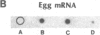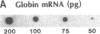Abstract
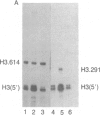
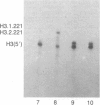
There are large amounts of histone mRNA present in mouse eggs. These RNAs are rapidly degraded, as are other mRNAs, after fertilization and prior to the second cleavage. During cleavage, the histone mRNA accumulates as the embryo divides. The same sets of histone genes are expressed in eggs and embryos, although there are large qualitative differences in the amounts of particular histone mRNAs. The function of the egg histone mRNA is unknown. The amount of histone mRNA in cleaving and blastocyst embryos is probably sufficient to code for the blastocyst histone proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Woodland H. R. Histone synthesis in early amphibian development: histone and DNA syntheses are not co-ordinated. J Mol Biol. 1974 Sep 15;88(2):263–285. doi: 10.1016/0022-2836(74)90481-1. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. V., Lengyel J. A. Changing rates of histone mRNA synthesis and turnover in Drosophila embryos. Cell. 1980 Oct;21(3):717–727. doi: 10.1016/0092-8674(80)90435-3. [DOI] [PubMed] [Google Scholar]
- Bachvarova R., De Leon V. Stored and polysomal ribosomes of mouse ova. Dev Biol. 1977 Jul 15;58(2):248–254. doi: 10.1016/0012-1606(77)90090-2. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bolton V. N., Oades P. J., Johnson M. H. The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J Embryol Exp Morphol. 1984 Feb;79:139–163. [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Ebert K. M., Brinster R. L. Rabbit alpha-globin messenger RNA translation by the mouse ovum. J Embryol Exp Morphol. 1983 Apr;74:159–168. [PubMed] [Google Scholar]
- Ecklund P. S., Levine L. Mouse sperm basic nuclear protein. Electrophoretic characterization and fate after fertilization. J Cell Biol. 1975 Aug;66(2):251–262. doi: 10.1083/jcb.66.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach G., Johnson M. H., Braude P. R., Taylor R. A., Bolton V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1(6):681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebelhaus D. H., Heikkila J. J., Schultz G. A. Changes in the quantity of histone and actin messenger RNA during the development of preimplantation mouse embryos. Dev Biol. 1983 Jul;98(1):148–154. doi: 10.1016/0012-1606(83)90343-3. [DOI] [PubMed] [Google Scholar]
- Giebelhaus D. H., Weitlauf H. M., Schultz G. A. Actin mRNA content in normal and delayed implanting mouse embryos. Dev Biol. 1985 Feb;107(2):407–413. doi: 10.1016/0012-1606(85)90322-7. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Marzluff W. F. Rapid reversible changes in the rate of histone gene transcription and histone mRNA levels in mouse myeloma cells. Mol Cell Biol. 1984 Feb;4(2):351–357. doi: 10.1128/mcb.4.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves R. A., Wellman S. E., Chiu I. M., Marzluff W. F. Differential expression of two clusters of mouse histone genes. J Mol Biol. 1985 May 25;183(2):179–194. doi: 10.1016/0022-2836(85)90211-6. [DOI] [PubMed] [Google Scholar]
- Kaye P. L., Church R. B. Uncoordinated synthesis of histones and DNA by mouse eggs and preimplantation embryos. J Exp Zool. 1983 May;226(2):231–237. doi: 10.1002/jez.1402260208. [DOI] [PubMed] [Google Scholar]
- Levey I. L., Stull G. B., Brinster R. L. Poly(A) and synthesis of polyadenylated RNA in the preimplantation mouse embryo. Dev Biol. 1978 May;64(1):140–148. doi: 10.1016/0012-1606(78)90066-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Maxson R. E., Jr, Wilt F. H. The rate of synthesis of histone mRNA during the development of sea urchin embryos (Strongylocentrotus purpuratus). Dev Biol. 1981 Apr 30;83(2):380–386. doi: 10.1016/0012-1606(81)90485-1. [DOI] [PubMed] [Google Scholar]
- Newrock K. M., Cohen L. H., Hendricks M. B., Donnelly R. J., Weinberg E. S. Stage-specific mRNAs coding for subtypes of H2A and H2B histones in the sea urchin embryo. Cell. 1978 Jun;14(2):327–336. doi: 10.1016/0092-8674(78)90118-6. [DOI] [PubMed] [Google Scholar]
- Pikó L., Clegg K. B. Quantitative changes in total RNA, total poly(A), and ribosomes in early mouse embryos. Dev Biol. 1982 Feb;89(2):362–378. doi: 10.1016/0012-1606(82)90325-6. [DOI] [PubMed] [Google Scholar]
- Sawicki J. A., Magnuson T., Epstein C. J. Evidence for expression of the paternal genome in the two-cell mouse embryo. Nature. 1981 Dec 3;294(5840):450–451. doi: 10.1038/294450a0. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Structure of a cluster of mouse histone genes. Nucleic Acids Res. 1983 Oct 11;11(19):6679–6697. doi: 10.1093/nar/11.19.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezky D. L., Angerer L. M., Angerer R. C. Accumulation of histone repeat transcripts in the sea urchin egg pronucleus. Cell. 1981 May;24(2):385–391. doi: 10.1016/0092-8674(81)90328-7. [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Adamson E. D. The synthesis and storage of histones during the oogenesis of Xenopus laevis. Dev Biol. 1977 May;57(1):118–135. doi: 10.1016/0012-1606(77)90359-1. [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Warmington J. R., Ballantine J. E., Turner P. C. Are there major developmentally regulated H4 gene classes in Xenopus? Nucleic Acids Res. 1984 Jun 25;12(12):4939–4958. doi: 10.1093/nar/12.12.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]