Abstract
Background
Respiratory failure is a leading cause of neonatal mortality in the developing world. Bubble continuous positive airway pressure (bCPAP) is a safe, effective intervention for infants with respiratory distress and is widely used in developed countries. Because of its high cost, bCPAP is not widely utilized in low-resource settings. We evaluated the performance of a new bCPAP system to treat severe respiratory distress in a low resource setting, comparing it to nasal oxygen therapy, the current standard of care.
Methods
We conducted a non-randomized convenience sample study to test the efficacy of a low-cost bCPAP system treating newborns with severe respiratory distress in the neonatal ward of Queen Elizabeth Central Hospital, in Blantyre, Malawi. Neonates weighing >1,000 g and presenting with severe respiratory distress who fulfilled inclusion criteria received nasal bCPAP if a device was available; if not, they received standard care. Clinical assessments were made during treatment and outcomes compared for the two groups.
Findings
87 neonates (62 bCPAP, 25 controls) were recruited. Survival rate for neonates receiving bCPAP was 71.0% (44/62) compared with 44.0% (11/25) for controls. 65.5% (19/29) of very low birth weight neonates receiving bCPAP survived to discharge compared to 15.4% (1/13) of controls. 64.6% (31/48) of neonates with respiratory distress syndrome (RDS) receiving bCPAP survived to discharge, compared to 23.5% (4/17) of controls. 61.5% (16/26) of neonates with sepsis receiving bCPAP survived to discharge, while none of the seven neonates with sepsis in the control group survived.
Interpretation
Use of a low-cost bCPAP system to treat neonatal respiratory distress resulted in 27% absolute improvement in survival. The beneficial effect was greater for neonates with very low birth weight, RDS, or sepsis. Implementing appropriate bCPAP devices could reduce neonatal mortality in developing countries.
Introduction
Severe respiratory distress is a common and serious complication of premature birth, neonatal pneumonia, and neonatal sepsis, which together account for over one-half of all neonatal deaths globally [1]. More than 50% of babies born at ≤31 weeks of gestation will develop respiratory distress syndrome (RDS) [2]. Respiratory distress is associated with over 80% of cases of neonatal pneumonia [3] and most cases of neonatal sepsis [4].
In the developed world, respiratory support is provided to neonates using either mechanical ventilation or Continuous Positive Airway Pressure (CPAP). Unfortunately, ventilators and CPAP machines are too expensive and technically complex for many resource-limited settings [5]. As a result, respiratory illness remains one of the most common causes of neonatal death in the developing world.
CPAP is a gentle and effective tool to treat even preterm and low birth weight infants in respiratory distress [6]–[11]. Well-resourced hospitals use ventilators, stand-alone CPAP devices, or tubing, wall air and oxygen to set up CPAP at the bedside [8]. In bubble CPAP (bCPAP), pressure is safely regulated by submerging the end of the tubing in a bottle of water. The depth of water determines the pressure in the system. This pressure helps recruit alveoli and increase functional residual lung capacity [12], thus lowering the baby's work of breathing. The result is better compliance, reduced airway resistance, conservation of surfactant, and stabilized chest and diaphragm [13]. Bubble CPAP has been used in developed countries for decades [8]. It reduces morbidity [6] and mortality [5], [8], as well as the need for mechanical ventilation [6], [7], [9]–[11], [14], [15]. It can be administered by trained nurses [14], [16], and is safer than mechanical ventilation [6], [14]. It also reduces hospital stay [6] and up-referrals [17]. As a result CPAP is increasingly used as a first choice for ventilatory support in tertiary centres [9], [14], [18].
A number of observational studies have shown that CPAP can be safely implemented in low-resource settings using commercially available devices designed for high-resource settings [14], [19]–[22]. Unfortunately, the cost and complexity of currently available CPAP devices is prohibitive for many low-resource settings.
We recently developed a novel, low-cost bCPAP system for low-resource settings that can be assembled for approximately $350, a fifteen-fold cost reduction compared to the average stand-alone CPAP. The device delivers the same therapeutic flow and pressure as bCPAP systems used in high-resource settings [23]. Here, we report a study to evaluate the efficacy of the new low-cost bCPAP system in improving survival in newborns with severe respiratory distress at Queen Elizabeth Central Hospital in Malawi. Malawi has a population of 15.4 million [24], with a preterm birth rate of 18% [25], neonatal mortality rate of 27 and infant mortality rate of 53 per 1000 live births [26]. The GDP is $805 per capita and 73.9% of the population live on less than $1.25 per day [27]. Queen Elizabeth Central Hospital is the main referral hospital in the southern region of the country; each year, about 10,000 babies are delivered at the hospital and over 3,000 are admitted to the neonatal ward [28].
Methods
Ethics Statement
The study protocol was approved by the University of Malawi College of Medicine Research and Ethics Committee (P.05/11/1079) and the Institutional Review Boards at Baylor College of Medicine (H-29059) and Rice University (11-198F) prior to study initiation. Written informed consent was obtained from parents or legal guardians before enrolling patients in the study.
Participants
This prospective, non-randomized controlled study, conducted at Queen Elizabeth Central Hospital, evaluated the efficacy and safety of a novel, low-cost bCPAP device to treat neonatal respiratory illness in a low-resource setting. In Malawi, nasal oxygen from an oxygen concentrator is the standard of care to treat respiratory insufficiency in neonates. The study evaluated whether bCPAP treatment improves survival for neonates with respiratory illness compared to standard nasal oxygen.
Infants admitted to the neonatal ward with severe respiratory distress as defined by the presence of severe chest in-drawing, central cyanosis, wheezing, grunting, or nasal flaring were eligible to participate. In addition, patients had to weigh 1,000 grams or more at enrollment, be breathing spontaneously, and be neurologically viable. The treating clinician also had to deem bCPAP appropriate treatment. Patients who presented with cleft palate, trachea-oesophageal fistula, diaphragmatic hernia, severe cardiac instability, and severe birth asphyxia were not eligible. Subjects were identified by clinicians working in the neonatal ward.
Procedures
Two low-cost bCPAP devices were installed in the ward. Figure 1 illustrates the study procedure and outcome groups. Patients were treated with bCPAP (treatment group) if a bCPAP system and trained clinical staff were available. If a bCPAP device or trained clinical staff were not available, the patient received the local standard of care, nasal oxygen (control group). This selection process was a sampling method whereby each patient was assigned to a group based on availability of a bCPAP device and appropriate staff when treatment was initiated. In some cases, a child in the control group was transitioned from nasal oxygen to bCPAP treatment after entering the study when a bCPAP device became available.
Figure 1. Flow chart summarizing study procedure and outcome groups.
The bCPAP device has been described previously [23]. It consists of an adjustable flow generator, a pressure-regulator, and a patient interface (Fig. S1). Two pumps provide continuous flow of room air. The output of an oxygen concentrator (Airsep, New Life Intensity, 10 LPM) is connected to an input port on the device; two flow regulators adjust the flow rate and proportion of oxygen delivered. A pressure control tube submerged in a bottle of water controls end-expiratory pressure. The device delivers a mixture of pressurized air and oxygen at flow rates ranging from 0–10 L/min, pressures varying from 5–8 cm H2O, and oxygen ranging from 21–65%. The pumps are designed to operate for two years, and repair involves simple replacement of a US $0.50 diaphragm.
The bCPAP delivered a pressurized air mixture via Hudson bi-nasal prongs that were attached to a stockinette hat using safety pins and elastic bands (Fig. S1). Nursing care for children receiving bCPAP included twice daily suctioning to clear the airways of mucus. Sterile nasal saline drops were administered every four hours to reduce mucosal drying. Nasal oxygen was delivered from an oxygen concentrator (Airsep, New Life Intensity, 10 LPM) via standard nasal cannulae. The flow rate was set using a flow regulator and typically varied from 1–2 L/min.
Bubble CPAP and oxygen were administered until the treating clinician determined that therapy was no longer necessary. All patient care, with the exception of respiratory support, was the same for the bCPAP and standard-of-care groups. Information, excluding personal identifiers, was recorded by clinical personnel on a standardized patient monitoring form and included age, date of birth, presumed diagnosis, gender, weight, HIV status, vital signs, mode and tolerance of feeding, physical examination and method and duration of respiratory support. Vital signs were repeated one hour after recruitment (control group) or commencing bCPAP (treatment group), and twice daily afterward until discharge or death. Patients were monitored for progress and complications.
A study physician (K.K.) reviewed every child's chart for study eligibility. Each participant was assigned a final primary diagnosis of respiratory distress syndrome (RDS), congenital pneumonia, acquired pneumonia, meconium aspiration, transient tachypnoea of the newborn (TTN), or stridor based on standard clinical criteria. Co-morbidities, including sepsis and jaundice, were also noted.
Statistical analysis
We planned to recruit a total of 50 patients in the treatment arm, matched 1∶1 with controls, giving a total sample size of 100. This would, using a one-sided Fisher's exact test, achieve at least 85% power to detect at least a 30% mortality reduction with bCPAP treatment compared with oxygen therapy with type I error = 0.05. We assumed mortality rates respectively in the treatment and control groups of 40% and 70%. To allow for potential loss due to data collection errors, we planned to enroll up to 110 patients.
Data were recorded on paper forms, then entered into Excel and ported to SPSS for analysis.
We compared demographic data, primary diagnosis, co-morbidities, and vital signs at entry for the two groups. A two-sided t-test for equality of means was performed (equal variances not assumed) to determine whether differences in continuous variables were statistically significant; for categorical variables, a two-sided Fisher's exact test was performed. Results were considered significant at the 5% level.
We calculated the survival rate with 95% confidence intervals (CIs) for the two groups. The hypothesis that the rate of survival was higher for babies receiving bCPAP than in the control group was tested using a one-sided Fisher's exact test. Logistic regression was used to calculate the odds ratio for survival with 95% CIs for babies receiving bCPAP compared to standard care. Kaplan-Meier survival curves were calculated to show the probability of survival following time from treatment initiation for the two groups.
Univariate logistic regression analysis was performed to explore whether demographic and clinical covariates were related to survival for all eligible participants. Results were considered significant at the 5% level using a two-sided Fisher's exact test for categorical variables and a t-test for continuous variables.
Subgroup analysis was conducted for three of the covariates that were related to survival (RDS, sepsis, VLBW). For each subgroup, the hypothesis that survival was higher for babies receiving bCPAP than in the control group was tested using a one-sided Fisher's exact test. Logistic regression was used to calculate the odds ratio for survival with 95% CIs for babies receiving bCPAP compared to standard care. The subgroup analyses were not defined a priori.
Multiple logistic regression analysis was used to calculate the survival rates, odds ratio for survival and associated 95% CIs for babies receiving bCPAP compared to standard care, adjusted for differences in baseline values of RDS, sepsis, VLBW.
Results
Eighty-nine neonates were enrolled from January to October 2012 (Table 1). Chart review identified two neonates who did not meet eligibility criteria because they suffered severe birth asphyxia. Of the 87 eligible participants, 62 were treated with bCPAP therapy and 25 received oxygen therapy. The 62 neonates receiving bCPAP included nine who were initially assigned to the control group, but ultimately received bCPAP therapy when a device became available. Data were analysed for all 87 eligible neonates.
Table 1. Number of study participants and demographic data for subjects meeting eligibility criteria.
| Demographic and Clinical Covariates | Treatment Group: Nasal Oxygen | Treatment Group: bCPAP | |
| Transitioned from Nasal Oxygen to bCPAP | bCPAP | ||
| Number of study participants | |||
| Number of subjects completing study | 25 | 9 | 55 |
| Number of subjects meeting eligibility criteria | 25 | 9 | 53 |
| Gender | |||
| % Male | 60.0% | 44.4% | 60.4% |
| % Female | 40.0% | 55.6% | 39.6% |
| Gestational Age | |||
| Average (weeks) | 33.0 weeks | 32.0 weeks | 33.2 weeks |
| Unknown (%) | 12.0% | 0.0% | 9.4% |
| Average Birth Weight (kg) | 1.68 kg | 1.37 kg | 1.79 kg |
| Very Low Birth Weight (> = 1.0 kg–<1.5 kg) (%) | 52.0% | 41.5% | 77.8% |
| Low Birth Weight (> = 1.5 kg–<2.5 kg) (%) | 28.0% | 41.5% | 22.2% |
| Birth Weight > = 2.5 kg (%) | 20.0% | 17.0% | 0.0% |
| Location of birth | |||
| Queen Elizabeth Central Hospital (QECH) (%) | 60.0% | 55.6% | 56.6% |
| Outside QECH (%) | 32.0% | 44.4% | 32.1% |
| Unknown (%) | 8.0% | 0.0% | 11.3% |
| Singletons vs. Multiples | |||
| Singletons (%) | 52.0% | 44.5% | 73.6% |
| Multiples (%) | 44.0% | 44.4% | 26.4% |
| Unknown (%) | 4.0% | 11.1% | 0.0% |
| Received bag & mask ventilation prior to therapy? | |||
| Yes (%)* | 4.0% | 22.2% | 24.5% |
| No (%) | 88.0% | 66.7% | 54.7% |
| Unknown (%) | 8.0% | 11.1% | 20.8% |
| HIV Status | |||
| Exposed | 20.0% | 33.3% | 22.7% |
| Unexposed | 64.0% | 44.4% | 67.9% |
| Unknown | 16.0% | 22.2% | 9.4% |
| Entry vital statistics | |||
| Average entry heart rate (beats per minute (bpm)) | 144 bpm | 148 bpm | 141 bpm |
| Average entry respiratory rate (bpm) | 54 bpm | 55 bpm | 54 bpm |
| Average entry oxygen saturation (%) | 92% | 91% | 88% |
| Primary Diagnosis | |||
| RDS | 68.0% | 100.0% | 73.6% |
| Congenital Pneumonia | 4.0% | 0.0% | 18.9% |
| Acquired Pneumonia | 4.0% | 0.0% | 0.0% |
| Meconium Aspiration | 8.0% | 0.0% | 5.7% |
| Transient Tachypnea of the Newborn | 12.0% | 0.0% | 0.0% |
| Stridor | 4.0% | 0.0% | 0.0% |
| Asphyxia | 0.0% | 0.0% | 1.8% |
| Co-Morbidities | |||
| Sepsis | 28.0% | 44.4% | 41.5% |
Difference in group receiving nasal oxygen and bCPAP significantly different (p = 0.015).
Table 1 summarizes the demographic and clinical covariates for the eligible neonates. Differences in the mean values of covariates for the group receiving bCPAP were not statistically significant compared to those for the control group with one exception: the fraction of babies who required bag and mask ventilation before recruitment was higher in the bCPAP group than in the control group (p = 0.015). Most participants had very low birth weight (VLBW) and were born at QECH. Over 25% of neonates in each group were from multiple births, and approximately 20% of neonates in each group were exposed to HIV.
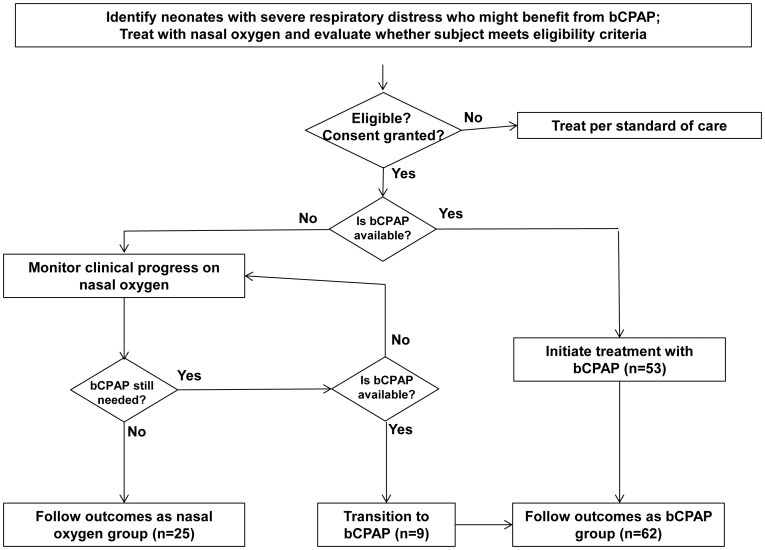
Figure 2 compares survival to discharge in the two groups. The survival rate in the control group was 44.0% (95% CI: 26–63%); survival in the bCPAP group was 71.0% (95% CI: 59–81%) (p = 0.018). Without adjustment, bCPAP is associated with a 3.1 fold increase in odds of survival (95% CI: 1.2–8.1, p = 0.02). Figure 2 also shows Kaplan-Meier survival curves of cumulative survival vs. time following initiation of treatment. These are different by the log rank test at p = 0.013.
Figure 2. Overall survival of eligible study participants receiving nasal oxygen vs. bCPAP.
(Left) Fraction of eligible study participants who survived to discharge and received nasal oxygen or bCPAP. When treated with bCPAP, the survival rate of infants with severe respiratory distress is significantly higher than for those treated with nasal oxygen (p = 0.018). Without adjustment, bCPAP is associated with a 3.1-fold increase in odds of survival (confidence interval 1.2–8.1, significance = 0.02). (Right) Kaplan-Meier survival curves showing cumulative survival vs. days since treatment initiation for infants with severe respiratory distress treated with bCPAP (n = 62) and those treated with nasal oxygen (n = 25).
Univariate logistic regression analysis indicated that five of the covariates in Table 1 were related to survival: a primary diagnosis of RDS (p = 0.002); co-morbidity of sepsis (p = 0.039); birth weight (p = 0.003); a birth weight in the VLBW range (p = 0.016); and gestational age (p = 0.023). Further subgroup analyses were performed by birth weight, presence of RDS and of sepsis. As gestational age was not available for approximately 10% of subjects, no subgroup analysis was conducted for this variable.
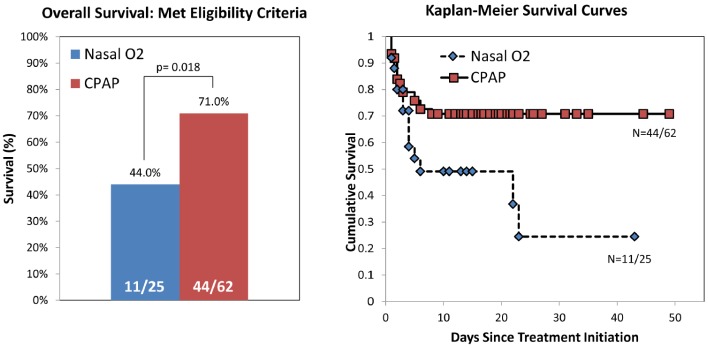
Figure 3 compares survival for eligible neonates with a primary diagnosis of RDS and those with sepsis. For neonates with RDS, survival was 23.5% (95% CI: 9–49%) in the control group, compared to 64.6% (95% CI: 50–77%) in the bCPAP group (p = 0.006). None of the seven neonates with sepsis in the control group survived, while survival was 61.5% (95% CI: 42–78%) in the bCPAP group (p = 0.005).
Figure 3. Survival of participants with RDS and sepsis receiving nasal oxygen vs. bCPAP.
(Left) Fraction of eligible study subjects with a primary diagnosis of respiratory distress syndrome (RDS) who survived to discharge and received nasal oxygen or bCPAP. (Right) Fraction of eligible study subjects with a co-morbidity of sepsis who survived to discharge and received nasal oxygen or bCPAP.
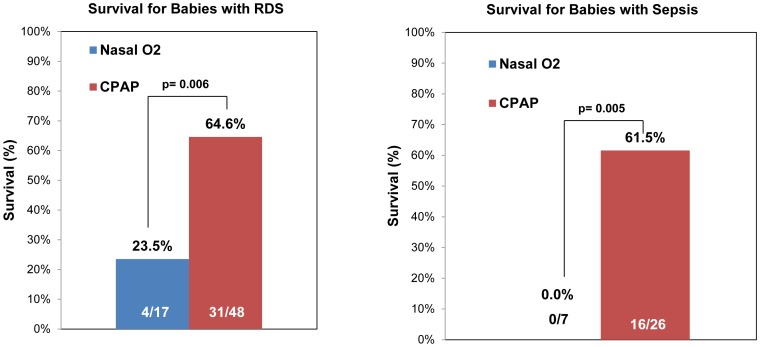
Figure 4 shows survival rates for eligible neonates in the two groups, stratified by birth weight. Improvements in survival are greatest for VLBW infants (≥1.0–<1.5 kg); in whom survival was 15.4% (95% CI: 4–45%) in the control group compared to 65.5% (95% CI: 47–80%) in the bCPAP group (p≤0.001). For babies with low birth weight (≥1.5 kg–<2.5 kg) and babies with birth weight ≥2.5 kg, differences in survival were not statistically different for the two groups.
Figure 4. Survival of participants by birthweight receiving nasal oxygen vs. bCPAP.
Fraction of eligible study subjects who survived to discharge and received nasal oxygen or bCPAP, stratified by birth weight. Results are reported for subjects with very low birth weight (> = 1.0 kg to <1.5 kg), low birth weight (> = 1.5 kg to <2.5 kg) and birth weights greater than or equal to2.5 kg.
Multiple logistic regression analysis was performed to adjust for baseline differences in RDS, sepsis, and VLBW; the adjusted survival rate for babies receiving bCPAP is 85% (95% CI: 69–94%), while that for standard care is 47% (95% CI: 23–73%) and bCPAP is associated with an adjusted 6.5 fold increase in odds of survival (95% CI: 1.9–22.7, p = 0.003).
Table 2 compares the secondary outcomes of treatment time and duration of hospital stay for all eligible subjects and for those who survived to discharge. On average, neonates receiving bCPAP spent five more days in the hospital and nearly three more days on treatment than those in the control group. On average, neonates who were transitioned from nasal oxygen to bCPAP spent twice as long in the hospital and twice as long on treatment as those who received bCPAP initially; the average delay in receiving bCPAP was 3.1 days. Similar trends in increased treatment and hospitalization time were seen for neonates who survived to discharge. The increase in treatment and hospitalization time in bCPAP could be explained by the differences in primary diagnosis between the two groups; children receiving bCPAP were more likely to suffer from RDS than those receiving nasal oxygen.
Table 2. Average duration of treatment and hospital stay (+/− standard deviation) for eligible subjects and survivors versus treatment group.
| All eligible neonates | Nasal Oxygen | bCPAP | Transitioned from nasal oxygen to bCPAP |
| Average hospital stay (days) | 9.10 (9.70) | 14.13 (10.87) | 22.00 (14.14) |
| Average time from study entry to treatment with bCPAP (days) | NA | 0.19 (0.42) | 3.11 (3.18) |
| Average total time on treatment (days) | 4.00 (4.37) | 6.91 (5.37) | 13.94 (11.45) |
| Average time receiving nasal oxygen (days) | 4.00 (4.37) | 1.84 (3.04) | 9.83 (9.68) |
| Average time receiving bCPAP (days) | NA | 5.07 (3.66) | 4.11 (2.87) |
Only mild complications were associated with bCPAP (Table S1), including nasal irritation, facial irritation, and epistaxis. Similar complication rates were observed in the control group. No clinical diagnoses of pneumothorax were made, but as routine x-rays and cranial ultrasound were unavailable, silent complications cannot be ruled out.
Discussion
This study demonstrates the potential impact of introducing a low-cost, appropriate bCPAP system in a low-resource setting where standard therapy is nasal oxygen. Using bCPAP to treat newborns with severe respiratory distress resulted in a 27% absolute improvement in survival to discharge. The benefit was more pronounced in neonates with VLBW, RDS, or sepsis. Only 24% of neonates with RDS treated with nasal oxygen survived to discharge, compared to 65% receiving bCPAP, reflecting similar rates of survival as observed in the US during the transition from nasal oxygen to bCPAP for treatment of RDS [5].
The value of CPAP therapy for neonatal RDS is well-accepted in high-resource settings but very few studies have examined the value of CPAP therapy in resource-limited settings lacking advanced respiratory support [16], [20], [29]. These studies have either included older children [16], examined immediate improvements in clinical endpoints rather than survival to hospital discharge [16], or been small, observational studies [20], [29].
Our study was carried out in a typical low-resource setting using a bCPAP that was conceived, designed and evaluated with input from local physicians and nurses, ensuring that the device addresses competencies and capacity of this under-resourced environment [23], The technical performance of the device was monitored throughout the study. Therapeutic flow and pressure met performance standards reliably without preventive maintenance, and no device failures occurred. In contrast, 40% of study oxygen concentrators failed during this same period when circuit boards were damaged by line voltage spikes. To meet cost and infrastructure constraints, the bCPAP device did not heat or humidify the mix of pressurized air and oxygen delivered via the nasal prongs. Instead, humidification was provided through routine use of nasal saline drops. This did not appear to result in significant complications (Table S1).
Our study has a number of limitations. As bCPAP is known to be an effective therapy [5], [6], [8], we did not perform a randomised trial. Instead, the decision to treat with bCPAP was based on availability of a bCPAP device. This design allowed potential bias. We planned to recruit equal numbers of patients in each arm to detect a difference between the two groups. However, since allocation of CPAP to eligible subjects was based on device availability actual enrollment was dependent on available equipment and staffing resources. Our original estimate was that CPAP devices would be available approximately half the time needed. To allow for potential loss due to data collection errors, we planned to enroll up to 110 patients. In practice, CPAP devices were available more frequently than not and thus more study participants received CPAP than nasal oxygen. The study was stopped when over 60 participants received CPAP; at this point, the projected number of CPAP patients had been enrolled plus the entire additional allowed margin to account for data loss.
In addition, this design could result in treating infants with more severe illness with bCPAP and those with less severe illness with nasal oxygen. Indeed a significantly higher proportion of infants treated with bCPAP required resuscitation prior to therapy (24.5%) than those who received nasal oxygen (4.0%). We monitored the status of children receiving nasal oxygen. Nine of them were transitioned to bCPAP when they failed to improve and a device became available. The outcomes of these nine children were analysed with the bCPAP group. All of the babies in the CPAP group received CPAP within 36 hours of being identified as needing CPAP, with a majority receiving it immediately. Five of the nine babies in the group that transitioned to CPAP also received CPAP treatment within 36 hours of being identified as needing CPAP. Because, in general, preterm infants who survive the first few postnatal days have an increased chance of long-term survival, transitioning babies into the CPAP group after a few days in the oxygen group might have created bias in favour of CPAP.
To account for this potential bias, we performed a per-protocol analysis, comparing outcomes for infants who received bCPAP to those who received nasal oxygen; babies who initially received oxygen but were later transitioned to CPAP are not included in the per-protocol analysis. The survival rate in the control group was 44.0%; survival in the bCPAP group was 69.8% (p = 0.014). For neonates with RDS, survival was 23.5% in the control group, compared to 61.5% in the bCPAP group (p = 0.004). Again, improvements in survival were found to be greatest for VLBW infants (≥1.0–<1.5 kg); in whom survival was 15.4% in the control group compared to 57.1% in the bCPAP group (p = 0.006).
Analysing these nine babies as part of the standard care group, analogous to intent-to-treat, we find that 69.8% (37/53) of children receiving bCPAP survived to discharge, compared to 52.9% (18/34) for standard care; the improvement in survival approaches significance (p = 0.087). Finally, we did not determine the optimal time to initiate or terminate treatment with bCPAP. Additional studies are needed to determine if earlier commencement of bCPAP could further improve outcomes in this setting.
Neonatal deaths account for 41% of global child mortality; the neonatal mortality rate has changed little in the last decade [30]. If our results are generalizable, we estimate that on the African continent, where nearly one million babies die each year within a week of birth [31], providing low-cost bCPAP in central and district hospitals could prevent 178,000 neonatal deaths. While the cost of the bCPAP device has been reduced, the cost and availability of consumables, staff support and support equipment remain a barrier to scale-up. Nonetheless, implementing such a system has the potential to improve neonatal care and health outcomes in low-resource settings.
Supporting Information
Photograph showing bubble CPAP device used in the study. bCPAP was delivered using Hudson prongs secured to a stretchy hat with safety pins and elastic bands. The bCPAP delivered a blended mix of air and oxygen from an oxygen concentrator at flow rates varying from 0–10 L/min and pressures ranging from 5–8 cm H2O.
(TIF)
Fraction of eligible participants in each treatment group who experienced minor complications during treatment.
(PDF)
Acknowledgments
We appreciate the contribution of Florence Mwenifumbo, Isabella Partridge, Chrissie Mbendera and Glissie Kantwela for their patient care and data collection. We also thank all doctors, nurses and staff working in the Gogo Chatinkha nursery for their contribution to and support for the study. We are grateful to the parents and guardians of the babies who took part in the study. Rice University and Texas Children's Hospital provided technical support, while the paediatric department of the University of Malawi and QECH provided ward accommodation, support for the study and care of the children.
Funding Statement
This work was made possible through the generous support of the Saving Lives at Birth partners: the United States Agency for International Development (USAID), the Government of Norway, the Bill & Melinda Gates Foundation, Grand Challenges Canada, and the World Bank. It was prepared by William Marsh Rice University and does not necessarily reflect the views of the Saving Lives at Birth Partners. Funding was also provided by the African Network for Drugs and Diagnostics Innovation (ANDI). 1. USAID Cooperative Agreement Award No. AID-OAA-A-13-00014, www.usaid.gov 2. African Network for Drugs and Diagnostics Innovation (ANDI), www.andi-africa.org. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lawn JE, Kerber K, Enweronu-Laryea C, Cousens S (2010) 3.6 Million Neonatal Deaths-What Is Progressing and What Is Not? Seminars in Perinatology 34: 371–386. [DOI] [PubMed] [Google Scholar]
- 2. St Clair C, Norwitz ER, Woensdregt K, Cackovic M, Shaw JA, et al. (2008) The probability of neonatal respiratory distress syndrome as a function of gestational age and lecithin/sphingomyelin ratio. Am J Perinatol 25: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duke T (2005) Neonatal pneumonia in developing countries. Archives of Disease in Childhood-Fetal and Neonatal Edition 90: 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gnanaratnem J, Finer NN (2000) Neonatal acute respiratory failure. Curr Opin Pediatr 12: 227–232. [DOI] [PubMed] [Google Scholar]
- 5. Kamath BD, MacGuire ER, McClure EM, Goldenberg RL, Jobe AH (2011) Neonatal Mortality From Respiratory Distress Syndrome: Lessons for Low-Resource Countries. Pediatrics 127: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Klerk AM, De Klerk RK (2001) Nasal continuous positive airway pressure and outcomes of preterm infants. Journal of Paediatrics and Child Health 37: 161–167. [DOI] [PubMed] [Google Scholar]
- 7. Nowadzky T, Pantoja A, Britton JR (2009) Bubble continuous positive airway pressure, a potentially better practice, reduces the use of mechanical ventilation among very low birth weight infants with respiratory distress syndrome. Pediatrics 123: 1534–1540. [DOI] [PubMed] [Google Scholar]
- 8. DiBlasi RM (2009) Nasal Continuous Positive Airway Pressure (CPAP) for the Respiratory Care of the Newborn Infant. Respiratory Care 54: 1209–1235. [PubMed] [Google Scholar]
- 9. Finer NN (2010) Early CPAP versus Surfactant in Extremely Preterm Infants (vol 362, pg 1970, 2010). New England Journal of Medicine 362: 2235–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morley CJ (2008) Nasal CPAP or intubation at birth for very preterm infants (vol 358, pg 700, 2008). New England Journal of Medicine 358: 1529–1529. [DOI] [PubMed] [Google Scholar]
- 11. Schmolzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, et al. (2013) Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ 347: f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Courtney SE, Pyon KH, Saslow JG, Arnold GK, Pandit PB, et al. (2001) Lung recruitment and breathing pattern during variable versus continuous flow nasal continuous positive airway pressure in premature infants: an evaluation of three devices. Pediatrics 107: 304–308. [DOI] [PubMed] [Google Scholar]
- 13. Rehan VK, Laiprasert J, Nakashima JM, Wallach M, McCool FD (2001) Effects of continuous positive airway pressure on diaphragm dimensions in preterm infants. J Perinatol 21: 521–524. [DOI] [PubMed] [Google Scholar]
- 14. Koyamaibole L, Kado J, Qovu JD, Colquhoun S, Duke T (2006) An evaluation of bubble-CPAP in a neonatal unit in a developing country: effective respiratory support that can be applied by nurses. J Trop Pediatr 52: 249–253. [DOI] [PubMed] [Google Scholar]
- 15. Gittermann MK, Fusch C, Gittermann AR, Regazzoni BM, Moessinger AC (1997) Early nasal continuous positive airway pressure treatment reduces the need for intubation in very low birth weight infants. Eur J Pediatr 156: 384–388. [DOI] [PubMed] [Google Scholar]
- 16. Wilson PT, Morris MC, Biagas KV, Otupiri E, Moresky RT (2012) A Randomized Clinical Trial Evaluating Nasal Continuous Positive Airway Pressure for Acute Respiratory Distress in a Developing Country. J Pediatr [DOI] [PubMed] [Google Scholar]
- 17. Buckmaster AG, Arnolda G, Wright IM, Foster JP, Henderson-Smart DJ (2007) Continuous positive airway pressure therapy for infants with respiratory distress in non tertiary care centers: a randomized, controlled trial. Pediatrics 120: 509–518. [DOI] [PubMed] [Google Scholar]
- 18. Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, et al. (2007) European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med 35: 175–186. [DOI] [PubMed] [Google Scholar]
- 19. Tagare A, Kadam S, Vaidya U, Pandit A, Patole S (2010) A pilot study of comparison of BCPAP vs. VCPAP in preterm infants with early onset respiratory distress. J Trop Pediatr 56: 191–194. [DOI] [PubMed] [Google Scholar]
- 20. Pieper CH, Smith J, Maree D, Pohl FC (2003) Is nCPAP of value in extreme preterms with no access to neonatal intensive care? J Trop Pediatr 49: 148–152. [DOI] [PubMed] [Google Scholar]
- 21. Koti J, Murki S, Gaddam P, Reddy A, Reddy MD (2010) Bubble CPAP for respiratory distress syndrome in preterm infants. Indian Pediatr 47: 139–143. [DOI] [PubMed] [Google Scholar]
- 22. Urs PS, Khan F, Maiya PP (2009) Bubble CPAP - a primary respiratory support for respiratory distress syndrome in newborns. Indian Pediatr 46: 409–411. [PubMed] [Google Scholar]
- 23. Brown J, Machen H, Kawaza K, Mwanza Z, Iniguez S, et al. (2013) A high-value, low-cost bubble continuous positive airway pressure system for low-resource settings: technical assessment and initial case reports. PLoS ONE 8: e53622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(2013) Malawi. World Bank [cited 2013 April 1]. Washington, D.C.: The World Bank Group. [Google Scholar]
- 25. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, et al. (2012) National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379: 2162–2172. [DOI] [PubMed] [Google Scholar]
- 26.You D, Rou New, J., Wardlaw, T. (2012) Levels and Trends in Child Mortality: Estimates Developed by the UN Inter-Agency Group for Child Mortality Esitmation. New York: United Nations Inter-Agency Group for Child Mortality Estimation; UNICEF. World Health Orgainzation, The World Bank, United Nations. [Google Scholar]
- 27.Malik K (2013) The Rise of the South: Human Progress in a Diverse World. Human Development Report 2013. New York: United Nations Development Programme.
- 28. Blencowe H, Kerac M, Molyneux E (2009) Safety, effectiveness and barriers to follow-up using an ‘early discharge’ Kangaroo Care policy in a resource poor setting. J Trop Pediatr 55: 244–248. [DOI] [PubMed] [Google Scholar]
- 29. van den Heuvel M, Blencowe H, Mittermayer K, Rylance S, Couperus A, et al. (2011) Introduction of bubble CPAP in a teaching hospital in Malawi. Ann Trop Paediatr 31: 59–65. [DOI] [PubMed] [Google Scholar]
- 30. Lawn JE, Kerber K, Enweronu-Laryea C, Massee Bateman O (2009) Newborn survival in low resource settings–are we delivering? BJOG 116 Suppl 1: 49–59. [DOI] [PubMed] [Google Scholar]
- 31.Zupan J, Åhman E, World Health Organization. (2006) Neonatal and perinatal mortality: country, regional and global estimates. Geneva: World Health Organization. iv, 69 p. p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photograph showing bubble CPAP device used in the study. bCPAP was delivered using Hudson prongs secured to a stretchy hat with safety pins and elastic bands. The bCPAP delivered a blended mix of air and oxygen from an oxygen concentrator at flow rates varying from 0–10 L/min and pressures ranging from 5–8 cm H2O.
(TIF)
Fraction of eligible participants in each treatment group who experienced minor complications during treatment.
(PDF)