The obesity epidemic has led to a marked increase in the incidence of type 2 diabetes mellitus, metabolic syndrome, and systemic hypertension. When present, these comorbidities contribute to the adverse cardiovascular outcomes that have been associated with obesity. In fact, for overweight individuals, the hazard ratio for cardiovascular mortality has been reported to be 1.4-2.8 (stratified by increasing body mass index) over 10-26 years of follow-up 1. This excess cardiovascular risk appears to be confined to obese individuals with coronary heart disease risk factors, particularly systemic hypertension. A recent landmark study of the Framingham Offspring cohort demonstrated convincingly that arterial stiffness, a vascular pathophenotype characterized by progressive stiffening of large conduit vessels that perturbs blood flow patterns in the distal vasculature, precedes increases in systolic blood pressure and incident hypertension 2. Other studies have established that arterial stiffness is present in individuals with obesity or metabolic syndrome and can predict future cardiovascular events 3. In some cases, weight reduction has been shown to decrease arterial stiffness, in part, through the loss of total body and abdominal adiposity, suggesting that vascular stiffness may be reversible 1. Thus, arterial distensibility may be considered as a measure of vascular health and progression to arterial stiffening may be viewed as a biomarker to predict future cardiovascular events 4.
While epidemiological studies highlight the association between obesity, arterial stiffness and hypertension, they are unable to inform on causality, particularly at a cellular or molecular level. To date, there have been few mechanistic studies examining the pathogenesis of arterial stiffness in obesity using models that recapitulate the phenomenon as it occurs in humans: high-fat, high-sugar caloric excess. In this issue of Hypertension, Weisbrod et al provide some of the first long-awaited evidence to demonstrate that obesity induces arterial stiffness through several complementary mechanisms, including inflammation, endothelial dysfunction, and extracellular matrix remodeling, to affect both vascular structure and function. Using a high-fat, high-sucrose fed mouse model of obesity and metabolic syndrome, they were also able to show that weight gain and metabolic derangements occur prior to or concomitant with arterial stiffening and that arterial stiffness is reversible with a return to a normal caloric dietary and weight loss 5.
The finding that inflammation and endothelial dysfunction are contributors to obesity-induced arterial stiffness is not surprising given their role in vascular dysfunction. There was infiltration of perivisceral adipose tissue with activated macrophages as well as elevated vascular expression of pro-inflammatory cytokines. There was also evidence of endothelial dysfunction and impaired vascular reactivity, indicating a decrease in bioavailable nitric oxide, as well as an increase in vascular oxidant stress as shown by oxidative posttranslational modification of sarco/endoplasmic reticulum Ca2+-ATPase 5. In addition to favoring vasoconstriction, these processes initiate vascular remodeling by stimulating cell proliferation, migration, hypertrophy, or extracellular matrix modification. There was vascular remodeling in obese mice, which developed aortic medial hypertrophy; however, this finding was not attributable to an increase collagen deposition and was not reversible by diet. While the etiology of the aortic hypertrophy remains unclear, it is evident that this alone did not account for the observed arterial stiffening. Instead there was evidence of extracellular matrix remodeling as demonstrated by the increase in activity of transglutaminase-2, which is nitric oxide sensitive and increases extracellular matrix cross-linking 5. Therefore, the mechanisms underlying arterial stiffness in this model appear to be related and may be co-dependent.
The findings from this study are in concert with prior works that have explored putative mechanisms to explain arterial stiffness related to other disease states (Fig. 1). Extracellular matrix remodeling that influences the integrity of collagen, elastin, or other components may contribute to vascular rigidity. For example, matrix metalloproteinase(s) activation promotes elastin cleavage and release of pro-inflammatory fragments that increase vascular stiffness 6. Extracellular matrix glycosaminoglycans can lose their viscoelastic properties while changes to fibronectin or integrin expression may increase stiffness by firming attachment of vascular smooth muscle cells to the extracellular matrix. Increased vascular rigidity may also be explained by the deposition of circulating calcific microparticles or the in situ formation of vascular microcalcifications 7.
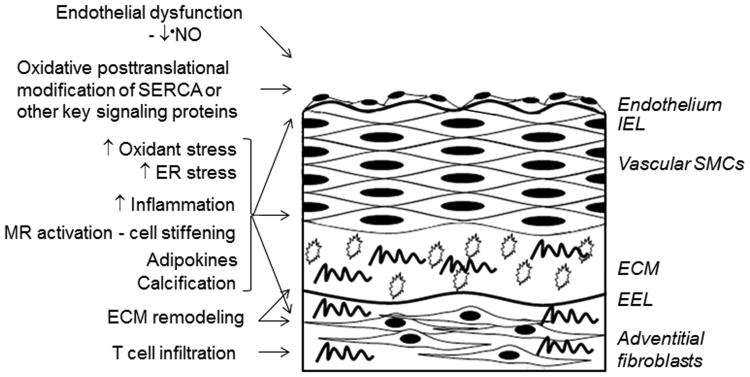
Figure 1.
Cellular and molecular mechanisms of arterial stiffening. Arterial stiffening appears to be an active process that results from complementary and possibly co-dependent mechanisms involving the endothelium, vascular smooth muscle cells (SMC), and adventitial fibroblasts. Adipocytes, inflammatory cells, and T cells have also been implicated as mediators of arterial stiffening through paracrine mechanisms. Decreased bioavailable nitric oxide (•NO), increased oxidant stress and oxidative posttranslational modifications of proteins, endoplasmic reticulum (ER) stress, and mineralocorticoid receptor (MR) activation have all been implicated in the pathogenesis of increased vascular rigidity. There may also be evidence of extracellular matrix (ECM) remodeling with increased collagen deposition (wavy lines), decreased integrity of collagen or elastin, and decreased viscoelasticity of glycosaminoglycans (open circles). IEL, internal elastic lamina; EEL, external elastic lamina.
Other studies have focused on the contributions of different cell types to arterial stiffness. Vascular smooth muscle cells themselves may become rigid and atomic force microscopy of cells isolated from hypertensive models has found an increase in cell membrane stiffness, similar to what was observed in the present study 6. This may occur as a result of mineralocorticoid receptor activation, which increases cellular water content and stiffness although short-term receptor blockade failed to improve vascular rigidity suggesting that the timing of this intervention maybe important 6, 8. Another possible mechanism is that of increased vascular cell endoplasmic reticulum stress, which would promote vascular cell stiffness by increasing the burden of aged and dysfunctional proteins and organelles in the cells 6. Owing to their role in hypertension, T cells have been proposed to mediate arterial stiffness and studies have linked interferon-γ and interleukin-6 to a loss of arterial distensibility 6. Adipocytes and adipokines have also been associated with arterial stiffening and the present study suggests an interaction between these mediators and vascular cells 9.
Obesity or its associated metabolic abnormalities may also initiate genetic or epigenetic changes that favor arterial stiffening. Arterial stiffness has been shown to be heritable and a genome-wide association study of 9 European ancestry cohorts identified a locus in the 3′-BCL11B gene desert on chromosome 14 that was associated with arterial stiffness. It was presumed that this area contained gene enhancers, regulatory elements, or microRNAs that regulate factors to modulate stiffness 10. While not investigated directly in the present study, future gene profiling from animal models of arterial stiffness may identify a cluster of coregulated genes localized to this area.
Perhaps the most intriguing finding from the current study is that weight loss and decreased adiposity improved arterial stiffness. With weight loss, indices of inflammation, endothelial dysfunction, and extracellular matrix remodeling returned to baseline pre-obesity levels. These changes occurred in association with an improvement in metabolic parameters so identifying a single responsible mediator may be difficult. It is, however, interesting to consider that weight loss alone might be as efficacious as pharmacological interventions aimed at improving vascular function and decreasing arterial stiffness. In future studies, it will be of importance to determine whether or not there is a remodeling threshold for the reversal of arterial stiffness beyond which diet alone will not be effective. Findings from this and other like mechanistic studies will help to establish additional genetic, biochemical, and structural remodeling processes that modulate obesity-related arterial stiffness and determine if diet alone or in combination with other pharmacotherapies is sufficient to ameliorate arterial stiffness.
Acknowledgments
Sources of Funding: This work is supported by National Institutes of Health grant HL105301 and the Thomas W. Smith MD Foundation.
This is a commentary on article Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62(6):1105-10.
Footnotes
Disclosures: NIH/NHLBI funding >$10,000
References
- 1.Lewis CE, McTigue KM, Burke LE, Poirier P, Eckel RH, Howard BV, Allison DB, Kumanyika S, Pi-Sunyer FX. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation. 2009;119:3263–3271. doi: 10.1161/CIRCULATIONAHA.109.192574. [DOI] [PubMed] [Google Scholar]
- 2.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safar ME, Balkau B, Lange C, Protogerou AD, Czernichow S, Blacher J, Levy BI, Smulyan H. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol. 2013;61:12–19. doi: 10.1016/j.jacc.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Briet M, Boutouyrie P. Arterial stiffness as surrogate end point: needed clinical trials. Hypertension. 2012;60:518–522. doi: 10.1161/HYPERTENSIONAHA.112.194456. [DOI] [PubMed] [Google Scholar]
- 5.Weisbrod RM, Shiang T, Al Sayah L, JL F, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell GF, Cohen RA, Seta F. Arterial stiffening preceds systolic hypertension in diet-induced obesity. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luft FC. Molecular mechanisms of arterial stiffness: new insights. J Am Soc Hypertens. 2012;6:436–438. doi: 10.1016/j.jash.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Safar ME, Lacolley P. Disturbance of macro- and microcirculation: relations with pulse pressure and cardiac organ damage. Am J Physiol Heart Circ Physiol. 2007;293:H1–7. doi: 10.1152/ajpheart.00063.2007. [DOI] [PubMed] [Google Scholar]
- 8.Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, Oberleithner K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. 2011;462:519–528. doi: 10.1007/s00424-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Windham BG, Griswold ME, Farasat SM, Ling SM, Carlson O, Egan JM, Ferrucci L, Najjar SS. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. Am J Hypertens. 23:501–507. doi: 10.1038/ajh.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell GF, Verwoert GC, Tarasov KV, Isaacs A, Smith AV, Yasmin, Rietzschel ER, Tanaka T, Liu Y, Parsa A, Najjar SS, O'Shaughnessy KM, Sigurdsson S, De Buyzere ML, Larson MG, ie MP, Andrews JS, Post WS, Mattace-Raso FU, McEniery CM, Eiriksdottir G, Segers P, Vasan RS, van Rijn MJ, Howard TD, McArdle PF, Dehghan A, Jewell ES, Newhouse SJ, Bekaert S, Hamburg NM, Newman AB, Hofman A, Scuteri A, De Bacquer D, Ikram MA, Psaty BM, Fuchsberger C, Olden M, Wain LV, Elliott P, Smith NL, Felix JF, Erdmann J, Vita JA, Sutton-Tyrrell K, Sijbrands EJ, Sanna S, Launer LJ, De Meyer T, Johnson AD, Schut AF, Herrington DM, Rivadeneira F, Uda M, Wilkinson IB, Aspelund T, Gillebert TC, Van Bortel L, Benjamin EJ, Oostra BA, Ding J, Gibson Q, Uitterlinden AG, Abecasis GR, Cockcroft JR, Gudnason V, De Backer GG, Ferrucci L, Harris TB, Shuldiner AR, van Duijn CM, Levy D, Lakatta EG, Witteman JC. Common genetic variation in the 3'-BCL11B gene desert is associated with carotid-femoral pulse wave velocity and excess cardiovascular disease risk: the AortaGen Consortium. Circ Cardiovasc Genet. 2012;5:81–90. doi: 10.1161/CIRCGENETICS.111.959817. [DOI] [PMC free article] [PubMed] [Google Scholar]