Abstract
Developing a universal vaccine for S. aureus is a top priority but to date we have only had failures in human clinical trials. Given the plethora of bacterial virulence factors, broad range of the health of humans at-risk for infections, lack of any information regarding immune effectors mediating protection for any manifestation of S. aureus infection and overall competence of this organism as a colonizer, commensal and pathogen, we may just simply have to accept the fact that we will not get a universal vaccine. Antigenic variation is a major challenge for some vaccine targets and for many conserved targets the organism can easily decrease or even eliminate expression to avoid immune effectors without compromise to infectivity and ability to cause disease. Studies of human immune responses similarly have been unable to identify any clear mediators of immunity and data from such studies can only eliminate those found not to be associated with protection or that might serve as a marker for individuals with a higher level of resistance to infection. Animal studies are not predictive of success in humans and unlikely will be except in hindsight if and when we develop an efficacious vaccine. Successful vaccines for other bacteria based on capsular polysaccharides have not worked to date for S. aureus, and laboratory studies combining antibody to the major capsular serotypes and the other S. aureus surface polysaccharide, poly-N-acetyl glucosamine, unexpectedly showed interference not augmentation of immunity. Potential pathways toward vaccine development do exist but for the foreseeable future will be based on empiric approaches derived from laboratory-based in vitro and animal tests and not on inducing a known immune effector that predicts human resistance to infection.
Keywords: vaccines, S. aureus, immunotherapy, clinical trials, antibody, T-cells
Introduction
To answer the title’s question, we will have to deal with the facts that S. aureus is just too variable in its expression of vaccine target antigens,1 is capable of infecting a wide range of animal and human tissues and thus able to survive in a wide enough varieties of niches in these hosts such that any selective pressures induced by vaccination can potentially be readily overcome by expansion of existing variants able to escape immune-selective pressures.2 While some studies have identified genes commonly found among a large majority of clinical isolates3,4 no single essential virulence factor needed for infection in most settings that can be targeted as a vaccine is known, exceptions being diseases mediated purely by toxins such as toxic-shock syndrome toxin,5,6 exfoliative dermatitis and mediators of staphylococcal food poisoning.7,8 Extensive genetic2 and hence antigenic variability in many potential antigens precludes their use as vaccines. Variability in the level of expression leading to a highly variable surfacome9,10 provides an easy means for bacterial escape from immune effectors by merely reducing levels of antigens to below that needed for elimination or killing of bacteria. S. aureus is also notorious for causing frequent reinfection with the same strain, indicating natural infection does not readily induce acquired immunity that can be defined and used to guide vaccine development. Further difficulties are encountered when using laboratory animals to evaluate S. aureus virulence and immunity as they are sufficiently different in their responses from those that occur in humans meaning that pre-clinical animal tests primarily function as systems of exclusion, used to judge what likely won’t work in humans but unable to predict what will work. Against these challenges we might find that, at best, we can develop vaccines to prevent specific types of S. aureus infections such as bacteremia or skin and soft tissue infections (SSTIs).
In spite of these barriers, many vaccinolgists would place the need for a highly effective vaccine against Staphylococcus aureus in the top 3–5 public health essentials. The organism is among the most frequent causes of infections in virtually all human, and many animal, tissues,11,12 causes considerable morbidity and mortality13-16 and community-acquired infections in otherwise healthy people continue unabated and may be increasing.17-19 So why has it not only been so difficult to develop a vaccine but, to date, numerous trials of a variety of vaccines in humans have all failed? Unfortunately failures provide little informative insight into their basis, as they are usually multi-factorial, and hypotheses about failure are untestable. Yet the failed S. aureus human vaccine trials conducted to date were all backed up by strong pre-clinical data,20-25 so, at best, we can conclude pre-clinical studies are insufficient to be predictive of success or failure in humans.
Recently, Proctor summarized the challenges for developing a universal S. aureus vaccine26 as have Jansen et al.27 and within these reviews excellent summaries of the attempts to date (Table 1 in Proctor26 and Table 1 in Jansen et al.27) and major challenges (Table 2 in Proctor26) are provided. Therefore there is no need to repeat these except to note the subsequent publication of the results of the Merck V710 (IsdB) vaccine trial in cardiothoracic surgery patients.28 The major point made in these and other reviews of S. aureus vaccine development26,29-31 is we have insufficient insight into the basis for virulence and immunity for this organism to rationally design vaccines targeting known protective immune effectors. The multitude of genetically variable virulence factors, the undefined mechanisms of host immunity which might indicate the antigens and immune effectors needed to produce an effective vaccine, the high recurrence rates seen in humans, particularly with methicillin-resistant S. aureus (MRSA) infections32-34 and evidence for inadequate immune responses following infection35-38 may, in fact, be insurmountable challenges to finding a broadly-effective vaccine for S. aureus. It may be time to consider that these challenges indicate there may not be a means to come up with an effective, broad-spectrum S. aureus vaccine with currently available technologies.
How Hopeless is it?
Difficult to know. Within his reviews,26,39 Proctor argued extensively that the pathways and mediators induced by vaccines that have been tried and failed in humans might simply elicit the wrong types of immune responses, He proposed a basis for an effective vaccine may be found in new insights regarding the role of interleukin-17 producing T-helper cells (Th17) and cell-mediated immunity in resistance to S. aureus infection. But evidence for this is fairly minimal, with some associations of human immune deficiencies and enhanced susceptibility to S. aureus skin and mucocutaneous infection40-43 and an observation that humans with B-cell deficiencies are not particularly more susceptible to S. aureus infections than individuals with intact immune systems.44,45 However, individuals with neutrophil deficiencies do have increased deep-seated S. aureus infections46 indicating inefficient opsonic killing contributes to certain types of S. aureus infection. But associations among various observations can easily confound obtaining insights that can be translated into evidenced-based vaccine approaches. As T cell function, particularly T-helper function, is at the core of all acquired immunity, defects in T-helper cells do not necessarily mean increased susceptibility to infection in the setting of T-cell deficiency would exclude a role for antibody-mediated immunity. Th-17 cells are also critical for effective neutrophil-dependent host responses,47 so defects in these cells would impair effective recruitment of this key effector of antibody-based immunity. Thus it may be that Th-17 and IL-17 responses may be necessary, but not sufficient, for effective immunity to S. aureus. More to the point, if most immunocompetent humans simply do not make a protective antibody response following either colonization or infection with S. aureus,48,49 then there is little value in drawing conclusions about the role of antibody in protection when comparing those who can’t (B-cell deficient) and those who don’t (everyone else) make protective antibody. This point was also discussed in detail by Jansen et al.27 So, if finding Th-17-inducing vaccine antigens50,51 is not the answer, it is not clear what is.
Immune Responses In Initial and Recurrent Infections: Can We Make Some Progress Here?
Analyzing antibody levels and T-cell responses present in acute and convalescent sera is a time-honored means to identify vaccine candidates but there are few such studies for S. aureus infections and all have focused on antibody responses.35-38,48,49 And with the high recurrence rate of S. aureus infections, many of these responses are either going to be ineffectual at preventing infection or potentially even promote infection by mechanisms such as neutralizing innate immune-activating properties of bacterial factors.52,53 Of note, in several of these studies35,36,48 antibody levels to many S. aureus antigens are already elevated in sera taken close to the time when a clinical sample yields a positive culture for S. aureus. This indicates that significant exposure to S. aureus that induced antibody responses had occurred prior to the time of actual microbiologic diagnosis of infection. Importantly, we must consider that the presence of elevated antibody levels early in the course of clinical infection indicates they are either too low or ineffective at protecting against infection. Although no cellular responses were measured, the common finding that antibody levels are already elevated at the onset of clinically-diagnosed infection might also suggest that cell-mediated immunity had also been stimulated but was without effect on preventing infection.
Numerous associations between patient outcomes and levels of antibodies to the S. aureus antigens have been made, but none of these associations indicate any cause and effect relationship between antibody level and protection from infection or reduction in severity of an outcome.35-38,48,49,54-57 Furthermore, often overlooked in these analysis is the significant overlap in antibody levels between the susceptible and resistant groups. For example, Adhikari et al.57 reported that patients with S. aureus bacteremia who went on to develop sepsis vs. those that did not had lower overall median antibody levels against five S. aureus toxins (α and δ-hemolysins, Panton-Valentine leukocidin (PVL), staphylococcal enterotoxin C-1, and phenol-soluble modulin-α3). Fritz et al.58 recently analyzed antibody levels to α-hemolysin and PVL in individuals with primary and recurrent SSTI and systemic infections and found no overall differences in an OD reading between those with primary vs. recurrent infections. While mean or median levels of antibodies to particular antigens might differ between groups that develop sepsis or recurrent infections vs. those that do not, many of the patients with sepsis or recurrent infections had antibody levels to toxins well above the medians in the non-septic/non-recurrent groups. This overlap indicates there is little likelihood that those antibodies were mediators of effective immunity. Also, in the study of Adhikari et al.57 all of the patients had S. aureus bacteremia, indicating the higher antibody levels were not protective against this manifestation of infection, and the likelihood of developing a vaccine to prevent S. aureus sepsis and not bacteremia is low. In order to have a meaningful difference in antibody levels associated with resistance to S. aureus infection there needs to be little overlap in titers between those at risk who do not get infected (higher) and those that do get infected (lower). When a significant proportion of the group developing an infection has antibody titers well within the range of those that do not, it highlights the inability of such associative studies to define a mediator or even a marker of immunity. Only in settings where a protective level of antibody or other effector is defined, above which infection is rare such as has been done with the successful capsular polysaccharide vaccines to other pathogens, can meaningful conclusions be drawn about the importance of an antibody titer in relation to protective immunity and vaccine development.
Another group of interest to study are patients that have been infected or even colonized with S. aureus that are at a high risk for a recurrent infection. Among patients with MRSA infections, or even just colonized during hospitalization, re-infection rates approaching 30% occur in the 6–18 mo after discharge,32-34 and identification of such cases markedly increases when vigorous means to detect post-discharge infections are used.32,59 Fritz et al.58 recently reported a recurrence rate of 62% in closely-followed individuals with primary or recurrent SSTI within 12 mo of the initial episode. Hospital-acquired, community onset infections identify patients potentially ideal for the study of acquired immunity to S. aureus. This is further substantiated by the finding that most of these recurrences are due to the same strain as the initially infecting one,34,38,59,60 meaning that serologic variation in antigens will not be a major factor in trying to identify protective immune responses, although this could be a problem for designing a universal vaccine if immunity to a single strain is targeted to a highly serologically variable antigen. If one therefore can compare immune responses among immunologically-intact humans with a properly diagnosed S. aureus infection or colonization in the hospital who then get a recurrent or subsequent infection with those that only have a primary infection or do not progress from colonization, insights into immune responses associated with resistance to reinfection might emerge. However, as noted above, the titers associated with becoming infected have to be, for the most part, lower than and not-overlapping to any large degree with the titers in the uninfected controls.
In this context, we examined the occurrence of antibody to the phage-encoded PVL, a major cytotoxin of the highly virulent USA300 and USA400 strains,61-63 among children with primary or recurrent skin and soft tissue infections (SSTI), the major manifestation of infection by PVL-producing MRSA. Due to its epidemiologic association with these S. aureus strains, PVL has been touted as a potential vaccine, but normal human sera and IVIgG have been found to have significant levels of neutralizing antibody to PVL.53,64 Among infected children, we found the highest levels of antibody to PVL in those with recurrent SSTI53 clearly indicating these antibodies are not protective and demonstrating there is an association between high levels of antibody to PVL and increased susceptibility to infection. Furthermore, most studies do not find patients with PVL infections do worse than those with non-PVL S. aureus infections63,65-67 suggesting it is not having a major impact on pathogenesis of human infection or natural immunity is sufficient to defuse its toxic manifestations. Of note, neutralizing antibody to PVL was found in a mouse skin infection model to promote, not inhibit, S. aureus infection68 due to the antibody’s ability to inhibit the immune-activating functions of PVL that initiate host innate immunity at low PVL concentrations, such as those encountered early in infection. Along the same lines, stains of S. aureus deleted for the PVL-encoding genes lukS and lukF were more, not less, virulent in a mouse model of pneumonia.52,69
The recently published study of Fritz et al.58 similarly looked at antibody reactivity (defined in the paper as the optical density readings obtained in an ELISA with a single 1:100 serum dilution) to α-hemolysin and PVL in various groups and overall found no differences in acute-phase titers in those with primary vs. recurrent infections and even found a decline in titers in the convalescent sera of those with SSTI but not invasive infection. However, when they examined patients with recurrent infections there were lower mean antibody levels at five time points post primary infection to α-hemolysin compared with those who did not get recurrent infections.58 But the overall differences in the mean OD readings were small and one can infer from the reported means and standard deviations that about 80% of the individuals with recurrent infection had a titer comparable to those without recurrent infections. Additionally, paired t-tests were used to analyze the differences in titers between enrollment and convalescent/post-infection sera, but this statistical analysis is not appropriate to apply to this type of longitudinal data, wherein samples were taken from individuals at multiple time periods and all compared with the same initial, pre-infection levels. Paired t-tests assume independence of each measurement and it is clear an individual with a high OD readings at one point does not have the same chance of having a low or high OD reading at the next time point, as antibody levels decline in a predictable manner in humans. Perhaps of greatest importance, the actual toxin-neutralizing activities of the antibodies were not measured to either α-toxin or PVL. As Foletti et al.70 showed, humans with the same ELISA titer to α-toxin had as much as a 100-fold difference in their α-hemolysin-neutralizing activity, so without functional characterization of the anti-toxin activity in the sera analyzed by Fritz et al.58 it is difficult to accept that any association of neutralization of toxins and resistance to S. aureus infection has been demonstrated. Similarly, although Adhikari et al.57 found significant differences in the ELISA binding titers to the individual proteins comprising PVL, LukF (p = 0.02) and LukS (p = 0.01) between patients with S. aureus sepsis and those that did not get septic, the P value for the difference in the toxin-neutralizing titer was only 0.17. Thus, in the study of Fritz et al.58 one must also take into account the possibility that patients with recurrent infections might actually have higher neutralizing titers to α-hemolysin than those without recurrent infections, which would indicate a possible negative association of neutralizing antibody to α-hemolysin and infection risk. Given that the Merck V710 vaccine trial28 revealed greater overall adverse events in the immunized group, greater rates of multiorgan failure and higher mortality rates than placebo recipients, the need for full characterization of the functional activity of any immunologic marker is paramount to avoid repetition of such unwanted outcomes from vaccine trials.
Animal Studies-Why They Really Can’t Be Improved For Vaccine Development
Because vaccine results obtained with mice, rats and rabbits do not predict human immunity. . Basically, animal and human susceptibility and the course of S. aureus infections are just too damn different from lab animals, and few other animals are routinely available to study vaccines. At most, lab animal studies serve as systems of exclusion, possibly defining what might not work. But even negative results in animals cannot predict human responses. When developing the meningococcal serogroup vaccines in the 1960s the purified capsular polysaccharides were not immunogenic in mice, rhesus monkeys, chimpanzees and only one of 5 gibbons made an antibody response following vaccination.71 But the investigators knew humans could make antibody from natural exposure.72,73 Importantly, the purified meningococcal serogroup A and C capsular antigens were immunogenic in nearly 100% of human volunteers74 so if one used animal results to conclude meningococcal polysaccharides were not good vaccine candidates we might not have benefited from this remarkable advance that ended the epidemic of meningococcal meningitis in military recruit camps.75,76 We just have to admit lab animals are “furry test tubes” and at best can be used to test hypothesis about in vivo activity of immune effectors such as functionality, the need for co-factors such as white blood cells, complement, Th17 cells, etc., and determine if a vaccine target is expressed in vivo in lab animals.
A major logical reason animal models cannot be improved to guide vaccine development is that we will only know which models are associated with human vaccine efficacy when we actually have an efficacious vaccine. As all of the failed S. aureus vaccines tested to date in humans28,77-79 have shown reductions in bacterial burdens and even protection from lethality21,23,80-82 in many animal models, it is clear these models have no predictive capacity for effectiveness in humans. Unless an efficacious S. aureus vaccine is developed for humans that has been tested in an animal model that would also show a failure of immunity to CP, ClfA, and IsdB, then the likelihood of finding an improved animal model is remote. Furthermore, many human manifestations of S. aureus infections induce symptoms that can be identified and communicated to a physician, this is not possible in animals. For example, someone awakening with a painful hand or finger who seeks medical care that turns out to be an S. aureus joint infection identifies a potentially serious situation where the individual is at risk for a significant compromise to the functionality of their hand. This, of course, could never be modeled in an animal, we have to infect them with a sufficient level of bacteria to get frank infections in most of the animals and these levels are usually many orders of magnitude above those that initiate infections in humans. At best one can hope that significant reductions in bacterial levels or other measures of infection in immunized animals are reasonable tests for potential vaccine efficacy without relying on the results to predict outcomes in a human vaccine trial.
Additionally, if we truly evaluate the results from animal infection models we often see in many studies that there is a reduction in bacterial levels, but in reality most, if not all, of the animals are infected.83-85 It might not matter in a human S. aureus vaccine trial if there are fewer bacteria in infected tissues of vaccinated patients, if they are still diagnosed with an infection based on a positive culture resulting from even low numbers of bacteria in the infected tissue it would be indicative of vaccine failure unless the reductions in bacterial burdens were accompanied by a significant effect on a measurable clinical outcome that had also been identified as an endpoint for the clinical trial.
Lethality studies in animals might be more predictive of efficacy in humans, and prevention of lethality would be an acceptable and readily identified endpoint for a clinical trial. But doses of S. aureus needed to induce lethality in lab animals are often quite high and lethality may not be due to bacterial burdens but rather acute toxicity from the bolus injection of these high infectious doses. It seems unlikely these challenge doses are representative of what initiates human infections with S. aureus. Furthermore, vaccines against CP antigens,23 immunity to ClfA82 and vaccination against IsdB80,81 have all shown protection from lethality in mice but these vaccines have all failed in human trials, so we can already conclude lethality models are not stringent tests for identifying vaccines likely to succeed.
Logical Assumptions and Historic Vaccine Success-Are They Indicators of a Path Toward Rationale Vaccine Design for S. aureus?
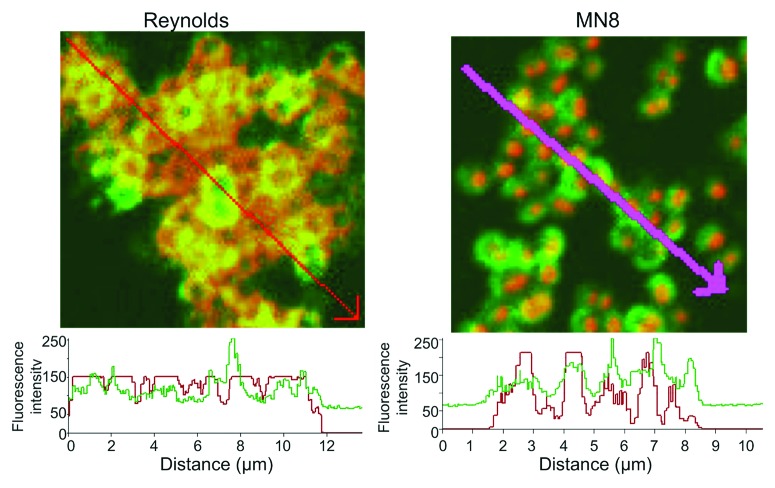
In spite of the lack of data associating any specific human immune response with protective immunity to S. aureus, some logical assumptions about potential vaccine candidates can emerge based on principles from other pathogens, notably the effectiveness of vaccines targeting capsular polysaccharides of Streptococcus pneumoniae,86 Hemophilus influenzae type b,87 Neisseria meningitidis88 and Salmonella enterica serovar typhi.89 The presence of capsule-specific immunity in resistant human populations was the basis for developing these successful vaccines, and thus one might predict analyzing antibody responses to S. aureus capsules could be highly informative. S. aureus strains can expresses either one of two capsular polysaccharides (CP), CP5 or CP8,29,90,91 or neither, along with the poly-N-acetyl glucosamine (PNAG) surface polysaccharide antigen.85,92,93 CP5 is produced by about 30% of strains, CP8 by about 50%94-96 and, as best as we can tell from our own immunologically-based studies,92 PNAG can be detected on the surface of nearly 100% of S. aureus clinical isolates. Near universal PNAG expression among invasive clinical isolates is also supported by genetic evidence3,4 indicating the presence of the intercellular-adhesin (ica) genes encoding the PNAG biosynthetic apparatus in almost all invasive S. aureus strains. An antigen designated 336 has been proposed as another S. aureus capsule, but it is, in fact, a cell wall teichoic acid antigen and not a true capsular polysaccharide.97 On in vitro grown S. aureus, the CP antigens and PNAG are both co-expressed on the cell surface as intercalated molecules (Fig. 1), with significant overlap in the pixels visualized by confocal microscopy indicative of the presence of both CP and PNAG antigens in close proximity on the bacteria (Fig. 2). These results also indicate there is comparable availability of these antigens to mediators of immunity. Thus, in contrast to the description of Jansen et al.27 in Table 1 of their review that PNAG is a biofilm antigen into which antibodies and immune effectors might have trouble penetrating, PNAG is, in fact, a capsular antigen like CP5 and CP8 and passive and active immunization against PNAG will most likely target infections wherein the bacteria are in the planktonic, not the biofilm, state.
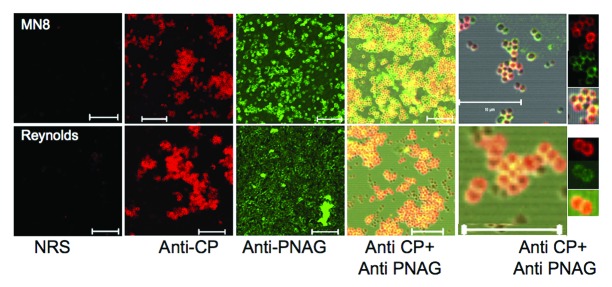
Figure 1. Reactivity of S. aureus CP8 strain MN8 and CP5 strain Reynolds to antibody to either the homologous CP antigen (Anti-CP), PNAG (Anti-PNAG) or both as detected by immunofluorescence. NRS = normal rabbit serum. Binding of rabbit antibody to CP5 or CP8 to S. aureus cells detected with anti-rabbit IgG secondary antibody conjugated to AlexaFluor (AF) 588 (red). Human mAb F598 to PNAG directly conjugated to AF 488 (Green) was used to detect PNAG on the bacterial surface. Co-localization of red and green pixels in samples reacted with antibody to both CP and PNAG antigens appears as an orange-yellow to yellow color. Far right panels show fluorescence in the individual red (Anti-CP), green (Anti-PNAG) or both channels for selected bacterial cells in the micrograph co-stained with antibody to CP and PNAG antigens. White bars = 10 µm.
Figure 2. Quantification of the fluorescence intensity of the reactivity of S. aureus CP5 strain Reynolds and CP8 strain MN8 to antibody to the homologous CP antigen and PNAG. Binding of primary rabbit antibody to purified CP5 or CP8 conjugate antigens to S. aureus cells detected with anti-rabbit IgG secondary antibody conjugated to AlexaFluor (AF) 588 (red). Human mAb F598 to PNAG directly conjugated to AF 488 (Green) was used to detect PNAG on the bacterial surface. Histograms depict analysis of the co-localization of red and green pixels in samples reacted with antibody to both CP and PNAG antigens across the distances, in microns (µm), depicted on the X-axis. Arrows on photomicrographs indicate regions analyzed.
Of note, in the reports on immune responses in various populations given CP-conjugate vaccines it appeared that natural antibody to CP5 and CP8 present in the pre-immunization sera was low, (< 10 µg/ml22,98-100) suggesting that most humans do not make much of an antibody response to CP5 or CP8 from natural exposure. In some contexts this would be encouraging, as it would indicate that an effective CP-specific vaccine could provide immunity not engendered by natural exposure. However, from the epidemiology of S. aureus infections it is clear that CP antigens are not essential virulence factors, as CP-non-expressing strains comprise 10–20% of clinical isolates, and the highly virulent USA300 clone of MRSA, increasingly found as a cause of both community- and hospital-acquired infections,101 does not make either CP5 or CP8.102 As non-essential virulence factors it may be that in the presence of antibody to CP antigens the infecting S. aureus strains phase-vary and stop making them without any loss of virulence. This concept is supported by the frequent recovery of isolates from human infections that have mutated the accessory-gene-response (agr) system103-106 needed for maximal CP production.107 Loss of agr-facilitated CP synthesis could allow for escape from antibody-mediated opsonic killing and such variation during infection is deserving of further investigation.
Might PNAG then serve as a conserved, broadly expressed vaccine target present of the surface as are the capsules of pathogens for which successful vaccines have been developed? Over the past 15 y PNAG has been extensively evaluated in pre-clinical settings as a S. aureus vaccine candidate.85,92,108-110 Overall, strong evidence for both protective immunity and function as a virulence factor in settings of experimental S. aureus infection has been obtained,85,108-111 which, while encouraging, still represent studies comparable to those for the S. aureus vaccines that have gone before and failed in human trials. A key breakthrough in the development of PNAG vaccines was the finding that immunity elicited to the native glycoform of the antigen, in which the vast majority of the amino groups on the second carbon of the N-acetyl glucosamine monomers are acetylated, does not elicit protective antibody.112 Eliminating most of the acetyl substituents results in a glycoform termed deacetylated PNAG (dPNAG) that readily elicits opsonic, protective antibody.84,85,113 The protective antibodies effectively deposit complement opsonins onto the bacterial surface.110 Natural antibody to the native glycoform of PNAG found in all human sera we have analyzed are non-opsonic and non-protective in 95% of the samples,35,36 indicating a basis for the escape of S. aureus and other PNAG-producing pathogens from natural immunity to this antigen.
Under these circumstances, wherein natural antibody to both the CP and PNAG surface antigens are either low or ineffective, it seemed quite promising to combine these into a multi-component vaccine that could potentially cover all strains of S. aureus. When we attempted to determine the additive and/or synergistic effects of having both antibody to CP and PNAG antigens in an immune serum, we were completely surprised to find they inhibited each other’s opsonic and protective activity when present in serum at specific ratios.35,36,108 Inhibition was due to a charge-dependent idiotype-anti-idiotype binding of the variable regions of the antibodies to the negatively-charged CP and positively charged, dPNAG to each other. Inhibition was not present if antibody to either CP or PNAG antigens predominated in a serum36 but in humans this could be a transient phenomenon, as not only did vaccine induced antibody to PNAG inhibit CP antibody functionality,36 but the natural, non-opsonic, non-protective antibodies to PNAG present in almost all normal human sera could also inhibit vaccine-induced antibody to CP antigens.35 Thus, once an initial spike in antibody to CP antigens induced by immunization declines over time, the predominant effect in human sera could be loss of functional activity of the antibody to S. aureus CP antigens.
Looking at development of antibody to CP and PNAG antigens in infected human sera provided further insight into the incompatible nature of antibody to both antigens being present in one serum. We found that among S. aureus infected humans, only those with bacteremia made opsonic antibody responses to either CP or PNAG antigens, but, in the majority of these cases, the ratios of antibodies were in the inhibitory range.35,36 To detect the CP- or PNAG-specific opsonic killing activity, we had to remove one or the other of these antibodies from the sera by absorption, but when combined back together the opsonic killing manifest in the absorbed sera were restored to the initial non-opsonic state. Thus, the problem was not in induction of a potentially protective opsonic response to either CP or PNAG antigens in patients heavily infected with S. aureus, the problem was the inability of these two effectors to peacefully co-exist. This inhibitory phenomenon may also have contributed to the failure of the CP5/CP8 conjugate vaccine trial in hemodialysis patients99 wherein a significant 57% (95% C.I. 10–81%) reduction in S. aureus infections among vaccinates was detected at 40 weeks post-immunization but not at 54 weeks, the pre-determined trial endpoint. From the 40–54 week period the decline in antibody levels to the CP antigens could have brought them into the inhibitory range, thus turning an efficacious signal at 40 weeks into a non-efficacious one 14 weeks later. A repeat trial of the CP5/8 conjugate vaccine in hemodialysis patients failed to show efficacy at any time point, and while no published data are available for analysis, it was suggested at meeting presentations that there was a manufacturing problem leading to less-than-expected immunogenicity of the batch of vaccine used in the repeat trial. Such a situation could also exacerbate the potential for natural antibody to PNAG to be in the inhibitory range for the immunization-induced antibody to CP antigens. Overall, another barrier to an otherwise theoretically promising approach to a S. aureus vaccine emerged and these results likely preclude developing any multi-component vaccine containing both CP and PNAG antigens.
What about inducing immunity to PNAG—would natural antibody to CP antigens be similarly inhibitory? This possibility does need to be evaluated but, as noted above, studies of antibody levels to the CP antigens in pre-immunization sera from conjugate-vaccine volunteers22,99,100 and normal humans35 indicates little natural antibody to S. aureus CP antigens arises from natural exposure. Also, we have reported that titers to CP5 and CP8 are markedly lower in most normal human sera when compared with titers to PNAG in the same sera.35 Of note, in a phase I safety and pharmacokinetic evaluation of a fully human IgG1 mAb to PNAG opsonic antibody to S. aureus was detected in all recipients within hours of infusion and maintained for 50 d,114 indicating over this time period no interference in functional activity emerged.
As of now, the development of vaccines and passive therapies targeting PNAG are progressing but whether they will be successful or meet the same fate as S. aureus human vaccines that have gone before them won’t be known for several years at a minimum. The fully human mAb is being evaluated to determine what phase II trial would be best for obtaining a potential efficacy signal as well as additional safety and pharmacokinetic data in individuals at-risk for S. aureus infection. The oligoglucosamine-conjugate vaccine is being synthesized for phase I trials projected to commence in 2014 and potentially earlier in economically-valuable animals. Impacting the development of PNAG-targeting vaccines is the finding that not only S. aureus and S. epidermidis make PNAG but gram-negative bacteria carrying a biosynthetic genetic locus termed pga can make PNAG.84,115-119 Surprisingly, we have recently identified a large number of major human bacterial pathogens that lack a 4-gene operon homologous to either the staphylococcal ica or gram-negative pga loci that make PNAG, and additionally found that fungal and eukaryotic parasites such as Candida albicans, Trichomonas vaginalis and Plasmodium falciparum make PNAG.120 How this will guide and impact the development of a PNAG vaccine or potentially even affect clinical trials of CP vaccines that could be incompatible with a PNAG vaccine is not yet known, but represent important questions that will affect S. aureus vaccine development as well.
The Challenges Coming From Finding An Effective Clinical Setting For Testing a Vaccine
The question of how to identify a clinical setting or population to provide the results needed to validate the efficacy of a S. aureus vaccine is not only lacking a clear answer but it is a moving target. The CP vaccine trials in hemodialysis patients were chosen for the anticipated high infection rates experienced by this population.78,99,121 Immunization of individuals undergoing cardiothoracic surgery for the evaluation of the V710/IsdB vaccine represented a clinical issue with a high medical need for an effective intervention as well as a setting where the at-risk individuals could be identified sufficiently ahead of time to allow for vaccination to proceed prior to surgery.28 Trials of passive therapy targeting ClfA and LTA in low-birth weigh neonates also represented a setting of high infection rates and medical need.122,123 None of these patient populations are ideal for vaccine/passive therapy evaluations but the choice is driven by numerous factors and it is unlikely there is an ideal population consisting of immunocompetent individuals that can respond to a vaccine that also have a high risk for S. aureus infection.
Further impacting clinical trial design is the changing nature of patient care that evolves as the vaccine trials are being planned and populations evaluated for basal S. aureus infection rates in order to ascertain that a sufficient signal can be garnered in a reasonable time period. Improvements leading to reductions in nosocomial infections such as better hand hygiene, surgical wound care,124-126 infection control including contact precautions with single room isolation or cohorting127 and introduction of interventions such as routine use of chlorhexidine baths128 can effectively reduce infection rates with S. aureus, particularly when applied to MRSA. Thus, what might look like a good population to conduct a S. aureus vaccine trial in at the start of the trial might have marked reductions in infection rates as the trial progresses, confounding the ability to get a robust signal as to a vaccine candidate’s efficacy.
One population that emerges as a potentially robust target group are patients with a significant exposure to a health-care setting that are at high risk for community onset S. aureus infections, notably bacteremia and osteomyelitis caused by MRSA within 12 mo of the exposure.32-34,59,60 This indicates a reasonable expectation of sufficient levels of infection such that a rationale clinical trial can be designed and implemented. Additionally, this is a heterogeneous group of individuals but they are identified during their hospitalization by an MRSA-positive culture from a superficial body site,34 so enrolling volunteers is facilitated by the availability of hospital-based cultures. Furthermore, although many of these individuals have significant underlying disease leading to an immunodeficient state, or have an underlying rapidly fatal condition, they can be excluded from early vaccine trials. Hemodialysis patients represent about 20% of this at-risk group34 and their ability to respond to or effectively utilize a vaccine-induced immune effector could be problematic, although in the CP vaccine trials in this poplation immunogenicity of the conjugate vaccines did not appear to be a problem in the published studies.22,99,100 Evaluations of immune responses and testing of all effectors needed for vaccine efficacy, such as phagocyte and complement function, in the blood of hemodialysis patients should be undertaken in initial phase II trials to insure that efficacy won’t be impacted by sub-optimal function or availability of needed co-factors or rapid loss of antibody due to dialysis. Importantly, the individuals at risk for community onset MRSA infections often are not discharged with a standard of care that includes such treatments as routine antibiotic administration, so potential treatments that could reduce the occurrence of infection among controls is minimized, although encouraging optimal practices such as chlorhexidine baths in such populations might impact infection rates. Routine monitoring by visits from home-health aides and keeping close track of post-discharge medical care32 should insure maximal determination of infection rates among vaccinees in this group. Better epidemiological studies of this population might be needed to substantiate that there is a sufficient number of relatively immunologically-intact individuals that could enroll in a S. aureus vaccine trial, but if neither numbers of available volunteers or other confounding or contraindicating factors are found among this post-discharge, MRSA group they likely represent a good cohort to evaluate in any S. aureus vaccine approach.
Potential Ways Forward
With all of the barriers, failures and difficulties encountered to date on developing a universal vaccine for S. aureus we would nonetheless like to find some means to either target the more severe manifestations of infection such as bacteremia, sepsis, bone infections and pneumoniae or prevent infections that are difficult to manage such as implant-related infections. Thus, instead of a universal S. aureus vaccine we may need to settle for one targeting specific clinical manifestations. Many individuals in the field advocate a multi-valent vaccine approach,38,129 which, while logical and could even cover different manifestations of S. aureus infection, the idea is still not predicated on any human data, only results from pre-clinical animal studies.81,108,129 Given the cost of drug development it is unlikely a systematic approach wherein human testing of both individual and combined vaccine components will ensue, so we might end up with an effective multi-component vaccine without any real knowledge of which of the antigens are essential. PNAG remains a potential single component vaccine if it induces infection-preventing immunity in humans to S. aureus as do capsular vaccines for other bacterial pathogens, but at the moment there are no data regarding susceptibility and resistance of humans to infection based on their PNAG antibody status. One encouraging finding in regards to PNAG expression among the variety of microbial pathogens that make this antigen is that limited, but consistent, findings indicate in vivo production during human infection.120 Of note, if immunity to PNAG can contribute to resistance to infection with S. aureus and other organisms, it is possible that a vaccine inducing immunity to this antigen could be widely used but augmentation of immunity specific to antigens from different organisms, such as α-hemolysin for S. aureus,120 shiga toxin for organisms producing this factor130,131 and Clostridium difficle toxins132 might be needed to engender maximal immunity to each pathogen. Overall, while the path to an effective human vaccine for S. aureus is so far strewn with failures and is not guided by any strong association between human immune effectors and resistance to infection, there is still a major effort and considerable investment within the biotechnology and pharmaceutical industry for pursuing active and passive therapies, engendering hope, but a cautious level of confidence, that we will ultimately be successful in this endeavor and be able to prevent at least some of the more severe or costly S. aureus infections.
Acknowledgments
I thank Dr. Colette Cywes-Bentley for provision of the confocal micrographs. Research results related to PNAG vaccines discussed in this review were supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID) grant numbers AI46706 and AI057159, a component of Award Number U54 AI057159, by a sponsored research agreement with Sanofi, Inc. and by an unrestricted gift from Alopexx Pharmaceuticals LLC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Conflict of Interest
GBP is an inventor of Intellectual Properties (IP) (Human monoclonal antibody to PNAG and PNAG vaccine) that are licensed by Brigham and Women’s Hospital (BWH) to Alopexx Vaccines LLC, and Alopexx Pharmaceuticals LLC. As an inventor of the IP, he has the right to receive a share of licensing-related income (royalties, fees) through BWH from Alopexx Pharmaceuticals and Alopexx Vaccines. GBP also holds equity in these two companies. GBP’s interests were reviewed and are managed by the BWH and Partners Healthcare in accordance with their conflict of interest policies.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/25182
References
- 1.Nanra JS, Timofeyeva Y, Buitrago SM, Sellman BR, Dilts DA, Fink P, et al. Heterogeneous in vivo expression of clumping factor A and capsular polysaccharide by Staphylococcus aureus: implications for vaccine design. Vaccine. 2009;27:3276–80. doi: 10.1016/j.vaccine.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 2.Golubchik T, Batty EM, Miller RR, Farr H, Young BC, Larner-Svensson H, et al. Within-host evolution of Staphylococcus aureus during asymptomatic carriage. PLoS One. 2013;8:e61319. doi: 10.1371/journal.pone.0061319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nethercott C, Mabbett AN, Totsika M, Peters P, Ortiz JC, Nimmo GR, et al. Molecular Characterization of Endocarditis-Associated Staphylococcus aureus. J Clin Microbiol. 2013;51:2131–8. doi: 10.1128/JCM.00651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, et al. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun. 2002;70:4987–96. doi: 10.1128/IAI.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre PF, Linnemann C, Weckbach LS, Staneck JL, Buncher CR, Vigdorth E, et al. Antibody responses to toxic-shock-syndrome (TSS) toxin by patients with TSS and by healthy staphylococcal carriers. J Infect Dis. 1984;150:662–6. doi: 10.1093/infdis/150.5.662. [DOI] [PubMed] [Google Scholar]
- 6.Christensson B, Hedström SA. Serological response to toxic shock syndrome toxin in Staphylococcus aureus infected patients and healthy controls. Acta Pathol Microbiol Immunol Scand B. 1985;93:87–90. doi: 10.1111/j.1699-0463.1985.tb02857.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson HM, Russell JK, Pontzer CH. Staphylococcal enterotoxin microbial superantigens. FASEB J. 1991;5:2706–12. doi: 10.1096/fasebj.5.12.1916093. [DOI] [PubMed] [Google Scholar]
- 8.Novick RP. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid. 2003;49:93–105. doi: 10.1016/S0147-619X(02)00157-9. [DOI] [PubMed] [Google Scholar]
- 9.Dreisbach A, Hempel K, Buist G, Hecker M, Becher D, van Dijl JM. Profiling the surfacome of Staphylococcus aureus. Proteomics. 2010;10:3082–96. doi: 10.1002/pmic.201000062. [DOI] [PubMed] [Google Scholar]
- 10.Dreisbach A, van der Kooi-Pol MM, Otto A, Gronau K, Bonarius HP, Westra H, et al. Surface shaving as a versatile tool to profile global interactions between human serum proteins and the Staphylococcus aureus cell surface. Proteomics. 2011;11:2921–30. doi: 10.1002/pmic.201100134. [DOI] [PubMed] [Google Scholar]
- 11.Klevens RM, Edwards JR, Gaynes RP, National Nosocomial Infections Surveillance System The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–30. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 12.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 13.Forstner C, Dungl C, Tobudic S, Mitteregger D, Lagler H, Burgmann H. Predictors of clinical and microbiological treatment failure in patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia: a retrospective cohort study in a region with low MRSA prevalence. Clin Microbiol Infect. 2013;19:E291–7. doi: 10.1111/1469-0691.12169. [DOI] [PubMed] [Google Scholar]
- 14.Peyrani P, Allen M, Wiemken TL, Haque NZ, Zervos MJ, Ford KD, et al. IMPACT-HAP Study Group Severity of disease and clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus strains not influenced by the presence of the Panton-Valentine leukocidin gene. Clin Infect Dis. 2011;53:766–71. doi: 10.1093/cid/cir541. [DOI] [PubMed] [Google Scholar]
- 15.de Kraker MEA, Davey PG, Grundmann H, BURDEN study group Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis. 2011;11:30–8. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 17.Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Curr Opin Microbiol. 2012;15:588–95. doi: 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Malachowa N, Kobayashi SD, DeLeo FR. Community-associated methicillin-resistant Staphylococcus aureus and athletes. Phys Sportsmed. 2012;40:13–21. doi: 10.3810/psm.2012.05.1960. [DOI] [PubMed] [Google Scholar]
- 19.Otto M. MRSA virulence and spread. Cell Microbiol. 2012;14:1513–21. doi: 10.1111/j.1462-5822.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74:2215–23. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184:1572–80. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 22.Welch PG, Fattom A, Moore J, Jr., Schneerson R, Shiloach J, Bryla DA, et al. Safety and immunogenicity of Staphylococcus aureus type 5 capsular polysaccharide-Pseudomonas aeruginosa recombinant exoprotein A conjugate vaccine in patients on hemodialysis. J Am Soc Nephrol. 1996;7:247–53. doi: 10.1681/ASN.V72247. [DOI] [PubMed] [Google Scholar]
- 23.Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–65. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisman LE, Fischer GW, Thackray HM, Johnson KE, Schuman RF, Mandy GT, et al. Safety and pharmacokinetics of a chimerized anti-lipoteichoic acid monoclonal antibody in healthy adults. Int Immunopharmacol. 2009;9:639–44. doi: 10.1016/j.intimp.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Burnie JP, Matthews RC, Carter T, Beaulieu E, Donohoe M, Chapman C, et al. Identification of an immunodominant ABC transporter in methicillin-resistant Staphylococcus aureus infections. Infect Immun. 2000;68:3200–9. doi: 10.1128/IAI.68.6.3200-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:1179–86. doi: 10.1093/cid/cis033. [DOI] [PubMed] [Google Scholar]
- 27.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: “Staphyloccocus aureus vaccines: Problems and prospects”. Vaccine. 2013;31:2723–30. doi: 10.1016/j.vaccine.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–78. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 29.Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol. 2012;34:335–48. doi: 10.1007/s00281-011-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:560–7. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen I, Dudney H, Creech CB. Searching for the holy grail of a staphylococcal vaccine. Hum Vaccin. 2010;6:1068–70. doi: 10.4161/hv.6.12.12917. [DOI] [PubMed] [Google Scholar]
- 32.Avery TR, Kleinman KP, Klompas M, Aschengrau A, Huang SS. Inclusion of 30-day postdischarge detection triples the incidence of hospital-onset methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2012;33:114–21. doi: 10.1086/663714. [DOI] [PubMed] [Google Scholar]
- 33.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–5. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 34.Duffy J, Dumyati G, Bulens S, Namburi S, Gellert A, Fridkin SK, et al. Community-onset invasive methicillin-resistant Staphylococcus aureus infections following hospital discharge. Am J Infect Control. 2013 doi: 10.1016/j.ajic.2012.10.020. In press. [DOI] [PubMed] [Google Scholar]
- 35.Skurnik D, Kropec A, Roux D, Theilacker C, Huebner J, Pier GB. Natural antibodies in normal human sera inhibit Staphylococcus aureus capsular polysaccharide vaccine efficacy. Clin Infect Dis. 2012;205:1709–18. doi: 10.1093/cid/cis624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skurnik D, Merighi M, Grout M, Gadjeva M, Maira-Litran T, Ericsson M, et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest. 2010;120:3220–33. doi: 10.1172/JCI42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med. 2010;207:1863–70. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol. 2012;15:92–9. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012;30:2921–7. doi: 10.1016/j.vaccine.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–18. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Ardura MI, Banchereau R, Mejias A, Di Pucchio T, Glaser C, Allantaz F, et al. Enhanced monocyte response and decreased central memory T cells in children with invasive Staphylococcus aureus infections. PLoS One. 2009;4:e5446. doi: 10.1371/journal.pone.0005446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 44.Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update) Blood Cells Mol Dis. 2010;45:246–65. doi: 10.1016/j.bcmd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 46.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 47.Kurebayashi Y, Nagai S, Ikejiri A, Koyasu S. Recent advances in understanding the molecular mechanisms of the development and function of Th17 cells. Genes Cells. 2013;18:247–65. doi: 10.1111/gtc.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolata J, Bode LG, Holtfreter S, Steil L, Kusch H, Holtfreter B, et al. Distinctive patterns in the human antibody response to Staphylococcus aureus bacteremia in carriers and non-carriers. Proteomics. 2011;11:3914–27. doi: 10.1002/pmic.201000760. [DOI] [PubMed] [Google Scholar]
- 49.den Reijer PM, Lemmens-den Toom N, Kant S, Snijders SV, Boelens H, Tavakol M, et al. Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS One. 2013;8:e53391. doi: 10.1371/journal.pone.0053391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narita K, Hu D-L, Mori F, Wakabayashi K, Iwakura Y, Nakane A. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect Immun. 2010;78:4234–42. doi: 10.1128/IAI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoong P, Pier GB. Immune-activating properties of Panton-Valentine leukocidin improve the outcome in a model of methicillin-resistant Staphylococcus aureus pneumonia. Infect Immun. 2012;80:2894–904. doi: 10.1128/IAI.06360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermos CR, Yoong P, Pier GB. High levels of antibody to panton-valentine leukocidin are not associated with resistance to Staphylococcus aureus-associated skin and soft-tissue infection. Clin Infect Dis. 2010;51:1138–46. doi: 10.1086/656742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verkaik NJ, Dauwalder O, Antri K, Boubekri I, de Vogel CP, Badiou C, et al. Immunogenicity of toxins during Staphylococcus aureus infection. Clin Infect Dis. 2010;50:61–8. doi: 10.1086/648673. [DOI] [PubMed] [Google Scholar]
- 55.Jacobsson G, Colque-Navarro P, Gustafsson E, Andersson R, Möllby R. Antibody responses in patients with invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis. 2010;29:715–25. doi: 10.1007/s10096-010-0919-x. [DOI] [PubMed] [Google Scholar]
- 56.Holtfreter S, Jursa-Kulesza J, Masiuk H, Verkaik NJ, de Vogel C, Kolata J, et al. Antibody responses in furunculosis patients vaccinated with autologous formalin-killed Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2011;30:707–17. doi: 10.1007/s10096-010-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, et al. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–23. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 58.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis. 2013;56:1554–61. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang SS, Hinrichsen VL, Datta R, Spurchise L, Miroshnik I, Nelson K, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One. 2011;6:e24340. doi: 10.1371/journal.pone.0024340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang SS, Diekema DJ, Warren DK, Zuccotti G, Winokur PL, Tendolkar S, et al. Strain-relatedness of methicillin-resistant Staphylococcus aureus isolates recovered from patients with repeated infection. Clin Infect Dis. 2008;46:1241–7. doi: 10.1086/529381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–41. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–62. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 63.Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, et al. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010;202:1866–76. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gauduchon V, Cozon G, Vandenesch F, Genestier AL, Eyssade N, Peyrol S, et al. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis. 2004;189:346–53. doi: 10.1086/380909. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasan A, Seifried S, Zhu L, Srivastava DK, Flynn PM, Shenep JL, et al. Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus infections in children with cancer. Pediatr Blood Cancer. 2009;53:1216–20. doi: 10.1002/pbc.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orscheln RC, Hunstad DA, Fritz SA, Loughman JA, Mitchell K, Storch EK, et al. contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin Infect Dis. 2009;49:536–42. doi: 10.1086/600881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bae I-G, Tonthat GT, Stryjewski ME, Rude TH, Reilly LF, Barriere SL, et al. Presence of genes encoding the panton-valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J Clin Microbiol. 2009;47:3952–7. doi: 10.1128/JCM.01643-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A. 2010;107:2241–6. doi: 10.1073/pnas.0910344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foletti D, Strop P, Shaughnessy L, Hasa-Moreno A, Casas MG, Russell M, et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus α-hemolysin. J Mol Biol. 2013;425:1641–54. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Gotschlich EC, Goldschneider I, Artenstein MS. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–84. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–48. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gotschlich EC, Goldschneider I, Artenstein MS. Human immunity to the meningococcus. V. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med. 1969;129:1385–95. doi: 10.1084/jem.129.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gold R, Artenstein MS. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull World Health Organ. 1971;45:279–82. [PMC free article] [PubMed] [Google Scholar]
- 76.Artenstein MS, Gold R, Zimmerly JG, Wyle FA, Schneider H, Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970;282:417–20. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- 77.Weisman LE, Thackray HM, Garcia-Prats JA, Nesin M, Schneider JH, Fretz J, et al. Phase 1/2 double-blind, placebo-controlled, dose escalation, safety, and pharmacokinetic study of pagibaximab (BSYX-A110), an antistaphylococcal monoclonal antibody for the prevention of staphylococcal bloodstream infections, in very-low-birth-weight neonates. Antimicrob Agents Chemother. 2009;53:2879–86. doi: 10.1128/AAC.01565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shinefield HR. Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: is an additional vaccine needed or possible? Vaccine. 2006;24(Suppl 2):S2–65, 9. doi: 10.1016/j.vaccine.2005.01.126. [DOI] [PubMed] [Google Scholar]
- 79.Vernachio J, Bayer AS, Le T, Chai YL, Prater B, Schneider A, et al. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob Agents Chemother. 2003;47:3400–6. doi: 10.1128/AAC.47.11.3400-3406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joshi A, Pancari G, Cope L, Bowman EP, Cua D, Proctor RA, et al. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother. 2012;8:336–46. doi: 10.4161/hv.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, et al. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine. 2010;28:6382–92. doi: 10.1016/j.vaccine.2010.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, et al. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun. 2003;71:6864–70. doi: 10.1128/IAI.71.12.6864-6870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brady RA, Mocca CP, Prabhakara R, Plaut RD, Shirtliff ME, Merkel TJ, et al. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of Staphylococcus aureus infection. PLoS One. 2013;8:e63040. doi: 10.1371/journal.pone.0063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gening ML, Maira-Litrán T, Kropec A, Skurnik D, Grout M, Tsvetkov YE, et al. Synthetic β-(1->6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect Immun. 2010;78:764–72. doi: 10.1128/IAI.01093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maira-Litrán T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-β-(1-6)-glucosamine. Infect Immun. 2005;73:6752–62. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toltzis P, Jacobs MR. The epidemiology of childhood pneumococcal disease in the United States in the era of conjugate vaccine use. Infect Dis Clin North Am. 2005;19:629–45. doi: 10.1016/j.idc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Ladhani SN. Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom. Clin Ther. 2012;34:385–99. doi: 10.1016/j.clinthera.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 88.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362:1511–20. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 89.Engels EA, Lau J. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2000:CD001261. doi: 10.1002/14651858.CD001261. [DOI] [PubMed] [Google Scholar]
- 90.Cocchiaro JL, Gomez MI, Risley A, Solinga R, Sordelli DO, Lee JC. Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Mol Microbiol. 2006;59:948–60. doi: 10.1111/j.1365-2958.2005.04978.x. [DOI] [PubMed] [Google Scholar]
- 91.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–34. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Döring G, et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–7. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 93.Maira-Litrán T, Kropec A, Abeygunawardana C, Joyce J, Mark G, 3rd, Goldmann DA, et al. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun. 2002;70:4433–40. doi: 10.1128/IAI.70.8.4433-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sutter DE, Summers AM, Keys CE, Taylor KL, Frasch CE, Braun LE, et al. Capsular serotype of Staphylococcus aureus in the era of community-acquired MRSA. FEMS Immunol Med Microbiol. 2011;63:16–24. doi: 10.1111/j.1574-695X.2011.00822.x. [DOI] [PubMed] [Google Scholar]
- 95.Melles DC, Taylor KL, Fattom AI, van Belkum A. Serotyping of Dutch Staphylococcus aureus strains from carriage and infection. FEMS Immunol Med Microbiol. 2008;52:287–92. doi: 10.1111/j.1574-695X.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 96.von Eiff C, Taylor KL, Mellmann A, Fattom AI, Friedrich AW, Peters G, et al. Distribution of capsular and surface polysaccharide serotypes of Staphylococcus aureus. Diagn Microbiol Infect Dis. 2007;58:297–302. doi: 10.1016/j.diagmicrobio.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 97.Verdier I, Durand G, Bes M, Taylor KL, Lina G, Vandenesch F, et al. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J Clin Microbiol. 2007;45:725–9. doi: 10.1128/JCM.01572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I, et al. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun. 1993;61:1023–32. doi: 10.1128/iai.61.3.1023-1032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 100.Fattom A, Fuller S, Propst M, Winston S, Muenz L, He D, et al. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine. 2004;23:656–63. doi: 10.1016/j.vaccine.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 101.Hudson LO, Murphy CR, Spratt BG, Enright MC, Elkins K, Nguyen C, et al. Diversity of methicillin-resistant Staphylococcus aureus(MRSA) strains isolated from inpatients of 30 hospitals in Orange County, California. PLoS One. 2013;8:e62117. doi: 10.1371/journal.pone.0062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, et al. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis. 2008;198:561–70. doi: 10.1086/590157. [DOI] [PubMed] [Google Scholar]
- 103.Shopsin B, Eaton C, Wasserman GA, Mathema B, Adhikari RP, Agolory S, et al. Mutations in agr do not persist in natural populations of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2010;202:1593–9. doi: 10.1086/656915. [DOI] [PubMed] [Google Scholar]
- 104.Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, et al. agr function in clinical Staphylococcus aureus isolates. Microbiology. 2008;154:2265–74. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shopsin B, Drlica-Wagner A, Mathema B, Adhikari RP, Kreiswirth BN, Novick RP. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J Infect Dis. 2008;198:1171–4. doi: 10.1086/592051. [DOI] [PubMed] [Google Scholar]
- 106.Smyth DS, Kafer JM, Wasserman GA, Velickovic L, Mathema B, Holzman RS, et al. Nasal carriage as a source of agr-defective Staphylococcus aureus bacteremia. J Infect Dis. 2012;206:1168–77. doi: 10.1093/infdis/jis483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luong T, Sau S, Gomez M, Lee JC, Lee CY. Regulation of Staphylococcus aureus capsular polysaccharide expression by agr and sarA. Infect Immun. 2002;70:444–50. doi: 10.1128/IAI.70.2.444-450.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One. 2012;7:e46648. doi: 10.1371/journal.pone.0046648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maira-Litrán T, Bentancor LV, Bozkurt-Guzel C, O’Malley JM, Cywes-Bentley C, Pier GB. Synthesis and evaluation of a conjugate vaccine composed of Staphylococcus aureus poly-N-acetyl-glucosamine and clumping factor A. PLoS One. 2012;7:e43813. doi: 10.1371/journal.pone.0043813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect Immun. 2006;74:2742–50. doi: 10.1128/IAI.74.5.2742-2750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kropec A, Maira-Litran T, Jefferson KK, Grout M, Cramton SE, Götz F, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:6868–76. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joyce JG, Abeygunawardana C, Xu Q, Cook JC, Hepler R, Przysiecki CT, et al. Isolation, structural characterization, and immunological evaluation of a high-molecular-weight exopolysaccharide from Staphylococcus aureus. Carbohydr Res. 2003;338:903–22. doi: 10.1016/S0008-6215(03)00045-4. [DOI] [PubMed] [Google Scholar]
- 113.Cerca N, Jefferson KK, Maira-Litrán T, Pier DB, Kelly-Quintos C, Goldmann DA, et al. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect Immun. 2007;75:3406–13. doi: 10.1128/IAI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vlock D, Lee JC, Kropec-Huebner A, Pier GB. Pre-clinical and initial phase I evaluations of a fully human monoclonal antibody directed against the PNAG surface polysaccharide on Staphylococcus aureus Abstracts of the 50th ICAAC 2010; Abstract G1-1654/329 2010. [Google Scholar]
- 115.Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186:2724–34. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parise G, Mishra M, Itoh Y, Romeo T, Deora R. Role of a putative polysaccharide locus in Bordetella biofilm development. J Bacteriol. 2007;189:750–60. doi: 10.1128/JB.00953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cerca N, Maira-Litrán T, Jefferson KK, Grout M, Goldmann DA, Pier GB. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc Natl Acad Sci U S A. 2007;104:7528–33. doi: 10.1073/pnas.0700630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Izano EA, Sadovskaya I, Vinogradov E, Mulks MH, Velliyagounder K, Ragunath C, et al. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathog. 2007;43:1–9. doi: 10.1016/j.micpath.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skurnik D, Davis MR, Jr., Benedetti D, Moravec KL, Cywes-Bentley C, Roux D, et al. Targeting pan-resistant bacteria with antibodies to a broadly conserved surface polysaccharide expressed during infection. J Infect Dis. 2012;205:1709–18. doi: 10.1093/infdis/jis254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cywes-Bentley C, Skurnik D, Zaidi T, Roux D, Deoliveira RB, Garrett WS, et al. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc Natl Acad Sci U S A. 2013;110:E2209–18. doi: 10.1073/pnas.1303573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fitzgerald SF, O’Gorman J, Morris-Downes MM, Crowley RK, Donlon S, Bajwa R, et al. A 12-year review of Staphylococcus aureus bloodstream infections in haemodialysis patients: more work to be done. J Hosp Infect. 2011;79:218–21. doi: 10.1016/j.jhin.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 122.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151:260–5, e1. doi: 10.1016/j.jpeds.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 123.Weisman LE, Thackray HM, Steinhorn RH, Walsh WF, Lassiter HA, Dhanireddy R, et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128:271–9. doi: 10.1542/peds.2010-3081. [DOI] [PubMed] [Google Scholar]
- 124.Pahys JM, Pahys JR, Cho SK, Kang MM, Zebala LP, Hawasli AH, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95:549–54. doi: 10.2106/JBJS.K.00756. [DOI] [PubMed] [Google Scholar]
- 125.Fry DE. The continued challenge of Staphylococcus aureus in the surgical patient. Am Surg. 2013;79:1–10. [PubMed] [Google Scholar]
- 126.Goyal N, Miller A, Tripathi M, Parvizi J. Methicillin-resistant Staphylococcus aureus (MRSA): colonisation and pre-operative screening. Bone Joint J. 2013;95-B:4–9. doi: 10.1302/0301-620X.95B1.27973. [DOI] [PubMed] [Google Scholar]
- 127.Marshall C, Richards M, McBryde E. Do active surveillance and contact precautions reduce MRSA acquisition? A prospective interrupted time series. PLoS One. 2013;8:e58112. doi: 10.1371/journal.pone.0058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–42. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–7. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsuji T, Shimizu T, Sasaki K, Tsukamoto K, Arimitsu H, Ochi S, et al. A nasal vaccine comprising B-subunit derivative of Shiga toxin 2 for cross-protection against Shiga toxin types 1 and 2. Vaccine. 2008;26:2092–9. doi: 10.1016/j.vaccine.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 131.Serna A, 4th, Boedeker EC. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol. 2008;24:38–47. doi: 10.1097/MOG.0b013e3282f2dfb8. [DOI] [PubMed] [Google Scholar]
- 132.Gerding DN. Clostridium difficile infection prevention: biotherapeutics, immunologics, and vaccines. Discov Med. 2012;13:75–83. [PubMed] [Google Scholar]