treatment of stress-induced noradrenergic dysfunction.
Keywords: WIN 55,212-2; microdialysis; norepinephrine; locus coeruleus; clonidine; electrophysiology
Introduction
Endogenous and exogenous cannabinoids acting through cannabinoid type 1 (CB1) receptors have been implicated in the regulation of a variety of behavioral and cognitive functions (D’Souza et al., 2008; Egashira et al., 2008; Senn et al., 2008) as well as emotional (Moreira and Lutz, 2008) and learning and memory processes (Freund et al., 2003; Gerdeman and Lovinger, 2003; Makela et al., 2006). A widespread function of CB1 receptor modulation involves inhibition of neurotransmitter release (Doherty and Dingledine, 2003; Schlicker and Kathmann, 2001; Szabo and Schlicker, 2005). However, increases in neurotransmitter release have also been reported following exposure to cannabinoid receptor agonists (Acquas et al., 2000; Fortin and Levine, 2007).
Our previous studies have shown that administration of the cannabinoid receptor agonist, WIN 55,212-2, significantly increases norepinephrine efflux in the frontal cortex (Oropeza et al., 2005; Page et al., 2007). Similarly, direct local infusion of WIN 55,212-2 into the PFC increases cortical norepinephrine efflux (Page et al., 2008), an effect that is inhibited by local (Page et al., 2008) or systemic (Oropeza et al., 2005) pre-treatment with the selective CB1 receptor antagonist, SR 141716A, demonstrating a specific role for CB1 receptors in these effects. In addition, we have shown that systemic administration of WIN 55,212-2 stimulates c-Fos expression in noradrenergic neurons in the locus coeruleus (LC) (Oropeza et al., 2005) and significantly increases expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of catecholamines, in the LC (Foote et al., 1983). WIN 55,212-2-induced neurochemical alterations were accompanied by changes in anxiety-like behaviors (Page et al., 2007). Our previous neuroanatomical studies characterized the cellular substrates for interactions between noradrenergic axon terminals and CB1r (Oropeza et al., 2007).
The endocannabinoid and noradrenergic systems are significantly and dynamically impacted by stress (Cassens et al., 1980; Flugge et al., 2004; Gorzalka et al., 2008; Hill and McEwen, 2010; Shinba et al., 2010) and noradrenergic transmission is responsible for cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis (McLaughlin et al., 2009). Under conditions of acute stress, norepinephrine is increased centrally and peripherally (Abercrombie and Jacobs, 1987; Ferry et al., 1999; Nestler et al., 1999; Page and Valentino, 1994; Sands et al., 2000; Valentino et al., 1998) while the endocannabinoid system (EC) tonically constrains activation of neural circuits, including the hypothalamic-pituitary-adrenal axis (Gorzalka et al., 2008; Steiner and Wotjak, 2008). However, disrupted noradrenergic and EC signaling is associated with an inability to adapt to chronic stress (Flugge et al., 2004; Gorzalka et al., 2008; Nestler et al., 1999; Hill and Gorzalka, 2004; Hill et al., 2008; Wong et al., 2000).
Dysfunction in the noradrenergic system has been implicated in a number of affective disorders (e.g. depression, anxiety), many of which are precipitated by chronic stress (Leonard and Myint, 2009; Morilak and Frazer, 2004). Upregulation in the activity of TH has been suggested to lead to changes in noradrenergic transmission that contributes to behavioral, cognitive, emotional and physiological manifestations of depression and anxiety (Sands et al., 2000; Duncko et al., 2001; Miller et al., 1996). Therefore, although norepinephrine transmission is critical for proper functioning of PFC neurons (Franowicz et al., 2002), high levels of catecholamine receptor stimulation during stress can impair cortical function and may contribute to exacerbating or precipitating a number of psychiatric disorders (Arnsten, 1997).
In the present study, we sought to determine whether CB1 receptors modulate stress-induced increases in norepinephrine efflux by assessing extracellular levels of norepinephrine in the PFC in rats that received a systemic injection of WIN 55,212-2 or vehicle prior to stress exposure. Indices of coping behaviors during a 15-minute swim were also measured. In addition, we examined a history of cannabinoid exposure and the impact of stress on cannabinoid-adrenergic interactions in the mPFC using in vitro electrophysiology.
Materials and Methods
Experimental animals
For microdialysis and behavioral studies, adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 250-300g were used. For electrophysiology experiments, male Sprague-Dawley rats (Taconic Farms Germantown, NY) initially weighing 50-75g were used because brain slice viability is much improved in the juvenile compared to adult rodent brain (Alger et al., 1984; Gibb and Edwards, 1994). However, cortical CB1 and α2-adrenergic receptors (α2-AR) are fully functional by this age in rats (Berrendero et al., 1998; Winzer-Serhan and Leslie, 1999). Animals were housed 2-3 per cage on a 12 hour light:dark schedule in a temperature-controlled (25°C) colony room. Rats were given ad libitum access to standard rat chow and water. The Thomas Jefferson and Temple University Institutional Animal Care and Use Committees (IACUC) approved the protocol and all studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and reduce the number of animals used.
Microdialysis
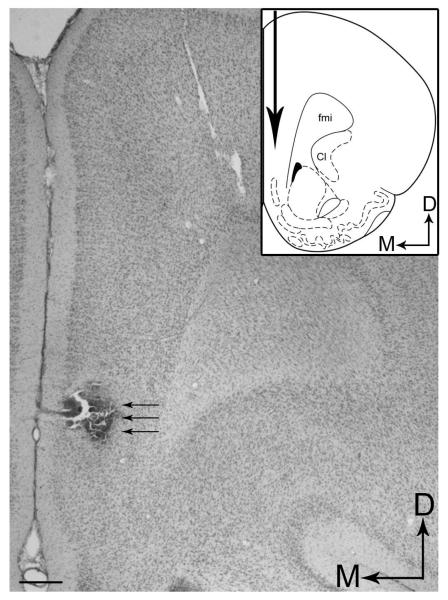
Following an acclimation period of approximately one week, rats were anesthetized with isoflurane (Abbott Laboratories, North Chicago, IL; 0.5-1.0%, in air) and via a specialized nose cone affixed to the sterotaxic frame (Stoelting Corp., Wood Dale, IL). A small burr hole was made in the skull centered at 3.2 mm anterior and ±0.7 mm lateral to bregma. The dura was removed, and the microdialysis probe was slowly lowered 5 mm from the brain surface into the infralimbic and prelimbic areas of the PFC (plates 8-10) (Paxinos and Watson, 1986) and secured with skull screws and dental acrylic. The inlet of the probe was connected to a fluid swivel (Instech Laboratories, Plymouth Meeting, PA) and the rat was placed into a cylindrical plexiglass container covered with bedding. Food and water were given ad libitum. Artificial cerebrospinal fluid (aCSF; Oropeza et al., 2005) was continuously perfused through the probe at a rate of 1.5 ul/min by a microliter infusion pump (Harvard Pump ‘11’ VPF Dual syringe, Harvard Apparatus, Holliston, MA). Rats were allowed to recover overnight. Approximately 24 hours following surgery, dialysate samples were collected every 20 minutes. After collecting dialysate samples for at least 2 hours to establish stable baseline levels, the cannabinoid agonist WIN 55,212-2 (Sigma-Aldrich Inc., St. Louis, MO) was administered to the rats via intraperitoneal injection at 3.0 mg/kg. Dilutions of the drug were prepared immediately prior to the start of each experiment by dissolving in 0.4% Tween 80 (Sigma-Aldrich Inc., St. Louis, MO). A control group was treated with vehicle only (water containing 0.4% Tween 80) and deionized water. Dialysate samples were collected and 60 minutes after cannabinoid injection, rats were gently moved from the animal apparatus to an adjacent Pyrex Cylinder (21× 46 cm; Fisher Scientific, Liberty Lane Hampton, NH), which was filled with room temperature water to a 30-cm depth. The 15-minute swim session was videotaped from above for subsequent analysis of the behavioral response. Following swim, rats were returned to the animal apparatus and sample collection continued for an additional 3 hours. Dialysate samples were stored at −80 °C for subsequent analysis by high performance liquid chromatography with electrochemical detection (HPLC-ED). At the conclusion of the experiment, rats were deeply anesthetized with pentobarbital (60mg/kg; Ovation Pharmaceuticals, Inc., Deerfield, IL) intraperitoneally and 2% pontamine sky blue dye (Alfa Aesar, Ward Hill, MA) was infused through the probe to mark its location. The rats were transcardially perfused with 10% formalin (Fisher Scientific, Pittsburgh, PA), decapitated and the brains removed for subsequent histological verification of probe placement. Figure 1 shows a representative example of the placement of the dialysis probe in the PFC. The data were not included if the placement was outside the infralimbic and prelimbic areas of the PFC.
Figure 1.
(A) A representative brightfield photomicrograph of a coronal section counterstained with pontamine sky blue dye showing histological verification of a microdialysis probe placement in the frontal cortex. Arrows depict the position of the implanted probe. Dorsal (D) and medial (M) orientation of the tissue section are indicated by arrows. Scale bar = 100 μm. Inset shows a schematic diagram adapted from the rat brain atlas of Paxinos (Paxinos and Watson, 1986) depicting the anterior posterior level of the representative microdialysis probe placement shown in panel A. Big arrow indicates the microdialysis probe placement. Arrows depict dorsal (D) and medial (M) orientation of the tissue section. Cl, claustrum; fmi, forceps minor corpus callosum.
Custom build vertical concentric microdialysis probes were used. A piece of fused silica (Polymicro Technologies, Phoeniz, AZ) was inserted through PE 10 tubing and semipermeable membrane made from hollow rayon fibers with a 223 μm o.d. and 35,000 MW cutoff was fixed over the fused silica and into the PE 10 tubing with epoxy. The open end of the dialysis fiber was sealed with a 0.5 mm epoxy plug, and 2 mm of the top of the membrane was coated with epoxy leaving an active area of 3 mm for exchange across the membrane. The in vitro recovery rate was determined by placing the probe in a beaker of aCSF containing a known concentration of NE standard. The concentration of norepinephrine in the dialysate was compared to the amount in the bath. Probes that did not correspond to an acceptable range of recovery (12-24%) were eliminated. Because the diffusion properties of neurochemicals in brain tissue are likely different from in vivo conditions, reported dialysate values were not corrected for recovery of the probe.
High performance liquid chromatography
Catecholamine levels were determined using HPLC-ED. Twenty-five μl of dialysate sample is diluted in 75μl of diluent containing the following antioxidants: ascorbic acid, L-cysteine, and oxalic acid. Samples and standards are kept at 4°C and injections of 50μl are made overnight using an ESA autosampler. Mobile phase is 0.113 M phosphate buffer, 0.13 mM EDTA, 0.17mM octyl sulphate, 4% acetonitrile, and pH of 3.1. Detection was performed using an ESA Coulochem II detector with conditioning cell (ESA model 5021) set at +350mV, and a duel electrode analytic cell (ESA model 5011A) with electrode 1 set at +80mV, and electrode 2 (working electrode) set at −320mV. Phenomonex (Phenomonex, Torrance, CA) reverse phase c18 Gemini column (150×4.6 mm, 3 mC, 110A) was used for separations, and EZChrom Elite software from Scientific Software Inc. was used to analyze the chromatograms.
Data analysis
The baseline value against which drug administration was compared to was derived from the average of three samples just prior to manipulation. The neurochemical data were expressed as the mean ± S.E.M. or absolute value (pg/25μl). The overall effect of treatment of WIN-55,212-2 on monoamine efflux in the PFC was analyzed using two-way analysis of variance (ANOVA) with repeated measures over time (P < 0.05). The absolute amount of neurotransmitter measured in dialysates (pg/sample) was used as the dependent variable for assessment of within group effects. Basal values plus the next five samples post drug injection including two post swim samples were used in the analysis. The frequency of behavioral responses in the swim test was compared between the drug-treated and vehicle treated animals using Tukey’s multiple comparison test. Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA).
Behavioral analysis
A time sampling technique was employed to analyze behavior during the 15-minute swim exposure whereby predominant behaviors in each 5-sec epoch of the 300-sec test was recorded (Detke et al., 1995). Climbing behavior consisted of upward directed movements of the forepaws along the side of the swim chamber. Swimming behavior was defined as movement (usually horizontal) throughout the swim chamber, which also included crossing over into another quadrant. Immobility was assigned when no activity was observed other than that required to maintain the rat’s head above water.
Electrophysiology
Brain slices were prepared from naïve subjects or immediately following exposure to a 15-min swim session as described above. In one subset of subjects, rats were treated with the synthetic CB1 receptor agonist WIN 55,212-2 (3 mg/kg, i.p.) or vehicle (5% DMSO in 0.9% saline) once daily for 7 days with the last injection occurring 1-hr prior to swim (or no swim) exposure.
Coronal slices (250 μM thick) containing the prefrontal cortex were cut on a Vibratome 3000 Plus (Vibratome, St. Louis, MO) and maintained as described previously (Kirby et al., 2007). Slices were transferred to a recording chamber (Warner Instruments, Hamden, CT) and continuously perfused with aCSF at 1.5-2.0 ml/min at 32-34°C maintained by an in-line solution heater (TC-324; Warner Instruments). Only one cell was recorded per brain slice. Pyramidal cells in layer V/VI of PFC were visualized using a Nikon E600 upright microscope fitted with a 40× water-immersion objective, differential interference contrast and infrared filter (Optical Apparatus, Ardmore, PA) connected to a CCD camera and computer monitor. Pyramidal cells were identified by their large, pyramidal-shaped soma and long apical dendrite projecting toward the brain surface as well as location within PFC layers V/VI. Whole-cell recording recordings were established as described previously (Kirby et al., 2007) using electrodes filled with an intracellular solution of the following (in mM): K-gluconate 120, KCl 10, MgCl2 1, EGTA 10, HEPES 10, MgATP 2, Na2GTP 0.5, NaPhosphocreatinine 10, 0.1% Biocytin, pH 7.3. Recordings were done using a HEKA patch-clamp EPC-10 amplifier (HEKA Elektronik, Pfalz, Germany) under current clamp (I = 0 pA) conditions. Series resistance was monitored throughout the experiment. If the series resistance was unstable or exceeded four times the electrode resistance, the cell was discarded. Signals were stored on-line using Pulse software, filtered at 1 kHz and digitized at 10 kHz. The liquid junction potential was approximately 9 mV between the pipette solution and the aCSF and was not subtracted from the data obtained.
At baseline, membrane potential was recorded and input resistance calculated using the current-voltage relationship. Neuronal excitability was assessed in each cell by injecting a series of current pulses (25-625 pA) to determine the current step that would evoke 2-3 action potentials in the cell. This current step was then used to test neuronal excitability throughout the rest of the experiment. Membrane potential, input resistance and neuronal excitability were also measured following bath application of the α2-AR agonist clonidine (10 μM). Previous studies have shown that α2-AR stimulation increases PFC pyramidal neuron excitability and input resistance (Andrews and Lavin, 2006; Carr et al., 2007; Cathel et al., 2012) via inhibition of a hyperpolarization/cyclic nucleotide gated channel-mediated inward current (Carr et al., 2007). To test the effects of acute CB1 receptor blockade in vitro, WIN 55,212-2 (1 μM) was added to the perfusion bath 6 min prior to clonidine. Following some electrophysiology experiments, the pyramidal cell morphology and location of recorded cells was confirmed using standard fluorescence immunohistochemistry techniques (Kirby et al., 2008).
Data analysis for electrophysiology
Electrophysiological recordings were analyzed using Clampfit 9.2 (Axon Instruments, Foster City, CA). Drug effects on membrane voltage, input resistance or neuronal excitability were tested by paired Student’s t-test (baseline vs single drug) or repeated measures ANOVA (baseline vs multiple drugs) followed by post-hoc Student-Newman Keuls tests when appropriate. When data did not have normal distribution or equal variance, equivalent nonparametric tests were employed (Wilcoxon signed-rank test and Friedman repeated measures ANOVA on ranks). Statistics for electrophysiology experiments were performed using SigmaStat 3.1 (Systat Software, Inc., San Jose, CA).
Results
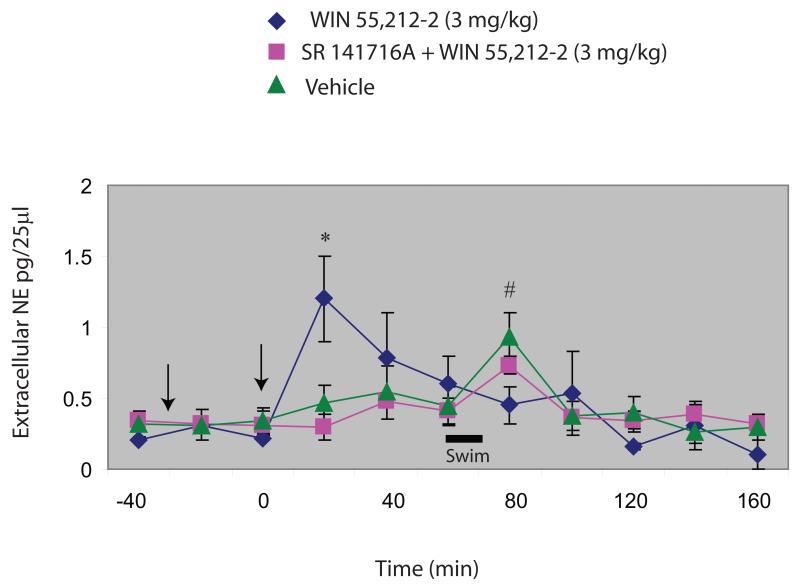
Administration of WIN 55,212-2 (3.0 mg/kg) produced a significant increase in norepinephrine efflux in the PFC (1.20 ± 0.32 pg/25ml) at 20 minutes post drug administration (Fig. 2), a result that is consistent with prior work from our laboratory (Oropeza et al., 2005; Page et al., 2008). The increase in norepinephrine release observed in cannabinoid-treated animals was significant compared to extracellular norepinephrine release observed in SR 141716A (a selective CB1 receptor antagonist) + WIN 55,212-2 and vehicle at 20 min post drug administration (P < 0.05). Consistent with previous findings from other laboratories (Berridge and Abercrombie, 1999; Page and Lucki, 2002), exposure to acute stress caused a marked increase in norepinephrine release in the PFC in control rats (0.935 ± 0.17 pg/25ml). Interestingly, in rats that had been pre-treated with WIN 55,212-2 (3.0 mg/kg) the increase in norepinephrine release elicited by the swim exposure was significantly decreased (0.45 ± 0.13 pg/25ml) when compared to vehicle (P < 0.05).
Figure 2.
The effect of pre-treatment with the CB1 receptor agonist WIN 55,212-2 on extracellular norepinephrine release in the rat PFC following a 15-minute exposure to swim was measured using in vivo microdialysis with HPLC-ED. Pre-treatment with 3.0 mg/kg of WIN 55,212-2 produced a significant increase in norepinephrine release in the PFC (1.20 ± 0.32 pg/25ml) at 20 minutes post drug administration. The increase in norepinephrine release observed in cannabinoid-treated animals was significant compared to extracellular norepinephrine release observed SR 141716A (a selective CB1 receptor antagonist) +WIN 55,212-2 and vehicle at 20 minutes post drug administration (P < 0.05). Exposure to acute swim stress (period of test illustrated by black bar) caused a marked increase in norepinephrine release in the PFC in vehicle (0.935 ± 0.17 pg/25ml). In cannabinoid-treated rats, the increase in norepinephrine release elicited by exposing the rats to swimming was attenuated (0.45 ± 0.13 pg/25ml) compared to vehicle (P < 0.05). *P < 0.05 vs SR 141716A+WIN and vehicle; # P < 0.05 vs WIN+swim.
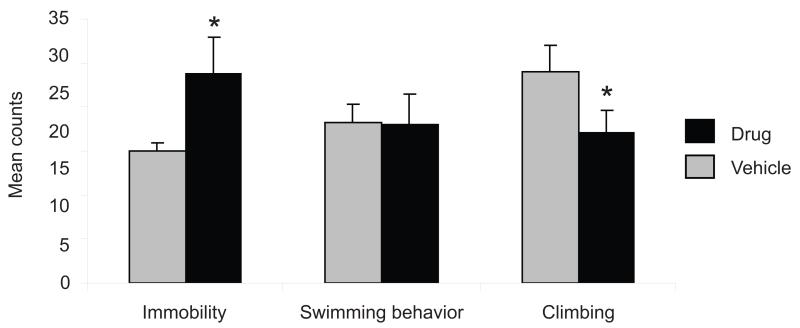
The effects of WIN 55,212-2 on behavior during the swim exposure are shown in Fig. 3. Animals treated with WIN 55,212-2 prior to swim exposure exhibited significantly increased immobility counts during the 5-min test session compared to controls (P < 0.01) and a decrease in climbing behavior (P < 0.05) with no significant difference in swimming behavior. This effect is most likely not attributed to WIN 55,212-2-induced alterations in locomotor activity as, at the dose used here, significant changes in locomotor behavior were not observed (data not shown) confirming previous report that acute low doses of WIN 55,212-2 have not been shown to alter motor activity (Drews et al., 2005).
Figure 3.
Effect of WIN 55,212-2 on rat behavior during swim stress. Mean counts of immobility, swimming, and climbing behaviors are shown. Behaviors were sampled every 5 s during the first 5 min of the 15-min test period (n=5 rats per group). Rats were treated with vehicle (gray bars) or 3 mg/kg of WIN 55,212-2 (black bars) 60 minutes prior to the swim test. The swim test significantly increased the frequency of immobility counts during the swim sessions when compared to controls (*P < 0.001). This was accompanied by a slight decrease in climbing behavior (*P < 0.05) with no significant difference in swimming behavior (P = 0.24).
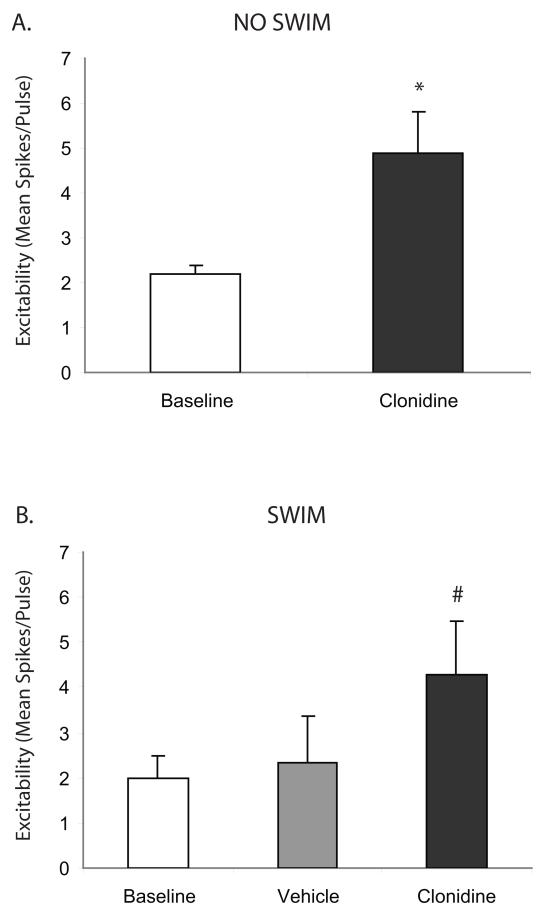
In vitro slice electrophysiology studies confirmed previous studies showing α2-AR increases in cortical pyramidal cell excitability (Fig. 4-5) (Carr et al., 2007). Furthermore, α2-AR-mediated elevations in cortical pyramidal cell excitability were unchanged by exposure to swim stress alone (Fig. 5). In both naïve (Fig. 5A) and swim-exposed subjects (Fig. 5B), α2-AR stimulation with clonidine (10 μM) significantly elevated cortical pyramidal cell excitability (P < 0.05) when compared to baseline.
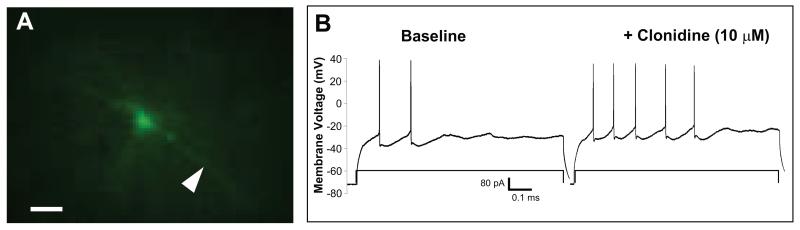
Figure 4.
Layer V/VI pyramidal neuron in PFC: morphology and electrophysiology. Panel A shows a layer V/VI pyramidal neuron in PFC filled with biocytin and visualized using Alexa 488-conjugated streptavidin. This cell has a large (~20 μm) pyramidal-shaped cell body with a large apical dendrite (arrowhead) extending toward the brain surface which is characteristic of cortical pyramidal neurons. Clonidine (10 μM) increases excitability in a PFC pyramidal neuron from a 2 to 5 spike response to an 80 pA current step. Scale bar = 50 μM.
Figure 5.
Swim stress does not affect α2-adrenergic receptor-mediated increase in PFC pyramidal neuron excitability. Clonidine (10 μM) significantly elevated mean pyramidal cell excitability (panel A; N = 6) in brain slices from naïve (non-stressed) subjects. In slices from animals exposed to a 15 min swim stress, this α2-adrenergic effect was unchanged (panel B; N = 12). The asterisk indicates a significant change from baseline by paired Student’s t-test (P < 0.05) and the pound sign indicates a significant difference from both baseline and vehicle by post-hoc Student-Newman-Keuls tests (P < 0.05). Data are represented as mean ± SEM.
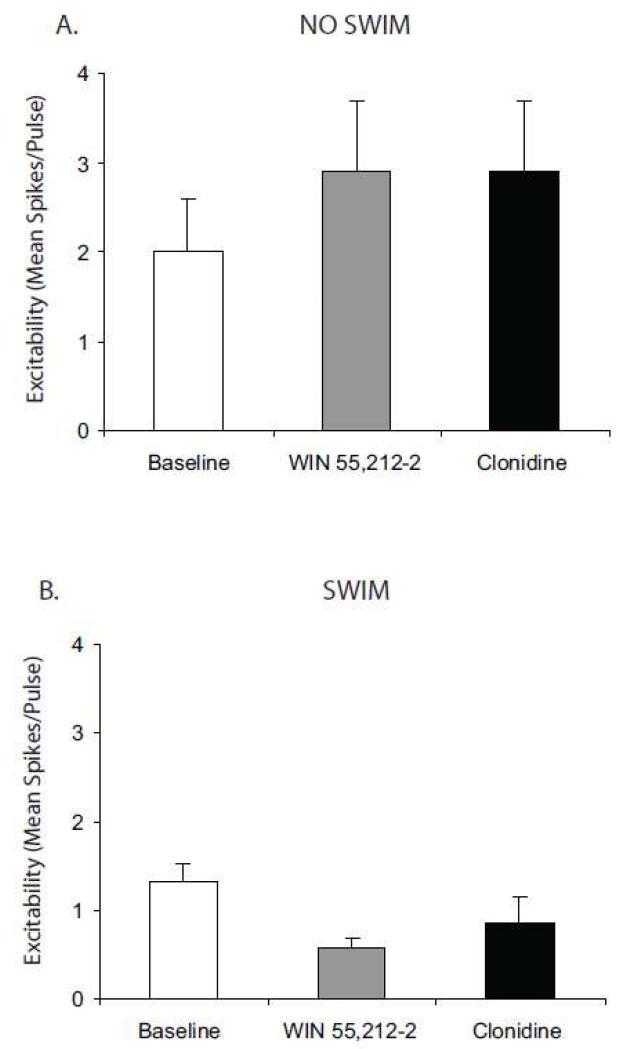
Bath application of WIN 55,212-2 (1.0 μM) blocked α2-AR-mediated elevations in cortical pyramidal cell excitability (Fig. 6). This effect was not changed by prior exposure to swim stress. Specifically, acute CB1 receptor stimulation with WIN 55,212-2 (1.0 μM), via bath application, blocked α2-AR-mediated elevations in cortical pyramidal cell excitability in slices from both unstressed (Fig. 6A) and stressed subjects (Fig. 6B).
Figure 6.
Acute CB1 receptor stimulation blocks α2-adrenergic receptor-mediated increase in PFC pyramidal neuron excitability in brain slices from unstressed and stressed subjects. Acute in vitro pretreatment with the CB1 agonist, WIN 55,212-2 (1.0 μM) blocks clonidine (10 μM)-induced elevation of PFC pyramidal neuron excitability in brain slices from naïve (unstressed) subjects (N = 7; panel A) and in slices from subjects exposed to a 15 min swim stress(N = 11; panel B). Data are represented as mean ± SEM.
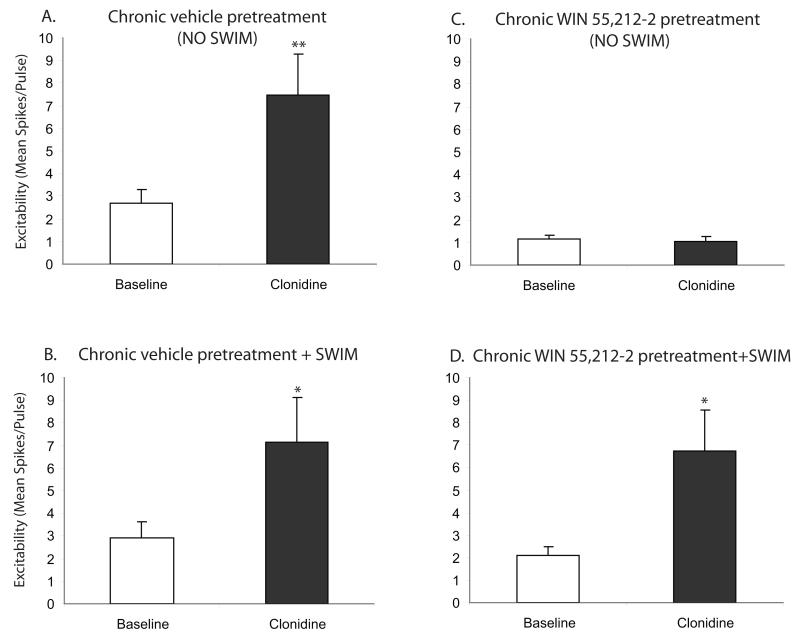
In contrast to the acute condition (bath application of WIN 55,212-2), swim stress impacted CB1-α2-AR interactions in subjects exposed to chronic with a history of cannabinoids in vivo (Fig. 7). Animals were administered WIN 55,212-2 (3 mg/kg, i.p.) or vehicle, once daily for 7 days and then either exposed to swim stress or remained in the home cage as non stress controls before preparation of slices for electrophysiology. Clonidine increased cortical pyramidal cell excitability in slices from chronic vehicle pretreated, unstressed (P < 0.01; Figure 7A) or stressed (P < 0.05; Figure 7B) animals. In unstressed animals, chronic WIN 55,212-2 pretreatment prevented this clonidine-mediated increase in cortical pyramidal cell excitability (P > 0.05; Figure 7C). However, in stressed animals, this ability of chronic WIN 55,212-2 pretreatment to block clonidine-mediated increase in cortical pyramidal cell excitability was lost (P < 0.05; Fig. 7D). In other words, stress exposure prevented the ability of chronic CB1 receptor activation to desensitize the α2-AR-mediated response in cortical pyramidal cells.
Figure 7.
Chronic CB1 receptor stimulation blocks α2-adrenergic receptor-mediated increase in PFC pyramidal neuron excitability in brain slices from unstressed but not stressed subjects. Clonidine (10 μM) significantly increased PFC pyramidal neuron excitability in slices from either unstressed (N = 17; panel A) or stressed animals pretreated with chronic vehicle (N = 13; panel B). Chronic WIN 55,212-2 treatment (3 mg/kg, i.p., once daily for 7 days) blocked clonidine’s effect on PFC neuron excitability in unstressed subjects (N = 10; panel C). By contrast, swim exposure prevented this CB1-α2-adrenergic receptor interaction. In slices from subjects exposed to a 15 min swim stress, chronic WIN 55,212-2 treatment did not alter the ability of clonidine to increase PFC neuron excitability (N = 13; panel D). Asterisks indicate a significant change from baseline by paired t-test (* P < 0.05; ** P < 0.01). Data are represented as mean ± SEM.
Discussion
This study demonstrates that pre-treatment with a cannabinoid receptor agonist diminishes stress-induced noradrenergic transmission in the mPFC, a region critical for cognitive flexibility and regulation of affect. Cannabinoid-pretreatment also resulted in a decrease in climbing during a single exposure to swim, an arousal-related behavior that has been attributed to availability of norepinephrine (Detke et al., 1995). In cortical slices, acute WIN 55,212-2 exposure blocked α2-AR mediated responses in cortical pyramidal neurons. Interestingly, stress exposure prevented the desensitization of α2-AR mediated responses produced by a history of cannabinoid exposure. Together, these data indicate the stress-dependent nature of cannabinoid interactions via both pre- and postsynaptic ARs.
The present study is in agreement with findings of others showing neurochemical and behavioral alterations in noradrenergic release following exposure to a single exposure of forced swim (Page et al., 2003). Our study did not use the forced swim test as a measure of antidepressant activity, but rather used a 15-minute swim exposure as a stressor that is known to engage noradrenergic afferents, specifically the coeruleo-cortical circuit (Page et al., 2003). Based on previous studies showing that the endocannabinoid and noradrenergic systems play a role in modulating stress and anxiety responses (Sands et al., 2000; Hill and Gorzalka, 2004; Duncko et al., 2001; Miller et al., 1996; Fride et al., 2005; Martin et al., 2002; Millan, 2003), it is tempting to speculate that, in response to an acute stress exposure, the cannabinoid system may engage noradrenergic signaling to increase coping behaviors. It is important to note that in the classical forced swim test paradigm, the Porsolt test, rats are subjected to two consecutive swim exposures. Studies using this test have shown that HU210, another CB1 agonist, or the anandamide-transporter inhibitor AM404, resulted in a reduction in immobility scores, suggestive of an antidepressant effect (Hill and Gorzalka, 2005). In the present study, we did not evaluate a second swim exposure but examined the effect of a cannabinoid agonist only with respect to acute stress-induced norepinephrine release or AR mediated responses.
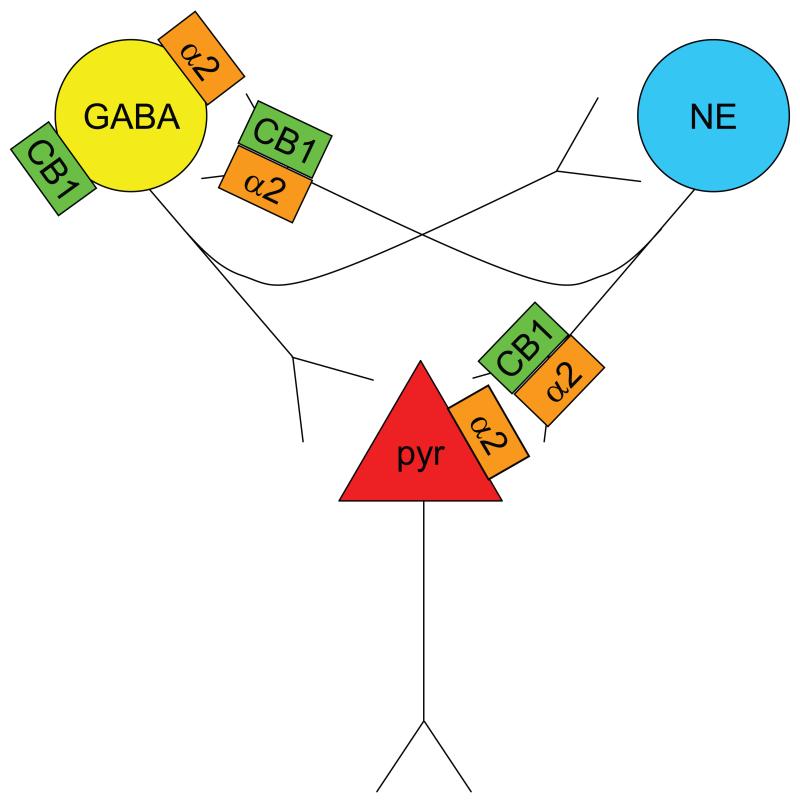
Using in vivo microdialysis, the present study reveals that systemic administration of WIN 55,212-2 prior to swim exposure prevents stress-induced cortical norepinephrine release, potentially via desensitization of inhibitory α2-adrenergic receptors located on norepinephrine terminals. Our previous studies have shown an increase in cortical norepinephrine efflux following acute exposure to a cannabinoid receptor agonist delivered either systemically (Oropeza et al., 2005) or directly within the PFC (Page et al., 2008). Functional interactions between cannabinoids and central noradrenergic systems have been well described (Oropeza et al., 2005; Page et al., 2007; Page et al., 2008; Oropeza et al., 2007; Carvalho et al., 2010a; Carvalho et al., 2010b; Gobbi et al., 2005; Mendiguren and Pineda, 2006; Mendiguren and Pineda, 2007; Muntoni et al., 2006). The exact mechanism underlying cannabinoid modulation of NE has yet to be determined but may involve direct influences of CB1 receptors that are localized to noradrenergic axon terminals in the PFC (Oropeza et al., 2007) that contribute to regulating norepinephrine release (Figure 8). Typically, CB1 receptors have been reported to be coupled with Gi/o proteins where they negatively regulate adenylate cyclase (Gαi/o-dependent inhibition) and positively regulate mitogen-activated protein kinase (Gβγ-dependent activation). However, promiscuous coupling of CB1 receptors has been reported where CB1 receptors stimulate adenylate cyclase via Gs (Turu and Hunyady, 2012). The microdialysis data support a mechanism whereby acute exposure to a CB1 receptor agonist followed by stress exposure decreases cortical norepinephrine efflux by inhibiting presynaptic inhibitory α2-adrenergic autoreceptors. This putative mechanism is supported by evidence showing a predominant pre-synaptic distribution of α2-AR in this region (Cerrito and Preziosi, 1985; Dennis et al., 1987; Pudovkina et al., 2001).
Figure 8.
Schematic diagram showing putative mechanisms underlying cannabinoid modulation of norepinephrine. Microdialysis and electrophysiology data indicate cannabinoid-induced de-sensitization of pre- and post-synaptic α2-ARs, respectively. Presynaptic α2-AR de-sensitization will lead to cannabinoid-induced increases in cortical NE efflux. Electrophysiological studies demonstrate blockade of α2-AR-mediated pyramidal cell excitability by WIN implicating de-sensitization of postsynaptic α2-ARs in the effect. Stress is impacting CB1-α2-AR interactions at both pre- and post-synaptic levels. In summary, the microdialysis study suggests that cannabinoids restrain stress-induced NE efflux. The electrophysiological studies suggest that cannabinoids restrain PFC cell excitability via de-sensitization of postsynaptic α2-ARs but stress can interfere with this interaction, potentially contributing to over-activation of pyramidal neurons in PFC. Alternatively, cannabinoids may desensitize α2-AR on GABA interneurons, resulting in increased GABA tone and potential restraint of both cortical excitability and NE efflux. If stress interferes with this desensitization, the effect would be decreased GABA tone, excess NE release and excitability of cortical neurons. Future studies are required to address local network interactions. Overall, cannabinoids are protective of the NE system and cortical excitability but stress can derail this protective effect, leading to psychopathology. Abbreviations: Acb-nucleus accumbens; α2-AR-α2-adrenergic receptor; aCSF-artificial cerebrospinal fluid; CB1-cannabinoid receptor 1; GABA-gamma amino butyric acid; HPLC-ED-high performance liquid chromatography with electrochemical detection; LC-locus coeruleus; mPFC-medial prefrontal cortex; NE-norepinephrine; PTSD-post-traumatic stress disorder; pyr-pyramidal neurons; TH-tyrosine hydroxylase;.
Alternative mechanisms underlying CB1 receptor modulation of norepinephrine include actions on cortical GABAergic neurons (Bodor et al., 2005) that impact on NE release (Figure 8). Cannabinoids may also impact cortical noradrenergic transmission via regulation of catabolism of monoamine neurotransmitters, such as actions on monoamine oxidase (Fisar, 2012). In addition to these direct cortical mechanisms, cannabinoids may impact noradrenergic transmission via actions on the coeruleo-cortical pathway through regulation of NE synthesis (Page et al., 2007; Moranta et al., 2004), alterations in CB1 receptor mRNA in LC neurons (Herkenham et al., 1990; Hohmann and Herkenham, 1999) or presynaptic influences within the LC, the source of NE afferents to the PFC. Finally, interactions may occur at the receptor level as recent data indicate that CB1 receptors form homo- and heteromeric complexes with other catecholamine receptors (Laviolette and Grace, 2006; Rasmussen et al., 2009). Physical interactions have been reported between CB1r and β2-AR and, in human embryonic kidney 293H cells, co-expression of β2-AR tempered the constitutive activity and increased cell surface expression of CB1rs (Hudson et al., 2012). The Gi-coupled CB1 receptor and dopamine D2 receptor are present in many of the same postsynaptic striatal neurons in which their dual in vitro activation elicits an unexpected stimulatory effect on cAMP (Glass and Felder, 1997; Kearn et al., 2005).
Complex bidirectional functional interactions have also been described between cannabinoid and dopaminergic pathways (Laviolette and Grace, 2006; Cheer et al., 2007; Lupica et al., 2004; Melis et al., 2004; Szabo et al., 2002). Administration of CB1 receptor agonists results in increased dopamine levels in the striatum, specifically in the shell of the nucleus accumbens (Acb) (Bossong et al., 2009; Chen et al., 1990; Fadda et al., 2006; Malone and Taylor, 1999; Ng Cheong Ton et al., 1988; Tanda et al., 1997). Evidence in support of a direct influence of cannabinoids on dopaminergic transmission is based on studies showing that release of dopamine results from CB1 receptor binding on gamma amino butyric acid (GABA) neurons, which disinhibits DA release in the Acb (Hoffman and Lupica, 2001). Indirect influences point to stimulation of DA neurons in the ventral tegmental area through cannabinoid receptor interaction with GABA and glutamate systems (Schlicker and Kathmann, 2001). The presynaptic mechanisms of CB1 receptor modulation underlying the suppression of transmitter release are likely distinct at different synapses. In addition, modulation of neurotransmitter release by cannabinoid receptor agonists can be different depending on neuronal firing rate (Roloff and Thayer, 2009).
The present electrophysiology data show that acute WIN blocks α2-AR-mediated pyramidal cell excitability. Carr and colleagues (Carr et al., 2007) showed that α2-AR-mediated increases in pyramidal cell excitability were independent of NE, implicating postsynaptic α2-ARs in the effect. The α2-AR is also localized post-synaptically on cortical neurons in the mPFC (Carr et al., 2007) as well as other regions including the hypothalamic magnocellular neurosecretory cells (Kuzmiski et al., 2009). Our data suggest that WIN may be desensitizing postsynaptic α2-ARs. Cannabinoid-induced attenuation in norepinephrine release following stressor exposure may appear paradoxical but could reflect differences in actions of cannabinoids that are state dependent and also involve pre- and postsynaptic receptors. In support of this idea, we found that CB1-α2-AR interactions were influenced by history of cannabinoid exposure and stress. In subjects exposed to acute stress, prior chronic CB1 receptor stimulation was no longer able to inhibit α2-AR function. These data suggest that cannabinoids normally restrain α2-mediated pyramidal cell excitability via postsynaptic α2-AR desensitization but that stress interferes with that process, potentially contributing to over-activation of these neurons.
There is growing evidence that the endocannabinoid system participates in the regulation of mood and is altered by stress. In support of this, the CB1 receptor is present in stress responsive circuits (PFC, amygdala and hypothalamus) that are essential to the expression of anxiety (Oropeza et al., 2005; Herkenham et al., 1990). Acute restraint stress has been shown to increase the synthesis of endogenous endocannabinoids in limbic forebrain areas (Martin et al., 2002; Haller et al., 2002; Patel et al., 2005). In addition, release of endocannabinoids has been shown to mediate opioid-independent stress-induced analgesia by actions in the periaqueductal gray (Hohmann et al., 2005). Complex interactions exist between the cannabinoid system and stress responsivity. Low doses of cannabinoid agonists administered in familiar, non-stressful environments, typically result in positive responses such as enhanced euphoria and a reduction in anxiety (Hollister, 1986). However, dysphoric reactions are commonly manifested as panic, anxiety and paranoia and occur in response to high doses of consumption (Hollister, 1986) or when the drug is administered in environments that are stressful (Gregg et al., 1976; Talbott and Teague, 1969). The effect of CB1r agonist pre-treatment on decreases in climbing behavior is consistent with involvement of the coeruleo-cortical pathway. Climbing behavior in the swim model is an active arousal-related behavior that has been attributed to availability of norepinephrine.
The present study has implications for advancing our understanding of cannabinoid-adrenergic interactions in the development of improved treatment strategies for stress-induced anxiety. The pathophysiology underlying anxiety disorders may be related to an inability to extinguish aversive memories (Lehner et al., 2009). Repeated re-consolidation of fear memories in limbic circuits and inability to extinguish fear memories (Jovanovic and Ressler, 2012) are thought to underlie the pathophysiology of post-traumatic stress disorder (PTSD). Consolidation of emotionally arousing memories involves, in part, noradrenergic circuits targeting the amygdala (Ferry et al., 1999; McGaugh et al., 1996), while extinction of memory is dependent on the mPFC (Mueller and Cahill, 2010; Mueller et al., 2008). Pharmacological manipulation of AR systems has provided symptomatic relief in PTSD patients (Byers et al., 2010; Taylor et al., 2008) suggesting that therapeutic improvement may result, in part, from attenuation of signaling of sensitized ARs. Moreover, the cannabinoid receptor agonist, nabilone, has recently been reported to be effective in management of symptoms of PTSD (Fraser, 2009). Taken with evidence that the endocannabinoid and noradrenergic systems interact in stress-related memory consolidation (Hill and McEwen, 2012; Campolongo et al., 2009) targeting interactions between these two systems may represent a novel approach for the treatment of stress-induced anxiety disorders. Given that the mPFC represents a critical region in mediating the extinction of traumatic/aversive memories, treatments that target this region may help alleviate symptoms of anxiety disorders by increasing extinction of such memories.
Research Highlights.
WIN 55,212-2 pre-treatment decreases stress-induced norepinephrine release in frontal cortex
Acute WIN 55,212-2 exposure desensitizes cortical α2-adrenergic receptors (α2-AR)
Cannabinoid modulation of adrenergic receptors is stress-dependent
Stress prevented the desensitization of α2-ARs produced by a history of cannabinoid exposure
Acknowledgements
Supported by NIDA DA 020129 (E.J.V.B.), DA 020126 (L.K.), Department of Veterans Affairs Research and Development Services Northwest Network Mental Illness Research, Education, and Clinical Center (P.S.) and Geriatric Research, Education, and Clinical Center (C.S.). The authors would like to thank Dr. Michelle Page for contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur. J. Pharmacol. 2000;401:179–185. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J. Neurosci. 1987;7:2844–2848. doi: 10.1523/JNEUROSCI.07-09-02844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE, Dhanjal SS, Dingledine R, Garthwaite J, Henderson G, King GL. In: Brain slice methods. Dingledine R, editor. Brain slice Plenum Press; New York, New York: 1984. pp. 381–438. [Google Scholar]
- Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Abercrombie ED. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience. 1999;93:1263–1270. doi: 10.1016/s0306-4522(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J. Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J. Clin. Psychopharmacol. 2010;30:225–229. doi: 10.1097/JCP.0b013e3181dac52f. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc. Natl. Acad. Sci. USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, Lavin A. alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J. Physiol. 2007;584:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Mackie K, Van Bockstaele EJ. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur. J. Neurosci. 2010a;31:286–301. doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology (Berl) 2010b;211:479–491. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassens G, Roffman M, Kuruc A, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism induced by environmental stimuli previously paired with inescapable shock. Science. 1980;209:1138–1140. doi: 10.1126/science.7403874. [DOI] [PubMed] [Google Scholar]
- Cathel AM, Carvalho AF, Van Bockstaele EJ, Kirby LG. Cannabinoid modulation of alpha2 adrenergic receptor function in rat prefrontal cortex. doi: 10.1111/ejn.12690. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrito F, Preziosi P. Rat brain alpha 2-pre- and postsynaptic receptors are different or differently modulated? J. Neurosci. Res. 1985;14:423–431. doi: 10.1002/jnr.490140405. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J. Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Dennis T, L’Heureux R, Carter C, Scatton B. Presynaptic alpha-2 adrenoceptors play a major role in the effects of idazoxan on cortical noradrenaline release (as measured by in vivo dialysis) in the rat. J. Pharmacol. Exp. Ther. 1987;241:642–649. [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. Functional interactions between cannabinoid and metabotropic glutamate receptors in the central nervous system. Curr. Opin. Pharmacol. 2003;3:46–53. doi: 10.1016/s1471-4892(02)00014-0. [DOI] [PubMed] [Google Scholar]
- Drews E, Schneider M, Koch M. Effects of the cannabinoid receptor agonist WIN 55,212-2 on operant behavior and locomotor activity in rats. Pharmacol. Biochem. Behav. 2005;80:145–150. doi: 10.1016/j.pbb.2004.10.023. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2008;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26:77–89. doi: 10.1016/s0306-4530(00)00040-8. [DOI] [PubMed] [Google Scholar]
- Egashira N, Ishigami N, Mishima K, Iwasaki K, Oishi R, Fujiwara M. Delta9-Tetrahydrocannabinol-induced cognitive deficits are reversed by olanzapine but not haloperidol in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:499–506. doi: 10.1016/j.pnpbp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, Fratta W. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17:1629–1632. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biol. Psychiatry. 1999;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Fisar Z. Cannabinoids and monoamine neurotransmission with focus on monoamine oxidase. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012 doi: 10.1016/j.pnpbp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Flugge G, Van Kampen M, Mijnster MJ. Perturbations in brain monoamine systems during stress. Cell Tissue Res. 2004;315:1–14. doi: 10.1007/s00441-003-0807-0. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb. Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J. Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD) CNS Neurosci. Ther. 2009;15:84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fride E, Suris R, Weidenfeld J, Mechoulam R. Differential response to acute and repeated stress in cannabinoid CB1 receptor knockout newborn and adult mice. Behav. Pharmacol. 2005;16:431–440. doi: 10.1097/00008877-200509000-00016. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br. J. Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb AJ, Edwards FA. Patch clamp recording from cells in sliced tissues. In: Standen NB, editor. Microelectrode Techniques, the Plymouth Workshop Handbook. Company of Biologists; Cambridge, UK: 1994. pp. 255–274. [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci. Biobehav. Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Gregg JM, Small EW, Moore R, Raft D, Toomey TC. Emotional response to intravenous delta9tetrahydrocannabinol during oral surgery. J. Oral Surg. 1976;34:301–313. [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB(1) receptor agonist HU-210 following chronic stress. Eur. J. Pharmacol. 2004;499:291–295. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacol. Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- Hudson BD, Hebert TE, Kelly ME. Physical and functional interaction between CB1 cannabinoid receptors and beta2-adrenoceptors. Br. J. Pharmacol. 2012;160:627–642. doi: 10.1111/j.1476-5381.2010.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry. 2012;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol. Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J. Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmiski JB, Pittman QJ, Bains JS. Metaplasticity of hypothalamic synapses following in vivo challenge. Neuron. 2009;62:839–849. doi: 10.1016/j.neuron.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol. Life Sci. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner M, Wislowska-Stanek A, Plaznik A. Extinction of emotional response as a novel approach of pharmacotherapy of anxiety disorders. Psychiatr. Pol. 2009;43:639–653. [PubMed] [Google Scholar]
- Leonard BE, Myint A. The psychoneuroimmunology of depression. Hum. Psychopharmacol. 2009;24:165–175. doi: 10.1002/hup.1011. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br. J. Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31:462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br. J. Pharmacol. 1999;128:21–26. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc. Natl. Acad. Sci. USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur. J. Pharmacol. 2009;624:71–76. doi: 10.1016/j.ejphar.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur. J. Pharmacol. 2006;534:83–88. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB(1) cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2007;52:617–625. doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR, Charney DS. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch. Gen. Psychiatry. 1996;53:117–128. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict. Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA. Differential effects of acute cannabinoid drug treatment, mediated by CB1 receptors, on the in vivo activity of tyrosine and tryptophan hydroxylase in the rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:516–524. doi: 10.1007/s00210-004-0921-x. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav. Brain Res. 2010;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J. Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol. Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, Gardner EL. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Valentino RJ. Locus coeruleus activation by physiological challenges. Brain Res. Bull. 1994;35:557–560. doi: 10.1016/0361-9230(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Page ME, Lucki I. Effects of acute and chronic reboxetine treatment on stress-induced monoamine efflux in the rat frontal cortex. Neuropsychopharmacology. 2002;27:237–247. doi: 10.1016/S0893-133X(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Page ME, Brown K, Lucki I. Simultaneous analyses of the neurochemical and behavioral effects of the norepinephrine reuptake inhibitor reboxetine in a rat model of antidepressant action. Psychopharmacology (Berl) 2003;165:194–201. doi: 10.1007/s00213-002-1269-x. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol. Biochem. Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci. Lett. 2008;431:1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1986. [DOI] [PubMed] [Google Scholar]
- Pudovkina OL, Kawahara Y, de Vries J, Westerink BH. The release of noradrenaline in the locus coeruleus and prefrontal cortex studied with dual-probe microdialysis. Brain Res. 2001;906:38–45. doi: 10.1016/s0006-8993(01)02553-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Kim E, Unterwald EM, Rawls SM. Methanandamide attenuates cocaine-induced hyperthermia in rats by a cannabinoid CB(1)-dopamine D(2) receptor mechanism. Brain Res. 2009 doi: 10.1016/j.brainres.2008.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff AM, Thayer SA. Modulation of excitatory synaptic transmission by Delta 9-tetrahydrocannabinol switches from agonist to antagonist depending on firing rate. Mol. Pharmacol. 2009;75:892–900. doi: 10.1124/mol.108.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands SA, Strong R, Corbitt J, Morilak DA. Effects of acute restraint stress on tyrosine hydroxylase mRNA expression in locus coeruleus of Wistar and Wistar-Kyoto rats. Brain Res. Mol. Brain Res. 2000;75:1–7. doi: 10.1016/s0169-328x(99)00255-7. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Senn R, Keren O, Hefetz A, Sarne Y. Long-term cognitive deficits induced by a single, extremely low dose of tetrahydrocannabinol (THC): behavioral, pharmacological and biochemical studies in mice. Pharmacol. Biochem. Behav. 2008;13:230–237. doi: 10.1016/j.pbb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Shinba T, Ozawa N, Yoshii M, Yamamoto K. Delayed increase of brain noradrenaline after acute footshock stress in rats. Neurochem. Res. 2010;35:412–417. doi: 10.1007/s11064-009-0070-1. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog. Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur. J. Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb. Exp. Pharmacol. 2005:327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Talbott JA, Teague JW. Marihuana psychosis. Acute toxic psychosis associated with the use of Cannabis derivatives. JAMA. 1969;210:299–302. doi: 10.1001/jama.210.2.299. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur. J. Neurosci. 1997;9:2077–2085. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am. J. Health Syst. Pharm. 2008;65:716–722. doi: 10.2146/ajhp070124. [DOI] [PubMed] [Google Scholar]
- Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol. 2012;44:75–85. doi: 10.1677/JME-08-0190. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation. Adv. Pharmacol. 1998;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha2A adrenoceptors during rat neocortical development. J Neurobiol. 1999;38:259–269. doi: 10.1002/(sici)1097-4695(19990205)38:2<259::aid-neu8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr., DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]