Abstract
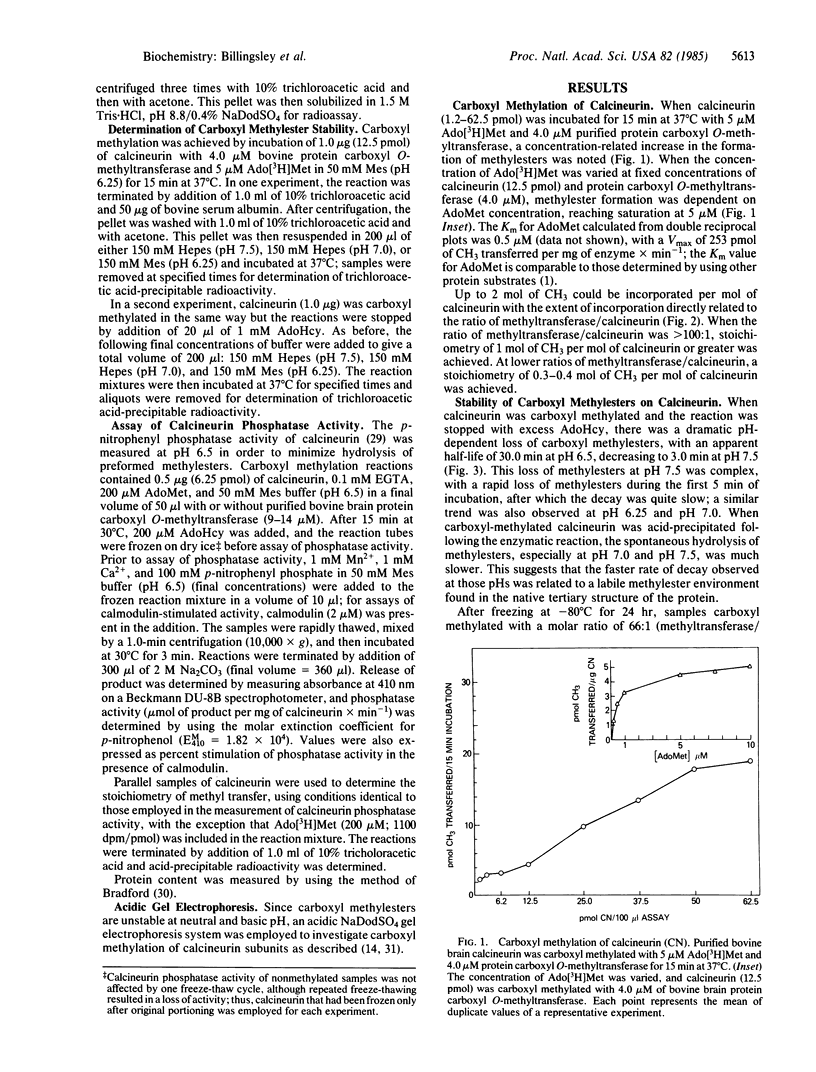
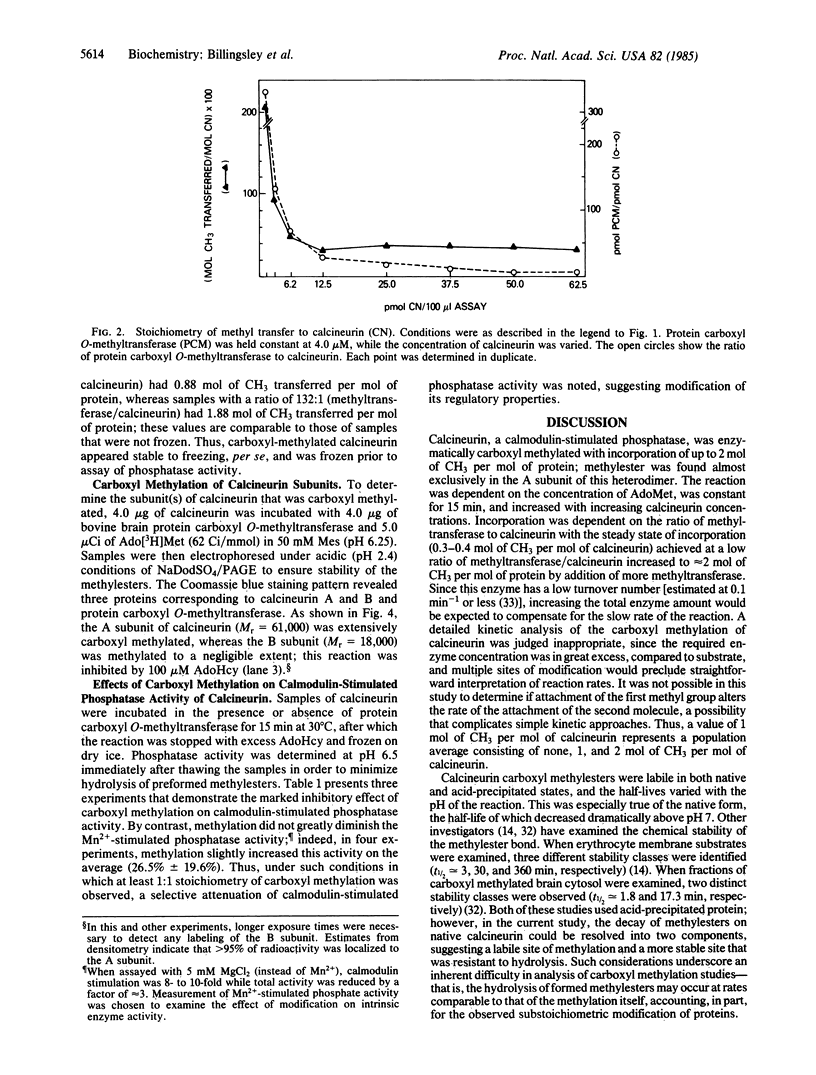
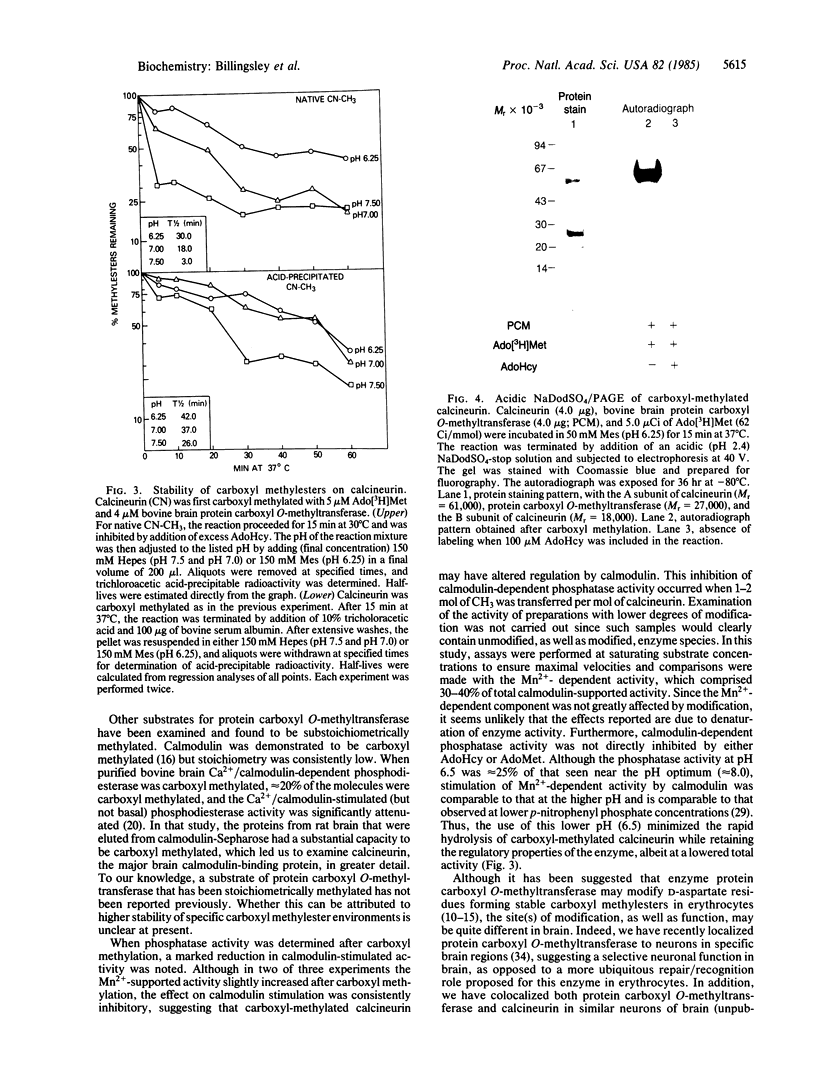
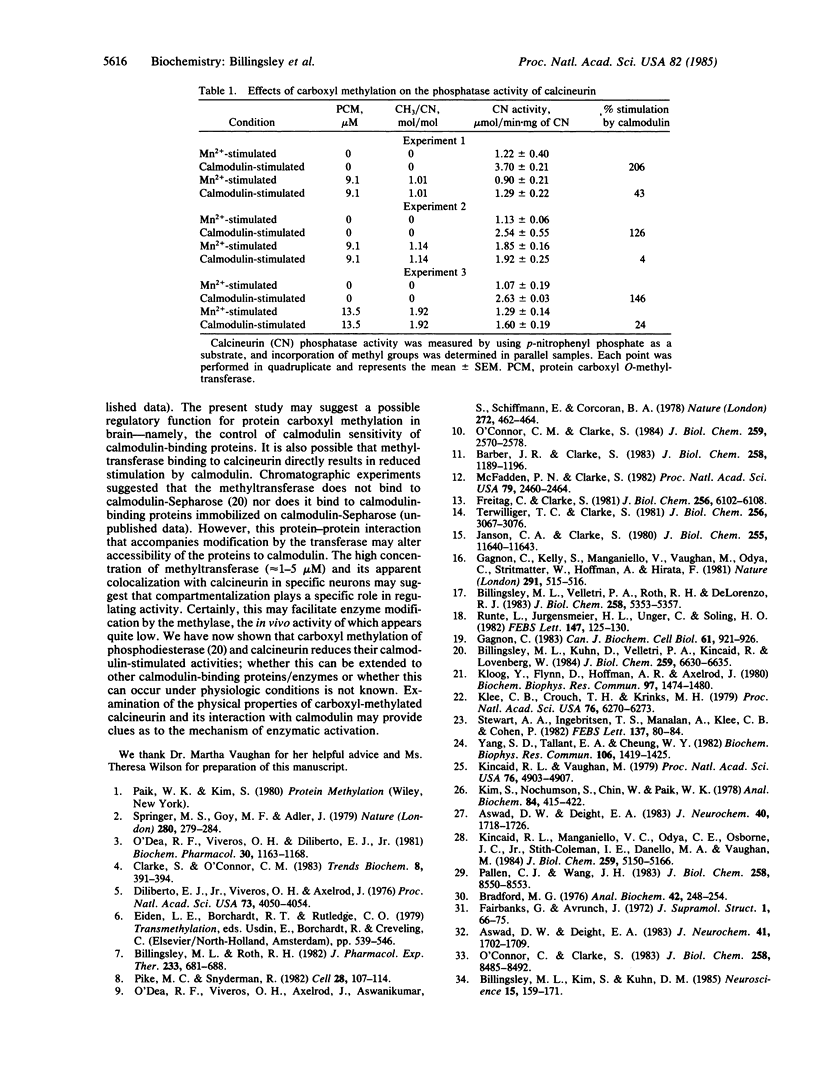
Calcineurin, a calmodulin-stimulated protein phosphatase, was a substrate for purified bovine brain protein carboxyl O-methyltransferase (protein O-methyltransferase; EC 2.1.1.24) and incorporated up to 2 mol of CH3 per mol of calcineurin. Carboxyl methylation was dependent on the concentrations of S-adenosyl-L-[methyl-3H]methionine and was prevented by addition of the carboxyl methylation inhibitor S-adenosylhomocysteine. The stoichiometry of methyl group incorporation was related to the ratio of methyltransferase/calcineurin. The rate of spontaneous hydrolysis of carboxyl methylester groups on calcineurin increased rapidly above pH 6.5 with those on native carboxyl-methylated calcineurin substantially more labile than for trichloracetic acid-precipitated calcineurin. Polyacrylamide gel electrophoresis in the presence of NaDodSO4 (pH 2.4) confirmed that the A subunit of calcineurin (Mr = 61,000) was the primary site of carboxyl methylation with little, if any, modification of the B subunit (Mr = 18,000). When carboxyl-methylated calcineurin (approximately 1-2 mol of CH3 per mol of protein) was assayed for p-nitrophenyl phosphatase activity at pH 6.5, a marked inhibition of calmodulin-stimulated activity was observed while there was little effect on Mn2+-stimulated phosphatase activity. Thus, calcineurin appears to be an excellent substrate for protein carboxyl O-methylation and this modification, which impairs calmodulin stimulation of phosphatase activity, may be of functional significance.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswad D. W., Deight E. A. Endogenous substrates for protein carboxyl methyltransferase in cytosolic fractions of bovine brain. J Neurochem. 1983 Dec;41(6):1702–1709. doi: 10.1111/j.1471-4159.1983.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Aswad D. W., Deight E. A. Purification and characterization of two distinct isozymes of protein carboxymethylase from bovine brain. J Neurochem. 1983 Jun;40(6):1718–1726. doi: 10.1111/j.1471-4159.1983.tb08147.x. [DOI] [PubMed] [Google Scholar]
- Barber J. R., Clarke S. Membrane protein carboxyl methylation increases with human erythrocyte age. Evidence for an increase in the number of methylatable sites. J Biol Chem. 1983 Jan 25;258(2):1189–1196. [PubMed] [Google Scholar]
- Billingsley M. L., Kim S., Kuhn D. M. Immunohistochemical localization of protein-O-carboxylmethyltransferase in rat brain neurons. Neuroscience. 1985 May;15(1):159–171. doi: 10.1016/0306-4522(85)90130-7. [DOI] [PubMed] [Google Scholar]
- Billingsley M. L., Roth R. H. Dopamine agonists stimulate protein carboxylmethylation in striatal synaptosomes. J Pharmacol Exp Ther. 1982 Dec;223(3):681–688. [PubMed] [Google Scholar]
- Billingsley M. L., Velletri P. A., Roth R. H., DeLorenzo R. J. Carboxylmethylation of calmodulin inhibits calmodulin-dependent phosphorylation in rat brain membranes and cytosol. J Biol Chem. 1983 May 10;258(9):5352–5357. [PubMed] [Google Scholar]
- Billingsley M., Kuhn D., Velletri P. A., Kincaid R., Lovenberg W. Carboxylmethylation of phosphodiesterase attenuates its activation by ca2+-calmodulin. J Biol Chem. 1984 May 25;259(10):6630–6635. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Diliberto D. J., Jr, Veiveros O. H., Axelrod J. Subcellualr distribution of protein carboxymethylase and its endogenous substrates in the adrenal medulla: possible role in excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4050–4054. doi: 10.1073/pnas.73.11.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Avruch J. Four gel systems for electrophoretic fractionation of membrane proteins using ionic detergents. J Supramol Struct. 1972;1(1):66–75. doi: 10.1002/jss.400010110. [DOI] [PubMed] [Google Scholar]
- Freitag C., Clarke S. Reversible methylation of cytoskeletal and membrane proteins in intact human erythrocytes. J Biol Chem. 1981 Jun 25;256(12):6102–6108. [PubMed] [Google Scholar]
- Gagnon C. Enzymatic carboxyl methylation of calcium-binding proteins. Can J Biochem Cell Biol. 1983 Aug;61(8):921–926. doi: 10.1139/o83-117. [DOI] [PubMed] [Google Scholar]
- Gagnon C., Kelly S., Manganiello V., Vaughan M., Odya C., Strittmatter W., Hoffman A., Hirata F. Modification of calmodulin function by enzymatic carboxylic methylation. Nature. 1981 Jun 11;291(5815):515–516. doi: 10.1038/291515a0. [DOI] [PubMed] [Google Scholar]
- Janson C. A., Clarke S. Identification of aspartic acid as a site of methylation in human erythrocyte membrane proteins. J Biol Chem. 1980 Dec 25;255(24):11640–11643. [PubMed] [Google Scholar]
- Kim S., Nochumson S., Chin W., Paik W. K. A rapid method for the purification of S-adenosylmethionine: protein-carboxyl O-methyltransferase by affinity chromatography. Anal Biochem. 1978 Feb;84(2):415–422. doi: 10.1016/0003-2697(78)90059-3. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Kincaid R. L., Vaughan M. Sequential adsorption-electrophoresis: combined procedure for purification of calcium-dependent cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4903–4907. doi: 10.1073/pnas.76.10.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog Y., Flynn D., Hoffman A. R., Axelrod J. Enzymatic carboxymethylation of the nicotinic acetylcholine receptor. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1474–1480. doi: 10.1016/s0006-291x(80)80031-3. [DOI] [PubMed] [Google Scholar]
- McFadden P. N., Clarke S. Methylation at D-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2460–2464. doi: 10.1073/pnas.79.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. M., Clarke S. Carboxyl methylation of cytosolic proteins in intact human erythrocytes. Identification of numerous methyl-accepting proteins including hemoglobin and carbonic anhydrase. J Biol Chem. 1984 Feb 25;259(4):2570–2578. [PubMed] [Google Scholar]
- O'Connor C. M., Clarke S. Methylation of erythrocyte membrane proteins at extracellular and intracellular D-aspartyl sites in vitro. Saturation of intracellular sites in vivo. J Biol Chem. 1983 Jul 10;258(13):8485–8492. [PubMed] [Google Scholar]
- O'Dea R. F., Viveros O. H., Axelrod J., Aswanikaumar S., Schiffmann E., Corcoran B. A. Raipid stimulation of protein carboxymethylation in leukocytes by a chemotatic peptide. Nature. 1978 Mar 30;272(5652):462–464. doi: 10.1038/272462a0. [DOI] [PubMed] [Google Scholar]
- O'Dea R. F., Viveros O. H., Diliberto E. J., Jr Protein carboxymethylation: role in the regulation of cell functions. Biochem Pharmacol. 1981 Jun 1;30(11):1163–1168. doi: 10.1016/0006-2952(81)90292-6. [DOI] [PubMed] [Google Scholar]
- Pallen C. J., Wang J. H. Calmodulin-stimulated dephosphorylation of p-nitrophenyl phosphate and free phosphotyrosine by calcineurin. J Biol Chem. 1983 Jul 25;258(14):8550–8553. [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Transmethylation reactions regulate affinity and functional activity of chemotactic factor receptors on macrophages. Cell. 1982 Jan;28(1):107–114. doi: 10.1016/0092-8674(82)90380-4. [DOI] [PubMed] [Google Scholar]
- Runte L., Jürgensmeier H. L., Unger C., Söling H. D. Calmodulin carboxylmethyl ester formation in intact human red cells and modulation of this reaction by divalent cations in vitro. FEBS Lett. 1982 Oct 4;147(1):125–130. doi: 10.1016/0014-5793(82)81025-9. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Protein methylation in behavioural control mechanisms and in signal transduction. Nature. 1979 Jul 26;280(5720):279–284. doi: 10.1038/280279a0. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Clarke S. Methylation of membrane proteins in human erythrocytes. Identification and characterization of polypeptides methylated in lysed cells. J Biol Chem. 1981 Mar 25;256(6):3067–3076. [PubMed] [Google Scholar]
- Yang S. D., Tallant E. A., Cheung W. Y. Calcineurin is a calmodulin-dependent protein phosphatase. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1419–1425. doi: 10.1016/0006-291x(82)91272-4. [DOI] [PubMed] [Google Scholar]