Abstract
Regulatory T (Treg) lymphocytes are important mediators of the allogeneic immune response, although the mechanisms by which they are controlled are not fully understood. Studies conducted in mice, including a recent article in Immunity by Laurence et al., have shown that STAT3 is an important factor involved in the instability of natural Treg (nTreg) lymphocytes and the generation of induced Treg (iTreg) lymphocytes. The authors used T lymphocytes obtained from Foxp3-GFP reporter mice, which allowed them to track the in vivo fate of the nTreg and iTreg lymphocyte populations in the inflammatory milieu of acute GvHD. They showed that nTreg lymphocytes lose the expression of FoxP3 within this inflammatory environment and that the loss of FoxP3 is, in part, STAT3-dependent. Ultimately, the absence of STAT3 permitted the conversion of transferred naive CD4+ T lymphocytes to iTreg lymphocytes, which correlated with a strikingly improved survival rate during GvHD. We herein discuss how the article by Laurence et al. offers a novel mechanism to explain how the inflammatory environment may alter the stability or phenotype of Treg lymphocytes.
Keywords: STAT3, regulatory T lymphocyte, graft-vs.-host disease, Foxp3, hematopoietic stem cell transplantation
Allogeneic hematopoietic stem cell transplantation (HSCT) is the most effective therapy for several types of hematological malignancies and primary immunodeficiencies. HSCT involves the deletion of the hematopoietic compartment using high-dose chemotherapy and irradiation and the reconstruction of a new hematopoietic system provided by the donor hematopoietic stem cells. However, the implanted cells also contain mature T lymphocytes that can evoke graft-vs.-host disease (GvHD), a life-threatening condition in patients undergoing allogeneic HSCT. GvHD remains the foremost impediment to the clinical application of HSCT. The frequency of severe acute GvHD is 50% among patients who receive implants with major histocompatibility complex (MHC) antigen-matched but unrelated donor allografts, and fewer than 20% of patients who develop severe GvHD survive for five years after transplantation. GvHD is evoked by alloreactive donor T lymphocytes that recognize the host minor and MHC antigens then propagate and injure target tissues. Donor T lymphocytes have been reported to facilitate engraftment of HSCs, reconstruct T lymphocyte immunity and mediate potential antitumor efficacy, known as the graft-vs.-leukemia (GVL) effect. The elimination of donor T lymphocytes leads to loss of engraftment of HSCs and abolishes the actions of T lymphocyte-mediated GVL. Furthermore, the administration of immunosuppressants to suppress GvHD following HSC transplantation impairs the T lymphocyte function and tumor recrudescence. Hence, recent approaches have focused on developing tailored approaches to maintaining the desirable effects of GVL while preventing the development of GvHD after HSC transplantation. Recent preclinical novel cell-based treatments have been developed to accomplish these outcomes and are currently being applied in clinics. Both CD4+ T lymphocytes (T helper lymphocytes; Th lymphocytes) and CD8+ T lymphocytes (T cytotoxic lymphocytes; Tc lymphocytes) mediate acute GvHD in murine models of GvHD following allogeneic HSTC.1 In addition to the traditional model of Th lymphocyte differentiation into either Th1 or Th2 lymphocytes, two additional lineages are generated in the presence of transforming growth factor β (TGF-β).2 Regulatory T (Treg) lymphocytes express the transcription factor FoxP3 and inhibit inflammatory responses in a STAT5-dependent manner.3 Treg lymphocytes consist of two types of populations: naturally occurring Treg (nTreg) lymphocytes and induced Treg (iTreg) lymphocytes. nTreg lymphocytes are preponderantly expressed during thymic development. Alternatively, in the presence of TGF-β and interleukin-2 (IL-2), iTreg lymphocytes are differentiated from naïve Th lymphocytes in vitro.4 The second lineage of additional Th lymphocytes is Th17 lymphocytes, which are distinguished by the excretion of IL-17 and are related to the pathogenesis of autoimmune diseases in a STAT3-dependent manner.5 The role of these Th lymphocytes in the pathology of GvHD remains controversial.6 Nevertheless, peculiar Th lymphocyte lineages may be involved in the specific tissue damage of GvHD, including gut and liver GvHD via the actions of Th1 lymphocytes,7 lung GvHD via the actions of Th2 lymphocytes7 and skin GvHD via the actions of Th17 lymphocytes.8 Adversely, Treg lymphocytes inhibit a broad array of T lymphocyte inflammatory disorders, including GvHD, in experimental murine models9,10 and studies using human T lymphocytes.11,12 The use of adoptive Treg lymphocyte transfer has been contemplated as a treatment for preventing GvHD.13 However, the ability to achieve an adoptive Treg lymphocyte remedy reaction depends on the reliability of the transplanted lymphocytes, which has recently cast doubt upon the efficacy of this procedure.14 The intent to control nTreg lymphocyte capabilities continues to be a debatable issue, with distinct groups drawing contrasting conclusions.15,16 Meanwhile STAT5 is an essential positive factor involved in the expression of FoxP3, of which STAT3 is a key suppresser.17 STAT3 binds to a silencer site within the Foxp3 gene, which induces a decrease in Smad3 binding.18 The significance of cytokine signaling via STAT3 has been shown in murine models of GvHD. The alloactivated T lymphocytes induced in murine models of acute GvHD are marked by phosphorylation of STAT3.19 The cytokines (IL-6, IL-21, and IL-23) that activate STAT3 cytokines are necessary for the onset of acute GvHD.20-22 In contrast, the suppression of IL-6 is accompanied by the presence of iTreg lymphocytes; however, the effects of IL-6 on implanted nTreg lymphocytes have not been examined.21
In a recent study, Laurence et al.23 demonstrated that murine recipients of allogeneic HSCT with T lymphocytes lacking STAT3 exhibit conspicuously persistent survival in comparison with mice that receive allogeneic transplants with control T lymphocytes. The findings of Th17 lymphocytes have conferred knowledge of the mechanisms underlying the pathogenesis of immune-mediated illness, and the significance of STAT3 in the development of Th17 lymphocytes has been corroborated in both rodents and humans. Therefore, the favorable anti-GvHD effects achieved by implanting STAT3-deficient T lymphocytes are presumably associated with the incompetence of these cells to secrete IL-17. However, a number of discoveries provide evidence against this mechanism as the primary machinery operating in this model. First, the cytokine secretion following allogeneic HSCT resembles that observed in recipients of control or STAT3-knockout T lymphocytes. Second, there is a manifest distinction between the number of Th17 lymphocytes within the colonic LP of syngeneic mice and that observed in allogeneic mice transplanted with wild-type T lymphocytes. In comparison with syngeneic recipients, there are notably scant IL-17-producing CD4+ T lymphocytes in allogeneic recipients, in spite of the presence of serious acute GvHD. These discoveries suggest that inflammatory colitis accompanied by acute GvHD is not dependent on Th17 lymphocytes and that the efficacy of eliminating STAT3 in donor T lymphocytes does not arise from the suppression of Th17 lymphocytes. Previous research has demonstrated that the implantation of nTreg lymphocytes decreases the severity of murine acute GvHD and other autoimmune diseases. One of the most remarkable observations by Laurence et al. is the diminution of FoxP3+ in transferred nTreg lymphocytes, with posterior diversion of these cells into cytokine-secreting effector lymphocytes. It remains controversial whether FoxP3+ Treg lymphocytes at particular sites of inflammation have the plasticity to differentiate into non-Treg lymphocytes, particularly proinflammatory Th lymphocytes, via the loss of the FoxP3 expression. This conversion could be harmful because FoxP3 Treg lymphocytes are thought to be more self-reactive in antigen specificity. Laurence et al. transplanted a pure cluster of nTreg lymphocytes and was able to easily differentiate distinct clusters of transferred lymphocytes based on congenic markers. With the lack of effector lymphocytes and the presence of GvHD, less than 10% of the implanted nTreg lymphocytes lost their FoxP3 expression. When effector T lymphocytes were supplemented, the FoxP3 expression was maintained in the syngeneic recipients and intimately lost in the allogeneic host mice.
The real query is how STAT3 achieves suppression of the expression of FoxP3 in T lymphocytes. Regarding the interplay between STAT3 and STAT5 in the development of Treg lymphocytes, Laurence et al. demonstrated that the existence of STAT3 blocks STAT5 binding to STAT5 and STAT3 binding sites on the FoxP3 loci. This finding suggests that the suppressive effects of STAT3 work in part by interfering with the competence of STAT5 to combine with the FoxP3 loci and facilitate the expression of genes, thereby highlighting a significant mechanism by which STAT3 is able to destabilize Treg lymphocytes.
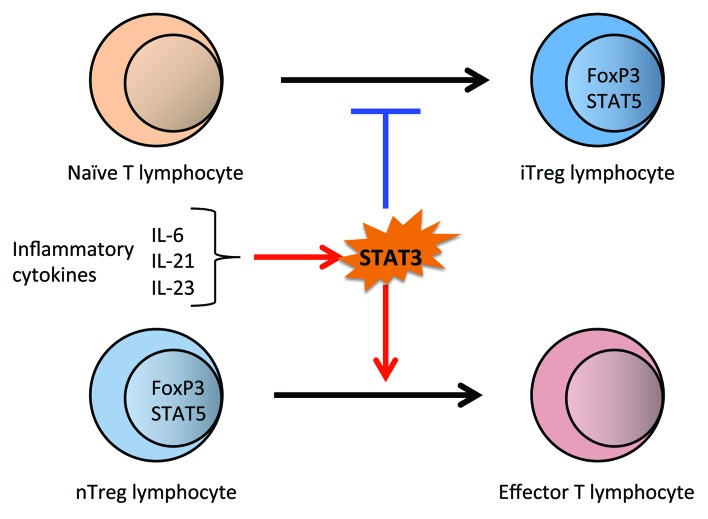
Contrary to the behavior of STAT3, STAT5 is understood to both expedite and sustain the FoxP3 expression of iTreg lymphocytes. STAT3-knockout nTreg lymphocytes, despite their in vivo continuity, are no more effective than wild-type nTreg lymphocytes in resolving GvHD. STAT3-knockout nTreg lymphocytes inhibit T lymphocyte expansion in vitro to a similar extent to that observed in control nTreg lymphocytes. In spite of the observation that implanted STAT3-knockout nTreg lymphocytes do not ameliorate the outcome of acute GvHD, transfer of STAT3-knockout naïve T lymphocytes is adequate for remarkably improving the prognosis, even in the absence of any additional nTreg lymphocytes. In murine GvHD models, wild-type donor cells primarily turn into effector T lymphocytes. However, an appreciable portion of implanted STAT3-knockout T lymphocytes express FoxP3, consequently exhibiting an iTreg lymphocyte phenotype. These results suggest that cytokines that elicit STAT3 signaling limit the number of FoxP3+ T lymphocytes via two routes: (1) enhanced capabilities of nTreg lymphocytes and (2) incompetence in the generation of new iTreg lymphocytes from naïve CD4+ T lymphocytes (Fig. 1). These data additively highlight the favorable potency of inhibiting IL-6 during GvHD and offer further mechanistic rationale for attempts to interfere with GvHD by directly suppressing the expression or DNA binding of STAT3 or redirecting the inhibition of STAT3 via neutralizing IL-6, IL-21, and IL-23.
Figure 1. The regulation of Treg lymphocyte plasticity by STAT3 in acute GvHD.
Previous reports have indicated that the inhibition of IL-6 results in decreased GvHD scores and improved survival in mice.21,24 Further clinical case reports and small clinical trials have ascribed some benefit to inhibiting IL-6 with anti-IL-6 receptor antibodies.25,26 Hence, as Laurence et al. suggested, IL-6 inhibition by anti-IL-6 receptors (IL-21 and IL-23 blockage) may be an effective method of GvHD treatment. In addition, it may be better to evaluate more specific inhibitors of JAK-STAT3 signaling in order to control GvHD.27,28
Meanwhile, the results of Laurence et al. are incompatible with the findings of Pallandre et al.,29 who demonstrated that in vivo STAT3 neutralization results in deterioration of acute GvHD. The most likely interpretation of this discrepancy is that, in the referenced research, CD4+ T lymphocytes were recovered from donor animals in which STAT3 was amputated using small interfering RNA in hematopoietic stem cells, while in the experiments of Laurence et al., STAT3 was amputated only in T lymphocytes. STAT3 amputation in hematopoietic stem cells turns on innate immunity, resulting in much more intense T lymphocyte responses, leading to autoimmunity.30,31 This is not the case when STAT3 is amputated in CD4+ T lymphocytes only, since these mice exhibit a normal phenotype, and the number of Treg lymphocytes is at a steady-state.32 In this respect, it is important to accentuate that all findings regarding the influence of amputating STAT3 signaling must be interpreted in the context of the effects on other cell populations and the host cytokine environment.
In order to ameliorate GvHD via STAT3 regulation, we must consider another cell population, dendritic cells. STAT3 is critical for the negative regulation of proinflammatory cytokine secretion by dendritic cells, and several studies have demonstrated that STAT3 plays a critical role in the negative regulation of dendritic cells.33-36 Dendritic cells are critically involved in the pathogenesis of GvHD and exhibit tolerogenic properties. Sun et al. demonstrated that histone deacetylase inhibition modulates the dendritic cell functions and regulates GvHD.34 In that report, the authors demonstrated that histone deacetylase inhibition acetylates and activates STAT3, which regulates dendritic cells by promoting the transcription of indoleamine 2,3-dioxygenase. These findings demonstrate a novel functional role for the posttranslational modification of STAT3 through acetylation and provide mechanistic insight into the histone deacetylase inhibition-mediated immunoregulation achieved by the induction of indoleamine 2,3-dioxygenase. Furthermore, Melillo et al. demonstrated that dendritic-specific Stat3-knockout mice develop cervical lymphadenopathy as well as mild ileocolitis. Consistent with this finding, Stat3-deficient DCs exhibit enhanced immune activity.35 In addition, Betts et al. demonstrated that anti-IL-6 receptors have no effect on human monocyte-derived dendritic cell maturation and activation, alloreactive T lymphocyte proliferation, Treg lymphocyte expansion or allogeneic Th1/Th17 responses in vitro and that JAK2 inhibition preserves the number of Treg lymphocytes and reduces the production of IL-6 and TNF-α in allogeneic MLRs, impairing the activation of central and effector memory T lymphocytes as well as the expansion of responder Th1 and Th17 lymphocytes.28,37 Therefore, it is sometimes difficult to correlate inconsistent immune effects with the disease response and clinical outcome.
As Laurence et al. demonstrated, STAT5 and STAT3 compete for promoter binding sites in CD4 T lymphocytes. Furthermore, the two transcription factors are believed to have opposite functions in the control of CD4 T lymphocyte differentiation. These findings help us understand that STAT3 not only plays a role in promoting Th17 lymphocytes, but also participates in the conversion of nTreg lymphocytes to effector lymphocytes and the inhibition of iTreg lymphocyte development among naïve CD4 lymphocytes. However, in their study, there are several remaining issues: (1) why iTreg lymphocytes derived from naïve precursors appear to be of paramount importance for improving the prognosis of acute GvHD, rather than the provision of nTreg lymphocytes, even STAT3-knockout nTreg lymphocytes, (2) why iTreg lymphocytes without STAT3 are able to regulate effector T lymphocytes, nTreg lymphocytes without STAT3 do not improve survival compared with nTreg lymphocytes with STAT3, (3) whether the immune suppressive function of iTreg lymphocytes toward effector T lymphocytes is different from that of nTreg lymphocytes, i.e., whether it is superior to that of nTreg lymphocytes—previous studies have demonstrated that STAT3 is a critical factor involved in the molecular pathway required for the FoXP3 expression in Treg lymphocytes and the Treg lymphocyte function29,38—and (4) what are the precise mechanisms and role of STAT3 and STAT5 in Treg lymphocyte development and induction in acute GvHD.
Presently, there is limited evidence demonstrating Treg lymphocyte plasticity in vivo as part of an efficient immune reaction. Using a single major cytokine for complete differentiation and commitment of T lymphocytes may have oversimplified the complexity and pliability of T lymphocytes. The disagreements observed between in vitro and in vivo systems accentuate the significance of comprehending the limitations of experimental systems. The development of novel and advanced technological tools will be essential in subsequent research, in particular, regarding the identification of machineries of plasticity. It is, as yet unclear which machineries are involved in plasticity and whether there are common triggers of plasticity among experimental systems or even between subsets. Unequivocally, further studies in this field will aid in understanding not only the enormous competence of the immune machinery, but also how the immune reaction functions best and how it can be harnessed.
Conducting further investigations of how cytokines and Th lymphocyte-specific transcription factors regulate the function and differentiation of FoxP3+ Treg lymphocytes is therefore a prerequisite for ameliorating GvHD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/24529
References
- 1.Vallera DA, Taylor PA, Sprent J, Blazar BR. The role of host T cell subsets in bone marrow rejection directed to isolated major histocompatibility complex class I versus class II differences of bm1 and bm12 mutant mice. Transplantation. 1994;57:249–56. doi: 10.1097/00007890-199401001-00017. [DOI] [PubMed] [Google Scholar]
- 2.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–9. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Regulatory T cells: history and perspective. Methods Mol Biol. 2011;707:3–17. doi: 10.1007/978-1-61737-979-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–12. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105:1289–98. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–74. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–6. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 11.Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, et al. Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 2010;8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutis T, van Rijn RS, Simonetti ER, Aarts-Riemens T, Emmelot ME, van Bloois L, et al. Human regulatory T cells control xenogeneic graft-versus-host disease induced by autologous T cells in RAG2-/-gammac-/- immunodeficient mice. Clin Cancer Res. 2006;12:5520–5. doi: 10.1158/1078-0432.CCR-06-0035. [DOI] [PubMed] [Google Scholar]
- 13.June CH, Blazar BR. Clinical application of expanded CD4+25+ cells. Semin Immunol. 2006;18:78–88. doi: 10.1016/j.smim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–71. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–75. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity. 2010;33:313–25. doi: 10.1016/j.immuni.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu SX, Alpdogan O, Lin J, Balderas R, Campos-Gonzalez R, Wang X, et al. STAT-3 and ERK 1/2 phosphorylation are critical for T-cell alloactivation and graft-versus-host disease. Blood. 2008;112:5254–8. doi: 10.1182/blood-2008-03-147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–84. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das R, Chen X, Komorowski R, Hessner MJ, Drobyski WR. Interleukin-23 secretion by donor antigen-presenting cells is critical for organ-specific pathology in graft-versus-host disease. Blood. 2009;113:2352–62. doi: 10.1182/blood-2008-08-175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–22. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tawara I, Koyama M, Liu C, Toubai T, Thomas D, Evers R, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17:77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gergis U, Arnason J, Yantiss R, Shore T, Wissa U, Feldman E, et al. Effectiveness and safety of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in a patient with refractory GI graft-versus-host disease. J Clin Oncol. 2010;28:e602–4. doi: 10.1200/JCO.2010.29.1682. [DOI] [PubMed] [Google Scholar]
- 26.Drobyski WR, Pasquini M, Kovatovic K, Palmer J, Douglas Rizzo J, Saad A, et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:1862–8. doi: 10.1016/j.bbmt.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HB, Oh K, Garmaa N, Seo MW, Byoun OJ, Lee HY, et al. CP-690550, a Janus kinase inhibitor, suppresses CD4+ T-cell-mediated acute graft-versus-host disease by inhibiting the interferon-γ pathway. Transplantation. 2010;90:825–35. doi: 10.1097/TP.0b013e3181f24e59. [DOI] [PubMed] [Google Scholar]
- 28.Betts BC, Abdel-Wahab O, Curran SA, St Angelo ET, Koppikar P, Heller G, et al. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood. 2011;118:5330–9. doi: 10.1182/blood-2011-06-363408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pallandre JR, Brillard E, Créhange G, Radlovic A, Remy-Martin JP, Saas P, et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J Immunol. 2007;179:7593–604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 30.Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A. 2003;100:1879–84. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 32.Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, et al. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol. 2007;19:695–702. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- 33.Reddy P, Sun Y, Toubai T, Duran-Struuck R, Clouthier SG, Weisiger E, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–73. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Chin YE, Weisiger E, Malter C, Tawara I, Toubai T, et al. Cutting edge: Negative regulation of dendritic cells through acetylation of the nonhistone protein STAT-3. J Immunol. 2009;182:5899–903. doi: 10.4049/jimmunol.0804388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol. 2010;184:2638–45. doi: 10.4049/jimmunol.0902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012;189:3848–58. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betts BC, St Angelo ET, Kennedy M, Young JW. Anti-IL6-receptor-alpha (tocilizumab) does not inhibit human monocyte-derived dendritic cell maturation or alloreactive T-cell responses. Blood. 2011;118:5340–3. doi: 10.1182/blood-2011-06-363390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–91. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]