Abstract
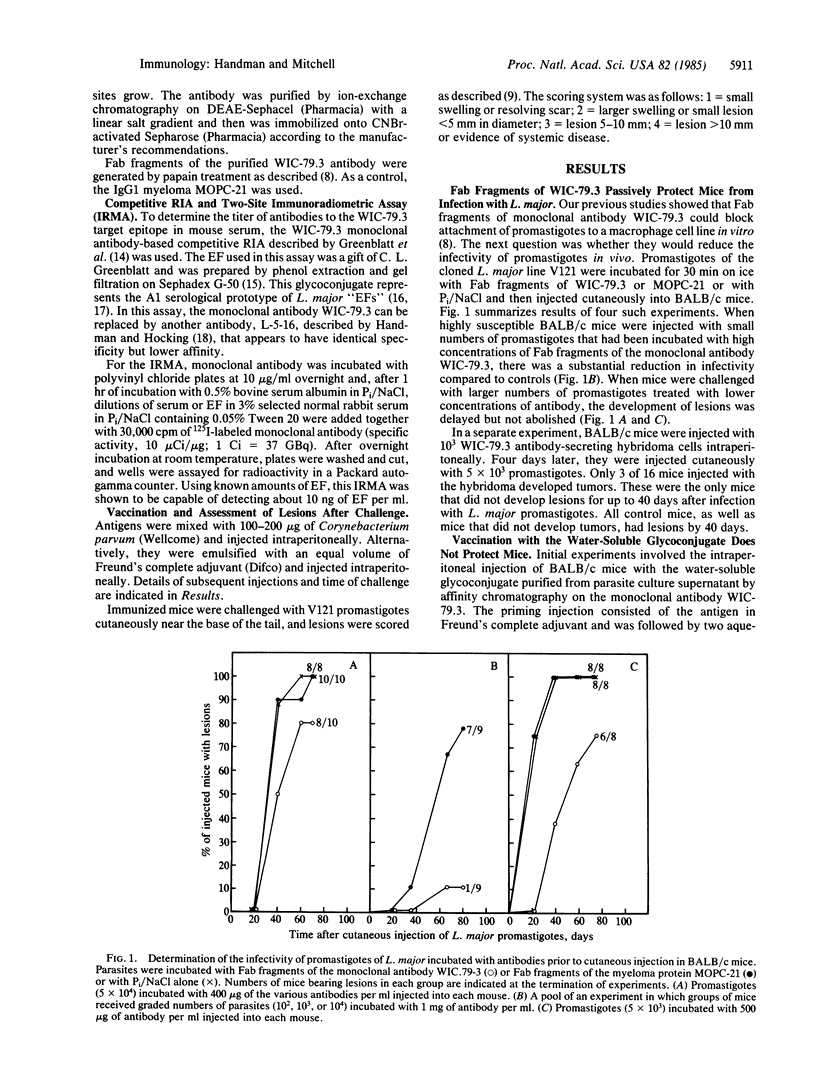
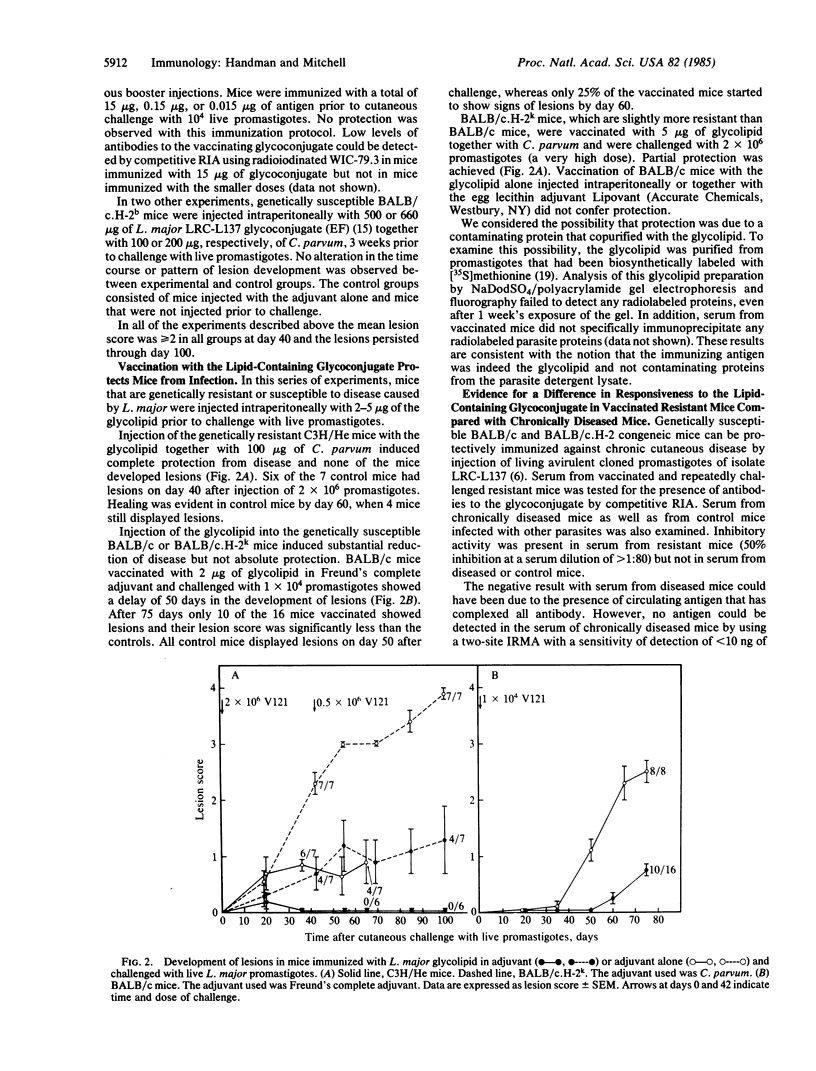
The Leishmania major receptor for macrophages is a lipid-containing glycoconjugate that is recognized by the monoclonal antibody WIC-79.3. When L. major promastigotes were incubated with Fab fragments of WIC-79.3 prior to injection into genetically susceptible mice, their infectivity was decreased. Fab fragments from an irrelevant control antibody of the same class had no effect. The L. major glycolipid was purified from detergent-solubilized promastigotes by affinity chromatography on immobilized WIC-79.3 and used to vaccinate mice that are genetically resistant or susceptible to disease. Genetically resistant mice could be protected totally from cutaneous disease with as little as 5 micrograms of glycolipid. A high but not absolute level of resistance was also induced in the susceptible mice, in which the disease is otherwise fatal. No protection was obtained with the carbohydrate fragment of the glycolipid alone or by injection of the glycolipid in the absence of adjuvant. Genetically susceptible mice, immunized and protected from disease as a result of multiple injections of live avirulent cloned promastigotes of L. major, produced antibodies to the glycolipid of L. major. No antibodies were detected in serum from chronically diseased mice. The data suggest that this functionally important antigen of L. major is a candidate vaccine against cutaneous leishmaniasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greenblatt C. L., Handman E., Mitchell G. F., Battye F. L., Schnur L. F., Snary D. Phenotypic diversity of cloned lines of Leishmania major promastigotes. Z Parasitenkd. 1985;71(2):141–157. doi: 10.1007/BF00926265. [DOI] [PubMed] [Google Scholar]
- Greenblatt C. L., Slutzky G. M., de Ibarra A. A., Snary D. Monoclonal antibodies for serotyping Leishmania strains. J Clin Microbiol. 1983 Jul;18(1):191–193. doi: 10.1128/jcm.18.1.191-193.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Howard J. G., Liew F. Y. Mechanisms of acquired immunity in leishmaniasis. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):87–98. doi: 10.1098/rstb.1984.0111. [DOI] [PubMed] [Google Scholar]
- Kellina O. I. O razlichiiakh v chuvstvitel'nosti inbrednykh myshei raznykh linii k Leishmania tropica major. Med Parazitol (Mosk) 1973 May-Jun;42(3):279–285. [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific suppressor T cells. J Immunol. 1982 Apr;128(4):1917–1922. [PubMed] [Google Scholar]
- Louis J. A., Zubler R. H., Coutinho S. G., Lima G., Behin R., Mauel J., Engers H. D. The in vitro generation and functional analysis of murine T cell populations and clones specific for a protozoan parasite, Leishmania tropica. Immunol Rev. 1982;61:215–243. doi: 10.1111/j.1600-065x.1982.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Curtis J. M., Scollay R. G., Handman E. Resistance and abrogation of resistance to cutaneous leishmaniasis in reconstituted BALB/c nude mice. Aust J Exp Biol Med Sci. 1981 Oct;59(Pt 5):539–554. doi: 10.1038/icb.1981.47. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. Leishmania tropica major in mice: vaccination against cutaneous leishmaniasis in mice of high genetic susceptibility. Aust J Exp Biol Med Sci. 1983 Feb;61(Pt 1):11–25. doi: 10.1038/icb.1983.2. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Spithill T. W. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust J Exp Biol Med Sci. 1984 Apr;62(Pt 2):145–153. doi: 10.1038/icb.1984.14. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Murine cutaneous leishmaniasis: resistance in reconstituted nude mice and several F1 hybrids infected with Leishmania tropica major. J Immunogenet. 1983 Oct;10(5):395–412. doi: 10.1111/j.1744-313x.1983.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Panosian C. B., Sypek J. P., Wyler D. J. Cell contact-mediated macrophage activation for antileishmanial defense. I. Lymphocyte effector mechanism that is contact dependent and noncytotoxic. J Immunol. 1984 Dec;133(6):3358–3365. [PubMed] [Google Scholar]
- Preston P. M., Dumonde D. C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin Exp Immunol. 1976 Jan;23(1):126–138. [PMC free article] [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A., Greenblatt C. L. Leishmanial serotypes as distinguished by the gel diffusion of factors excreted in vitro and in vivo. Isr J Med Sci. 1972 Jul;8(7):932–942. [PubMed] [Google Scholar]
- Steinberger A., Slutzky G. M., El-On J., Greenblatt C. L. Leishmania tropica: protective response in C3H mice vaccinated with excreted factor crosslinked with the synthetic adjuvant, muramyl dipeptide. Exp Parasitol. 1984 Dec;58(3):223–229. doi: 10.1016/0014-4894(84)90038-9. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Panosian C. B., Wyler D. J. Cell contact-mediated macrophage activation for antileishmanial defense. II. Identification of effector cell phenotype and genetic restriction. J Immunol. 1984 Dec;133(6):3351–3357. [PubMed] [Google Scholar]
- Zehavi U., Abrahams J. C., Granoth R., Greenblatt C. L., Slutzky G. M., El-On J. Leishmanial excreted factors (EFs): purification by affinity chromatography. Z Parasitenkd. 1983;69(6):695–701. doi: 10.1007/BF00927419. [DOI] [PubMed] [Google Scholar]
- Zuckerman A. Current status of the immunology of blood and tissue Protozoa. I. Leishmania. Exp Parasitol. 1975 Dec;38(3):370–400. doi: 10.1016/0014-4894(75)90123-x. [DOI] [PubMed] [Google Scholar]
- de Ibarra A. A., Howard J. G., Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982 Dec;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]


