Abstract
Approximately 50% of all patients with cancer receive radiation therapy at some point during the course of their treatment, and the majority of these patients are treated with curative intent. Despite recent advances in the planning of radiation treatment and the delivery of image-guided radiation therapy, acute toxicity and potential long-term side effects often limit the ability to deliver a sufficient dose of radiation to control tumours locally. In the past two decades, a better understanding of the hallmarks of cancer and the discovery of specific signalling pathways by which cells respond to radiation have provided new opportunities to design molecularly targeted therapies to increase the therapeutic window of radiation therapy. Here, we review efforts to develop approaches that could improve outcomes with radiation therapy by increasing the probability of tumour cure or by decreasing normal tissue toxicity.
Ionizing radiation is a commonly used modality for treating cancers (BOX 1). The majority of patients are treated with external beam radiation therapy, in which a radiation source external to the patient generates ionizing radiation that is directed towards the tumour. Modern radiation therapy is delivered mainly via linear accelerators, which generate high-energy X-rays that can be collimated to selectively shape the treatment field. Intensity modulated radiation therapy (IMRT) uses non-uniform, computer-optimized radiation fields to deliver a high dose of radiation to the tumour while limiting the radiation to normal tissues1. With IMRT, the high-dose region conforms better to the tumour, but a larger volume of normal tissue is exposed to low-dose radiation. The long-term effects of this radiation on normal tissues are not known.
Patients are typically treated with small 1.8–2 Gy fractions over the course of 4–8 weeks to limit toxicity to normal tissues. However, advances in treatment planning and delivery have made it possible to safely deliver a small number of high doses (15–20 Gy) to tumours. This treatment modality has been termed `stereotactic body radiation therapy' or radiosurgery. Stereotactic body radiation therapy, which is currently being used clinically for some early-stage cancers and oligom etastatic disease, may be more effective than standard radiation therapy for some cancers2. Although normal tissue toxicity limits the use of stereotactic body radiation therapy in certain anatomical locations3–5, it has been successfully utilized for many cancer types including non-small-cell lung cancer, prostate cancer, renal cell carcinoma and hepatocellular carcinoma6–9.
An emerging technique in radiation oncology is the use of high-energy charged particles to treat tumours10. Particle therapy offers a physical advantage over X-ray irradiation11. Unlike X-rays, which deposit radiation distal to the tumour target as they exit the patient, charged particles stop abruptly within the tissue and deposit the majority of their energy within a small area called the Bragg peak. This dose profile delivers radiation to the tumour while sparing normal tissues from exit irradiation. This may be especially useful for treating tumours that are adjacent to dose-limiting structures, such as the brainstem, or for treating children with cancer who may be at a relatively high risk of developing radiation-induced cancers. Protons are the most commonly used particle therapy11. Although protons are approximately equivalent to X-rays in terms of biological effectiveness, they have a Bragg peak that offers improved sparing of normal tissues. Protons are currently utilized for a broad range of tumours, including paediatric tumours, uveal melanomas, skull base tumours and prostate tumours12. Recently, a retrospective study of SEER (surveillance, epidemiology and end results) Medicare-linked data suggested that there was an increased incidence of gastrointestinal side effects in patients who were treated with protons13. A randomized clinical trial at Massachusetts General Hospital, Boston, USA, and the University of Pennsylvania, Philadelphia, USA, is currently underway to compare the effectiveness of protons and IMRT for the treatment of prostate cancer.
Carbon ions, which are used to treat patients with cancer in Japan and Germany, are also charged and therefore deposit energy with a Bragg peak. However, these larger particles cause concentrated damage that is more lethal to irradiated cells than the damage inflicted by X-rays or protons. Thus, for a given dose, carbon ions have a higher relative biological effectiveness (RBE). In addition, the cellular damage caused by carbon ions may be less dependent on oxygen to stabilize free radicals within cells. As a result, the oxygen enhancement ratio (OER) for heavy particles is lower than for X-rays.
In contrast to external beam radiation therapy, brachytherapy involves the implantation of a radiation source temporarily or permanently into the tumour site. Because the radiation exposure decreases with the square of the distance from the source, brachytherapy is a highly conformal therapy. Therefore, this approach may be particularly useful in com bination with radiosensitizing drugs because the entrance and exit dose (to normal tissues) associated with external beam radiation therapy is eliminated. Brachytherapy has been commonly used to treat prostate, breast and gynaecological cancers. Recent changes in source availability and the implementation ol remote afterloaders have resulted in the introduction of high-dose-rate brachytherapy into routine clinical practice at many institutions14. Unlike traditional low- to medium -dose-rate brachytherapy, which is delivered via perm anent implants, high-dose-rate systems utilize sources with dose rates that are similar to linear accelerators. As a result, the sources are implanted temporarily and the doses are often fractionated. New approaches to deliver radiation selectively to tumours via isotope-conjugated antibodies or nanoparticles are under development.
Advances in all aspects of radiation oncology are making it more feasible to com bine radiation treatment with targeted drugs. Limiting toxicity to normal tissues starts with treatment planning and optimized dose distributions15. Imaging tumours before, during and after radiation delivery makes it possible to make adjustments to account for changes in tum our position, size and shape16. Precise patient immobilization and localization on the treatment couch, as well as on-board X-ray, ultrasound and infrared imaging to ensure patient positioning, limit treatment errors and allow radiation to be delivered with smaller margins17, As the doses delivered to normal tissues are limited, radiation oncologists can focus more on sensitizing tumours than on sparing normal tissues.
History of radiation modulators
The potential for concurrent therapies to enhance the local control of tum our recurrance has long been recognized and numerous approaches have been attempted. Initial experiments in the 1960s and prospective trials in the 1970s investigated sequential and concurrent chemotherapy and radiation with the hypothesis that chem otherapy-mediated inhibition of DNA repair mechanisms would lead to synergistic effects18. Since then, continued experiments have demonstrated that drugs such as cisplatin, 5-fluorouracil and mitomycin C radiosensitize tumours including those from head and neck cancers, lung cancers and gastrointestinal cancers19. Although these concurrent chemotherapies increase the rates of local control and, in some cases, overall survival, because they are not specific for tumour cells they also increase radiation toxicity to normal tissues20. Improved radiosensitizers will therefore need to selectively increase tum our cell killing and local control while minimally affecting normal tissues.
Hypoxia: an example of selective tumour targeting
Historically, tumour hypoxia has been one o f the most frequently targeted characteristics of tumours to improve the efficacy of radiation. Unlike most normal tissues, tumours experience considerable hypoxia owing to fluctuations in blood flow and increased metabolicdemand21. Low oxygen availability decreases the efficacy of radiation and adversely affects the prognosis of patients with cancer22,23. Hypoxic sensitizers have long been recognized as potential radiosensitizers24, and numerous attempts have been made to decrease the negative effect of hypoxia on the outcomes of radiation therapy.
Generally, hypoxic drugs can be divided into three categories: drugs that increase delivery of oxygen to tumours; hypoxic cell radiosensitizers; and direct hypoxic cell cytotoxins25. Approaches to increase oxygen delivery to tumours include breathing oxygen under nonnobaric and hyperbaric pressure, blood transfusions, nicotinamide administration and the use of erythropoietin. Although there is some evidence that these approaches may improve local control, additional studies are needed to justify the implementation of cumbersome treatment techniques — such as hyperbaric oxygen administration — into clinical practice26. Notably, combining erythropoietin treatment with radiation therapy for head and neck cancers led to significantly worse outcomes in patients, presumably because the erythropoietin receptor is also expressed on squamous cell carcinoma tumour cells27,28. Therefore, in addition to promoting the production of red blood cells, erythropoietin may cause proliferation of head and neck cancer cells and/or protect them from cell death.
The nitroimidazoles are a family of electron-affinic drugs that mimic the effect of oxygen by reacting with DNA free radicals, and this sensitizes hypoxic cells to radiation. Nimorazole has been shown to improve the effect of radiation therapy on supraglottic and pharynx tumours in a Danish head and neck cancer study29 and is commonly used in clinics in Denmark. However, another nitroimidazole — etanidazole — was tested in a Radiation Therapy Oncology Group (RTOG) trial but it did not significantly improve outcomes30. As a result, the use of nitroimidazoles is not common in clinical practice in the United States.
A theoretical study suggested that hypoxic cell cytotoxins might be the best way to target hypoxic cells during radiation therapy31. Tirapazamine showed promise in early clinical trials, but no therapeutic benefit was observed in a recent Phase III clinical trial of unselected patients with head and neck cancers32. The idea that tirapazamine may be more effective in patients with hypoxic tumours led to attempts to retrospectively identify biomarkers of patients with hypoxic tumours. A subgroup of patients with elevated plasma levels of hepatocyte growth factor (HGF) and interleukin-8 (IL-8) seemed to benefit more from tirapazamine33, but prospective studies will be required to determine whether plasma biomarkers or functional imaging34 can be used to identify patients with hypoxic tumours that may benefit from hypoxic cell cytotoxins.
Numerous clinical trials have been completed to evaluate the effect of hypoxia modification on treatment outcome. Many of these trials have been inconclusive because they were small and underpowered. In a systematic review incorporating all of these trials, modification of hypoxia was shown to significantly improve locoregional control and overall survival without affecting radiation-related complications25. Therefore, further work to identify biomarkers of hypoxia will be important to enrich for patients who may benefit from hypoxia modification in future clinical trials of radiation therapy.
Amifostine: a clinical success story
Although numerous drugs that modify radiation response have been developed and clinically tested, amifostine is one of the few modifiers of radiation response that has received approval from the US Food and Drug Administration. This thiol drug acts as a free radical scavenger and radio-protector35. Amifostine concentrates more rapidly in normal tissues than in tumours36, and is not taken up by cells until it is dephosphorylated by alkaline phosphatase37. As a result, amifostine preferentially protects normal tissues — but not tumours — from radiation damage38.
Amifostine has been tested in Phase III clinical trials for head and neck cancer, non-small-cell lung cancer and pelvic malignancies39–41. Numerous randomized controlled studies have suggested that amifostine may protect against radiation-induced toxicity in patients with head and neck cancer35; this has led the American Society of Clinical Oncology to recommend that amifostine be considered for the prevention of xerostomia during fractionated radiotherapy. However, amifostine has not been recommended for the prevention of mucositis during concurrent platinum-based chemoradiotherapy for head and neck cancer42. As concurrent chemoradiotherapy is the standard of treatment for this type of cancer, this has limited the clinical application of amifostine as a radioprotector. However, amifostine has also been shown to decrease the incidence of nephrotoxicity43, ototoxicity44 and neurotoxicity45 in patients receiving cisplatin-based chemotherapy regimens in the absence of radiation therapy.
Limitations of current radiation modulators
To date, one of the major factors limiting the implementation of radiosensitizers and radioprotectors in clinical care has been the nonspecific mechanism of action of many radiation modulators. Drugs that affect radiosensitivity by targeting key cell survival pathways are likely to affect both tumours and normal tissues. Thus, selectivity is an important goal for designing improved radiation modulators. As our knowledge of the genes that are altered in cancer increases and as we gain further insight into the signalling pathways that mediate the DNA damage response after radiation in tumour and normal tissues, it may become more feasible to select drug targets that regulate radiosensitivity specifically in tumour cells or normal cells and tissues. However, the response of different cell types to radiation varies46. Therefore, a target of radiosensitivity that is identified in a certain tumour type may not translate to all clinical situations. Tumours arising in different tissues show varied responses to radiation47,48, and this should be considered when testing radiation modulators in the clinic.
Most radiation modulators are initially developed in preclinical models. However, not all preclinical models recapitulate human disease to the same extent49. It is important to account for the limitations of each preclinical model when therapies are translated from cells to animals and ultimately to patients. For example, the tumour microenvironment has been shown to contribute to tumour initiation, progression and response to therapy50. The tumour microenvironment can also affect the response of tumours to radiation51, and the immune system may be important for clearing tumours following radiation therapy52–54. Thus, cell culture models, xenografts established in immunocompromised mice and even syngeneic implantation of tumours may not accurately capture all of the important components of human tumours that regulate the response to radiation.
A solution may be to test radiation modulators in genetically engineered mouse models (GEMMs), in which primary cancers develop in the native tumour microenvironment in immunocompetent mice55. Numerous studies have suggested that GEMMs may more faithfully recapitulate the tumour stroma and microenvironment of human cancer than xenograft models56–58. Importantly, GEMMs have been shown to closely model the response of human cancers to systemic therapy in clinical trials59–60. However, even with GEMMs the limitations of testing drugs on mouse tumours must be taken into account. The pharmacology of drugs and the role of therapeutic targets may not be conserved across species. Nevertheless, as primary tumour models improve, it is likely that they will improve the translation of promising preclinical data on radiation modulators into successful clinical trials.
New insights into tumour biology
Tumour heterogeneity and cancer stem cells
Recent discoveries in cancer biology have suggested new mechanisms by which tumours recur after radiation therapy. Tumour heterogeneity has long been recognized as a mechanism by which tumours evade radiation therapy61. Heterogeneity arising from differences in the type of cancer (that is, sarcoma, carcinoma or glioma, and so on) can alter the response ol individual tumours to radiation therapy47,48. Even among tumours of the same histological subtype, the radiation response can vary substantially from patient to patient. Such differences between patients may be due to the mutations within each tumour as the tumour genotype may influence the response to radiation therapy and the effectiveness of radiosensitizers62–64. This observation should be taken into consideration when radiation modulators are tested in heterogeneous patient and tumour populations. Even at the individual tumour level, heterogeneity can be quite extensive. Single-cell sequencing has demonstrated that a given tumour contains many distinct clones with diverse mutations65. Each of these mutations has the potential to modify responsiveness to radiation, so it is possible that multiple radiation modulators may be necessary in order to sensitize an entire tumour to radiation therapy.
Within a tumour, individual cells have varying abilities to contribute to tumour regrowth following radiation therapy. Indeed, lineage-tracing experiments suggest that a small subset of tumour cells drives tumour growth and regrowth following therapy66–68. These tumour-propagating cells, or cancer stem cells, which have been identified in several different types of tumours, seem to be maintained in a stem-cell-like state by their niche within tumours69. Although these cells must be eliminated for a cancer to be cured, accumulating evidence suggests that cancer stem cells may be inherently radio-resistant70–72. Like haematopoietic stem cells73, cancer stem cells may reside in hypoxic niches. Moreover, these cells are generally quiescent and may show increased activation of the DNA damage response74. However, by understanding the unique properties of these cells, it may be possible to design drugs that sensitize cancer stem cells to radiation therapy. For example, drugs that disrupt the stem cell niche or force these cells out of quiescence and into the cell cycle have the potential to act as radiosensitizers75.
Synthetic lethality
Targeting the pathways that are required for cellular survivalis likely to be toxic to normal tissues. However, many important pathways — including the DNA damage response pathway — exhibit redundancy in normal cells. Growing evidence suggests that many tumours have mutations that cause the loss of a signalling pathway, which may remove this redundancy. As a result, targeting the remaining pathway in the tumour cell can induce synthetic lethality (fig.1). Because the normal cells retain the redundant signalling pathway, this approach can provide a therapeutic window to kill cancer cells while sparing normal tissues76. As DNA is the main target for radiation-induced cell killing77, and there is considerable redundancy in the ability of cells to repair DNA damage78, targeting DNA damage response pathways is a promising approach for the selective radiosensitization of tumour cells79.
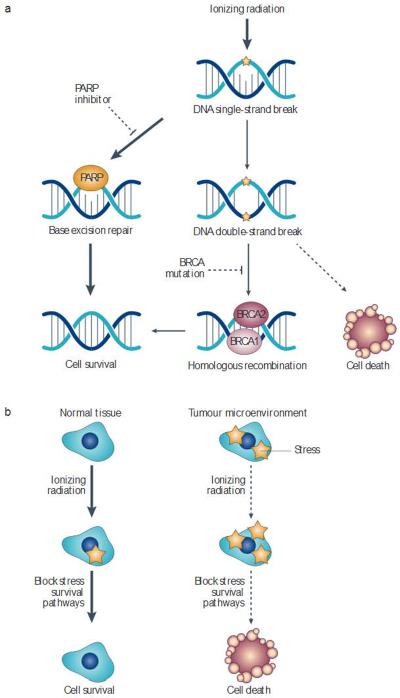
Figure 1. Selectively targeting tumour cells through synthetic lethality.
a | Ionizing radiation causes single-strand and double-strand DNA breaks. Some single-strand DNA breaks can be repaired by base excision repair. Poly(ADP-ribose) polymerase (PARP) inhibitors block base excision repair, causing some single-strend breaks to become double-strand breaks. In normal cells, BRCA (breast cancer susceptibility)-mediated homologous recombination can repair these breaks However, cancer cells with mutations in BRCA1 or BRCA2 are unable to repair this damage, allowing PARP inhibitors to radiosensitize these cellsvia synthetic lethality. b | Similarly synthetic lethality could potentially be used to sensitize cellsin the harsh tumour microenvironment Drugs that block stress survivel signalling pathways should preferentially kill tumour cells that are exposed to stresses such as hypoxia, oxidative stress or cell-cell contact abnormalities (right side) but not normal cells, which exist in a less harsh microenvironment (left side). These drugs have the potential to kill tumour cells in the absence of radiation, but radiation provides an additional stress that could enhance the probability of tumour eradication.
The application of poly(ADP-ribose) polymerase (PARP) inhibitors to tumours with BRCA1 (breast cancer susceptibility 1) and BRCA2 mutations demonstrates the potential of synthetic lethality in selectively targeting cancer cells. PARP1 has an important role in base excision repair of single-strand DNA breaks80. PARP inhibition may increase the number of endogenous and radiation-induced double-strand DNA breaks owing to the impaired repair of single-strand breaks81. In normal cells, these double-strand breaks can be repaired via homologous recombination and non-homologous end joining. However, many familial breast cancers are due to mutations in the BRCA1 or BRCA2 genes, which are required for the efficient repair of double-strand DNA breaks by homologous recombination82. Therefore, many BRCA1- and BRCA2-mutant breast cancers are profoundly sensitive to PARP inhibition83–85, which appears to be due to defective homologous recombination specifically in the tumour cells86. As a result, PARP inhibition may also be useful in tumours with other mutations associated with homologous recombination87,88. There is evidence that other mutations in the DNA damage response pathways can also sensitize cells to synthetic lethality. For example, cells with a mutated Fanconi anaemia pathway have increased sensitivity to ataxia telangiectasia mutated (ATM) inhibition89.
As the harsh tumour microenvironment can present unique challenges for cellular survival, drugs could also trigger synthetic lethality by targeting the pathways used by cells to withstand the stresses found in the tumour microenvironment, such as hypoxia, oxidative stress or cell–cell contact abnormalities. Tumour cells exposed to these environmental stresses, but not normal cells (which exist in a less harsh microenvironment), should be preferentially killed by inhibitors that block stress survival signalling pathways. These examples demonstrate the feasibility of targeting the loss of pathway redundancy to induce synthetic lethality and selectively target cancer cells.
Promising radiosensitization targets
Numerous approaches to radiosensitize tumours have shown promise using in vitro assays. Before these therapies can move into the clinic, the safety and efficacy of these drugs needs to be shown using relevant in vivo models. Targets for radiosensitization can be grouped into two broad categories: targets within the tumour cell, such as phosphoinositide 3-kinase (PI3K)-like kinases (PI3KKs) and survival pathways; and targets within the tumour microenvironment.
PI3K-like kinases
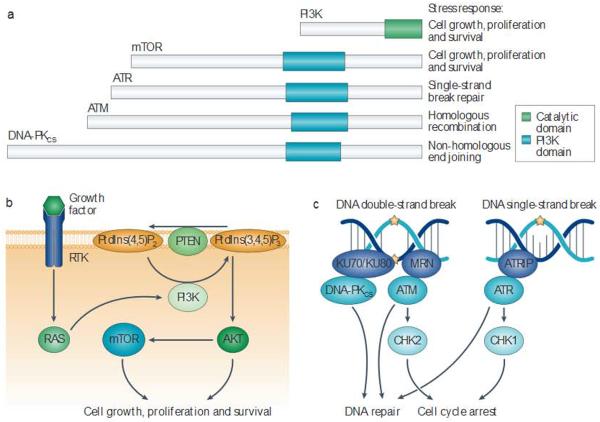
The PI3KK family contains several serine/threonine kinases with highly conserved catalytic domains that have homology to PI3K90. This includes mammalian target of rapamycin (mTOR), DNA-dependent protein kinase catalytic subunit (DNA-PKCS), ATM and ATR (ataxia telangiectasia and RAD3 related) (fig. 2). Following radiation, these proteins have crucial roles in responding to DNA damage, orchestrating DNA repair and promoting cellular survival. Selective inhibition of ATM, the gene that is mutated in the radiation hypersensitivity syndrome ataxia telangiectasia, has been shown to sensitize human tumour cell lines to radiation in vitro91,92, Importantly, ATM loss sensitizes both p53-null and wild-type tissues93, and cancer stem cells may be particularly sensitive to ATM inhibition94. Small-molecule inhibitors that are designed to target one member of the PI3KK family may also inhibit other family members, thereby leading to synergistic radiosensitization (fig. 2). Although inhibition of some PI3KK family members in normal tissues can be tolerated, inhibition of all the Pl3KKs may cause toxicity in normal cells. For example, cells lacking ATR are generally non-viable95,96 because ATR is critical for resolving stalled DNA replication forks to allow efficient progression through the S phase of the cell cycle97. However, cells that are under oncogene-induced replicative stress may be more sensitive to ATR inhibition than normal cells98. It was shown that a small-molecule ATR inhibitor in combination with radiotherapy prolonged the growth delay of pancreatic cancer xenografts compared to radiation therapy alone, without causing toxicity to normal tissues99. Nevertheless, to minimize toxicity to normal tissues, it will probably be important to design small molecules that inhibit some — but not all — members ol the PI3KK family.
Figure 2. Inhibiting P13 Kand the P13 K-like protein kinase family.
a | The phosphoinositide 3-kinase (H3K)-like protein kinase family includes several cellular kinases with catalytic domains that are homologous to PI3K(these are known as PI3K domains) This protein family coordinates diverse cellular responses that are crucial for the response to ionizing radiation. b | PI3K and mammalian target of rapamyc in (mTOR) are activated by numerous growth factors to stimulate cellular growth, survival and proliferation. Growth factors bind to receptor tyrosine kinases. Leading to downstream activation of PI3K by the RAS family of small GTFases. Active PI3K phosphorylates phosphatidylinositol-4,5-bisphophate (Ptdlns(4,5)P2) in the cellular membrane, converting it to the second messenger phosphatidylinositol-3,4,5-trisphophate (Ptdlns(3,4,5)P3). Ptdlns(3,4,5)P3 can activate the protein kinase AKT, leading to increased cell growth, proliferation and survivalby activating several proteins including mTOR. c | DNA-dependent protein kinase catalytic subunit (DNA-PKCCS), ataxia telangiectasia mutated (ATM) and ATR (ataxia telangiectasia and RAD3-related) respond to DNA damage by activating cell cycle arrest and engaging distinct DNA repair programmes. Because the catalytic domain is conserved across these diverse kinases, it is possible that a single drug could target several family members, thereby inhibiting some or all of these DNA repair and cell survival pathways. KU70 and/or KU80, the MRN complex(composed of MREll, RAD50 and Nijmegen breakage syndrome protein 1 (NBSl)) and ATR-interacting protein (ATRIP)sense DNA damage and promote the activation of DNA-PKCS, ATM and ATR. respectively. These kinases then phosphorylate several target sinside the cell, including checkpoint kinase | (CHK1) and CHK2.PTEN, phosphat ase and tensin homolog.
NVP-BEZ235 was designed as a combined PI3K–mTOR inhibitor and demonstrated potent anticancer activity as a monotherapeutic agent100. The drug is currently in Phase II clinical trials for advanced solid tumours and has shown efficacy as a radiosensitizer in preclinical models101. Recent studies have demonstrated that its radiosensitizing effect is partially mediated via inhibition of the DNA damage response proteins ATM and DNA-PKCS (ref. 102). However, there is evidence that PI3K activation alone can cause resistance to radiation103. Delivery of NVP-BEZ235 before irradiation may provide additional benefit owing to normalization of the tumour vasculature104. However, NVP-REZ235 may be a nonspecific inhibitor of the PI3KK family because it has also been shown to inhibit ATR98; this may limit its utility because ATR inhibition is associated with toxicity.
Survival pathways
Several survival pathways have been shown to be activated following ionizing radiation, including PI3K, mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB) and transforming growth factor-β (TGFβ) pathways105,106. In addition to regulating cell death, many of these pathways can affect the tumour microenvironment, and activate DNA damage repair104,107–109. As a result, inhibition of these pathways has the potential to increase the radiosensitivity of cancer cells through multiple mechanisms. However, inhibition of cellular survival could affect the radiosensitivity of normal tissues as well, thus decreasing the therapeutic index of radiation.
The PI3K–AKT pathway is activated by various mutations that are commonly found in cancer, including receptor tyrosine kinase activations, activating mutations in PI3K, loss of the tumour suppressor phosphatase and tensin homolog (PTEN) and activating RAS mutations110. Importantly, activation of the P13K–AKT pathway contributes to resistance to radiation111,112. As a result, selective inhibitors of this pathway increase the radiosensitivity of cancer cells, and many drugs targeting this pathway are currently in preclinical development or in clinical trials113. The specific activating mutation in tumours needs to be taken into account when targeting the PI3K–AKT signalling pathway, as drugs that block signalling upstream of the mutant protein may not be effective.
For example, epidermal growth factor receptor (EGFR) is a tyrosine kinase that signals upstream of RAS and PI3K. EGFR is commonly overexpressed in squamous cell carcinomas of the head and neck. The EGFR-specitic antibody cetuximab (Erbitux; Bristol-Myers Squibb/Lilly) significantly improved local regional control and overall survival by radiotherapy in a Phase III clinical trial of patients with head and neck eancers114,115 However, the inclusion of EGFR inhibitors in chemotherapy for patients with KRAS-mutant non-small-cell lung cancers actually led to worse clinical outcomes in comparison with patients who were treated with chemotherapy alone116. Similarly, patients with colon cancers only respond favourably to cetuximab if their tumours do not contain KRAS mutations117.
The intracellular kinases extracellular signal-regulated kinase 1 (ERK1) and ERK2 are activated downstream of RAS signalling following the exposure of cells to radiation118. As ERK signalling can promote growth and survival, several studies have attempted to inhibit ERK to enhance radiation-induced cell killing. Interestingly, the effect of inhibiting ERK1 or EREC2 signalling depends on thecell type and timingofinhibition105. Although it has the potential to radiosensitize tumour cells, some cells are not affected by ERK inhibition and others actually seem to be protected from radiation following ERK inhibition.
As many tumours develop resistance to apoptosis during cancer progression, programmed cell death has been shown to have only a modest role in the treatment response of most solid tumours to radiation therapy119. However, antagonism of anti-apoptotic proteins may reactivate cellular apoptosis m achinery and sensitize tumours to radiation-induced apoptosis120. As with PI3KK inhibitors, combined inhibition of several anti-apoptotic proteins may be more effective at radiosensitizing tumour cells. ART-737, a combined inhibitor of B cell lymphoma 2 (BCL-2), BCL-2-like protein 1 (BCL-2L1) and BCL2-L2, synergized with radiation to kill cancer cells in xenograft tum ours121. Phase I studies of ABT-263, an analogue of ABT-737 with improved oral availability, demonstrated safety and encouraging efficacy in small-cell lung cancer and lymphoid malignancies122–124.
Another anti-apoptotic protein, myeloid cell leukaemia sequence 1 protein (MCL1), has been shown to block radiation-induced apoptosis in some cell types and may be more important than BCL-2 for sensitizing haematopoietic tumours, as many haematopoietic cells require MCL1 for survival125–126. Antisense nucleotides targeting MCL1 synergize with radiation to increase cell death127, and the combination of MCL1 inhibition together with ABT-737 potentiates tum our cell a poptosis in vtro128. In addition, AT-101, a BH3 mimetic that targets MCL1 and other BCL-2 proteins, radiosensitized small-cell lung cancer cell lines in vitro129 and enhanced growth delay after radiation therapy in a xenograft model of prostate cancer130. Similarly, the pan-BCL-2 inhibitor obatoclax increased radiation-induced apoptosis in breast cancer cell lines with elevated expression of BCL-2 family mRNA131.
Approximately half of all human cancers have alterations in the TP53 gene, resulting in loss or inactivation of tumour suppressor p53 protein112. In tumours expressing wiki-type TP53, p53 function is generally inhibited through other mutations in the p53 pathway. HDM2 (known as MDM2 in mice) is an E3 ubiquitin ligase that binds directly to p53, blocking its transcriptional activity and leading to its degradation153. As HDM2 tightly regulates p53 activity, small-molecule HDM2 inhibitors have been designed to reactivate the p53 pathway in cancer cells expressing wild-type p53 (ref. 134). This approach could be used to reactivate p53-mediated cell death pathways, which can be triggered by radiation.
Checkpoint inhibitors
The activation of cell cycle checkpoints is an important consequence of the DNA damage response because cell cycle arrest may prevent mitosis with double-strand breaks, which can lead to the loss of critical chromosomal material in a process termed mitotic catastrophe135. In vitro, abrogation of a single cell cycle checkpoint does not alter cellular radiosensitivity — as measured by clonogenic survival — after radiation. For example, the radio sensitivity of BRCA1-deficient cancer cells, which are incapable of triggering an S phase checkpoint, can be rescued with a mutant BRCA1 that is incapable of triggering the S phase checkpoint136. This suggests that BRCA1-mediated radiosensitivity is not due to loss of the S phase checkpoint. Complementary experiments have dem onstrated that cells that have mutant BRCA1 and are incapable of triggering the early G2/M checkpoint have the same radiosensitivity as wild-type cells, as measured by clonogenic survival137. These experiments suggest that loss of BRCA does not increase radiosensitivity through loss of cell cycle checkpoints but instead through other mechanisms such as impaired DNA damage repair.
The cyclin-dependent kinase inhibitor p21 (also known as CDKNlA) is required for the radiation-induced G1 cell cycle checkpoint and, at least in some cells, also for a sustained G2 checkpoint138. Interestingly, loss of p21 does not affect the clonogenic survival of cells irradiated in vitro. However, when these same cells are grown as xenografts, tumours lacking p21 are sensitized to radiation therapy in in vivo139,140. Similarly, the deletion of p21 sensitizes mice to radiation injury of some normal tissues. For example, mice lacking p21 are sensitized to the radiation-induced gastrointestinal syndrome141–145 and to radiation-induced cardiac, injury144. Taken together, these studies underscore the importance of studying radiation responses using both in vitro and in vivo systems.
WEE1 is a protein kinase that prevents cells from entering mitosis following radiation by phosphorylating cyclin-dependent kinase 1 (CDK1)145. Several studies have shown that inhibiting WEE1 increases mitotic catastrophe following radiation and can sensitize tumour cells to radiation in vitro and in vivo146–148. However, these inhibitors also target other kinases, which could affect cellular radiosensitivity149.
Simultaneously targeting several cell cycle checkpoints may increase radiosensitivity. For example, it is possible that combined loss of the S and G2 checkpoints may cause premature mitotic entry150. The dual checkpoint kinase 1 (CHK1) and CHK2 inhibitor AZD7762 has been shown to radiosensitize human cancer cells and xenografts151. However, this may be due to its direct effects on homologous recombination152. In addition, inhibitors ot ATM and downstream targets such as cell division cycle 25 (CDC25) may potentiate radiosensitivity in part by targeting cell cycle checkpoints153.
Tumour microenvironment
Most attempts to sensitize tumours by manipulating the tumour microenvironment have focused on the tumour vasculature, as well as on the immune system (fig. 3). The vasculature is essential for tumour growth, and numerous groups have demonstrated that it is structurally and functionally abnormal in tumours154. Despite extensive evidence regarding the contribution of the vasculature to tumour development, the amount of vascular disruption following radiation therapy and the contribution of vascular collapse to tumour cure by radiation therapy remains controversial155.
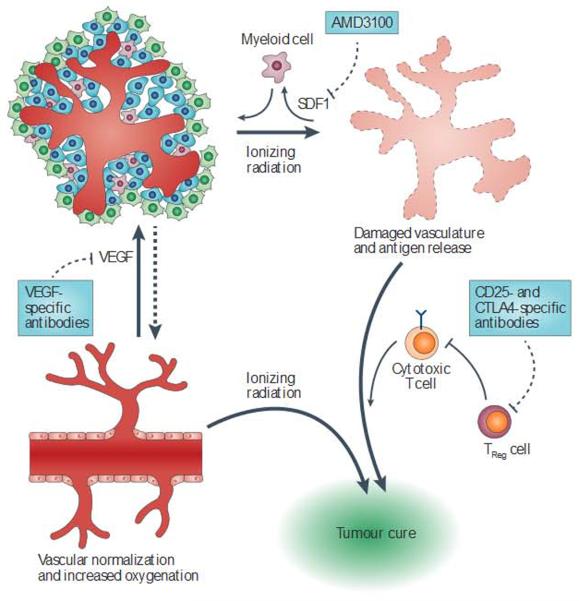
Figure 3. Enhancing tumour cure by modulating the tumour microenvironment.
The tumour microenvironment can regulate the response of tumours to radiation by altering tumour oxygenation and vascular rebuilding, and by regulating the clearance of surviving cells by the immune system. Targeted drugs can alter the interaction between tumour cells and their microenvironment, which may enhance tumour cell killing and increase the probability of a cure. Anti-angiogenic therapies such as blockade of vascular endothelial growth factor (VEGF) can cause vascular normalization, leading to a window of increased tumour oxygenation. Myeloid cells are recruited to inradiated tumours by cytokines suchasstromal cell-derived factor 1 (SDF1). Blocking their recruitment may impair vascular regrowth. Irradiated tumours release antigens that can be recognized by the immune system, leading to the destruction of tumour cells by cytotoxic T cells. Regulatory T(TReg) cells can abrogate this response,so targeting these cells with CD25- and cytotoxic T lymphocyte antigen 4 (CTLA4)-specific antibodies may enhance tumour cure by radiation.
Detailed experiments using syngeneic tumours have suggested that microvascular collapse owing to endothelial cell apoptosis may contribute to the response of tumours to radiation therapy156–158. Unlike typical radiation-induced cell death, which is dependent on DNA damage, endothelial cell apoptosis has been reported to be triggered by membrane damage, which leads to ceramide-mediated apoptosis159. Interestingly, endothelial cells may be particularly sensitive at higher doses160, so this mechanism ol endothelial cell death may be most pertinent to hypofractionaled regimens in stereotactic body radiation therapy. Although other groups have suggested that endothelial cells do not contribute to tumour cure162–163, drugs that trigger endothelial cell apoptosis could potentially increase the radiosensitivity of tumour tissues.
Regardless of the amount ol vascular damage caused by radiation, vascular function must be re-established for a tumour to regrow following radiation therapy164. Endothelial cells may be established through the local sprouting of existing blood vessels or by the recruitment of vascular progenitor cells from outside the irradiated field165. Although several studies have reported that endothelial progenitor cells from the bone marrow contribute to the formation of the tumour vasculature166–168, other studies have emphasized that bone marrow-derived cells do not contribute to the formation of the vascular endothelium169. Similarly, the contribution of each of these mechanisms following radiation remains controversial170, with no studies definitively demonstrating that vascular progenitor cells contribute to vessel formation after radiation, and one study emphasizing that bone marrow-derived cells do not contribute to revascularization following radiation171.
Several studies have suggested that myeloid cell recruitment after radiation contributes to vascular regrowth171–173. The recruitment of myeloid cells appears to be dependent on stromal cell-derived factor 1 (SDF1; also known as CXCL12)-CXC-chemokine receptor 4 (CXCR4) signalling in many tumours171,172,174, and inhibition of this signalling axis with AMD3100 represents a promising therapeutic target post-irradiation175. Notch signalling also regulates tumour angiogenesis, and an antibody against a Notch ligand — delta-like ligand 4 (DLL4) — impaired tumour regrowth by promoting non-functional tumour angiogenesis176.
Vascular normalization via anti-VEGF (vascular endothelial growth factor) therapies has long been championed as a mechanism for enhancing the response of tumours to radiation154. Preclinical experiments have demonstrated that anti-VEGF therapies can enhance the response of tumours to radiation in animal models177. Notably, there may be a tumour oxygenation window after anti-angiogenesis therapy, during which radiation therapy will be most effective178. However, clinical studies have not compared different treatment schedules, which may be important for therapies that normalize the vasculature to have maximum efficacy179.
Anti-VEGF therapies have measurable effects on human tumours180 and are currently being investigated in combination with radiation therapy in clinical trials. A Phase II trial of bevacizumab, radiation therapy and fluorouracil in rectal cancer demonstrated promising efficacy181. More recently, a Phase II study combining bevacizumab with radiation therapy and temozolomide for newly diagnosed glioblastoma demonstrated improved progression-free survival. However, overall survival was not improved182. Of note, small molecules can be designed to target both VEGF signalling and cell survival pathways. For example, vandetanib is a dual VEGF receptor 2 (VEGFR2) and EGFR inhibitor that is currently in Phase II clinical trials for glioblastoma183.
As described above, TP53 encodes a transcription factor — p53 — that responds to ionizing radiation by initiating a spectrum of cell-type specific responses, including cell cycle arrest, senescence, apoptosis and DNA damage repair184. As a result of these functions, p53 is a key molecule for determining cellular responses to ionizing radiation185. The function of p53 is complex and tissue-dependent186,187 (fig. 4). Although p53 protects the gut and cardiac endothelial cells from radiation141,142,144, it can trigger apoptosis in haematopoietic and lymphoid tissues188,189. As TP53 is one of the most commonly mutated genes in cancer190, it may be possible to inhibit p53 in tumour stromal cells without affecting the radiation response of tumour parenchymal cells, which already express mutant p53. For example, one study reported that p53 inhibition in the tumour stroma increases the sensitivity of transplanted syngeneic tumours to radiation, which is probably due to the increased sensitivity of endothelial cells to radiation191.
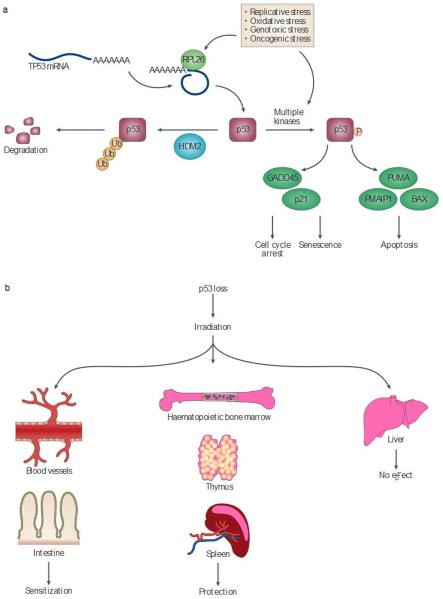
Figure 4. The tissue-dependent role of p53 in the response of cells to radiation.
a | In unstressed cells, HDM2 ubiquitylates the tumour suppressor p53, leading to its degradation. Numerous cellular stresses increase the translation of TP53 mRNA and the phosphorylation of p53 protein, increasing its stability. p53 acts predominantly as a transcription factor that upregulates the expression of target genes to arrest cell growth or lead to cellular senescence and apoptosis, depending on the cellular context. Ribosomal protein |26 (RPL26) binds to TP53 mRNA and increases its translation. The p53 transcriptional targets GADD45 (growth arrest and DNA-damage-inducible protein 45) and p21 lead to cell cycle arrest, whereas BCL-2-associated X protein (BAX) and the BH3-only proteins PUMA (p53-upregulated modulator of apoptosis) and PMAIP1 (PMA-induced protein 1) can trigger apoptosis. b | The consequence Of p53 loss on the cellular radiation response varies depending on the type of tissue As a result, p53 inhibitors may be useful as both radiosensitizers and radioprotectors.
Immunomodulation is an emerging approach for increasing the probability of tumour cure following radiation therapy192. Accumulating evidence suggests that the immune system is capable of recognizing tumours, impacting on cancer development193–195. As tumours develop in immunocompetent animals, they evolve to avoid cell death from immunosurveillance196. However, radiation treatment may present a unique opportunity to prime the immune system against tumours. There have been anecdotal reports that local irradiation of a tumour can reduce tumour growth outside the field of radiation. This abscopal effect is rare but it has been reported for many malignancies197–201. The immune system seems to be the main driver of this effect, as the radiation of primary tumours in combination with a growth factor that stimulates dendritic cell production has been shown to result in the regression of non-irradiated tumours202. Importantly, this effect was shown to be dependent on T cells.
Immunological mechanisms can also contribute to the regression of primary tumours after radiation therapy. It is well established that radiation increases the release of pro-inflammatory cytokines203–205 and tumour-associated antigens206,207. These immune cues may trigger the immune system to eliminate surviving cancer cells following radiation therapy. Indeed, it has been demonstrated that tumour-antigen-specific immune responses develop during radiation treatment208. In addition, CD8 T cell depletion largely diminishes the therapeutic effect of radiation in mouse tumour models54. Notably, fractionated radiotherapy was less effective than hypofractionated radiotherapy in mice with T cells, but not in mice that were deficient in recombination activating gene (Rag) — Rag-knockout mice; this suggests that stereotactic body radiation therapy may be more effective at stimulating immune responses than standard fractitonated radiotherapy.
Many tumours have been shown to recruit regulatory T cells to inhibit the development of pro-inflammatory tumour immune responses209. This subset of T cells may be an important therapeutic target because CD25- and cytotoxic lymphocyte antigen 4 (CTLA4)-specific antibodies, which target regulatory T cells, enhance the efficacy of radiation therapy210,211. Therefore, combining immunomodulators with radiation treatment that is optimized to prime the immune system represents a promising approach to improve local control after radiation therapy and potentially affect the development of cancer at distant sites.
Radiation protectors and mitigators
Radiation causes both acute toxicity (occurring within days to weeks after radiation) and late toxicity (occurring months to years after exposure) to normal tissues, which often limits the dose of radiation that can be delivered to tumours. Thus, the use of radiation protectors to selectively protect normal tissues (fig. 5) is a complementary approach to sensitizing tumours to radiation, allowing more cancers to be cured by exposing them to higher doses of radiation. In addition to the clinical setting of treating patients with cancer, radioprotectors could be useful as countermeasures against radiological attacks and in other pathophysiological settings to protect against damage to normal tissues. For example, during reperfusion injury after ischaemia, reactive oxygen species (ROS) are generated that damage cells and activate cell death pathways via mechanisms that are similar to ionizing radiation212. Thus, radioprotectors may prove to be useful in protecting normal tissues from insults other than radiation therapy.
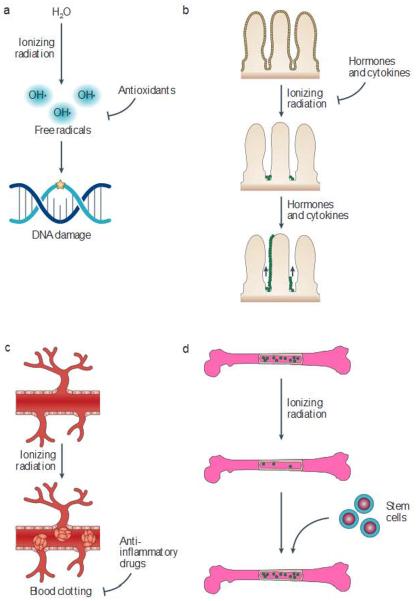
Figure 5. Strategies to protect and mitigate normal tissues from radiation damage.
a |Antioxidants can scavenge free radicals that damage DNA b | Hormones and cytokines can be administered before or shortly after radiation to enhance cellular survival and facilitate tissue repopulation. c | Anti-inflammatory drugs can be used to reduce autoinflammatory responses and vascular damage that results in late radiation effects, d | Stem cell scan be used to reconstitute tissues that have been damaged by radiation. Amifostine, an antioxidant, is approved by the US Food and Drug Administration for use in combination with radiation therapy, and paliformin (a recombinant form of keratinocyte growth factor) has been approved for preventing mucositis from total-body irradiation. Several other hormones, cytokines and anti-inflammatory drugs have shown promise as mitigators of radiation injury in animal models. Although bone marrow transplantation has been established as a mitigator of the haematopoietic syndrome, substantial work will be necessary to enable the use of stem cells to regenerate solid tissues following exposure to radiation.
When describing radiation countermeasures, a US National Cancer Institute Workshop recom mended distinguishing among radiation protectors, radiation mitigators and treatments for radiation injury213. These terms are defined by the timing of drug administration in relation to the exposure to radiation and the development of symptoms from radiation injury. Protectors are given before exposure to radiation, mitigators are given during or after exposure (but before the appearance of symptoms) and treatments are administered after symptoms from radiation injury develop. Although all of these drug classes would potentially be useful for treating patients in the clinic, the US government is actively seeking mitigators — which are effective when administered more than 24 hours after exposure to radiation — for the national stockpile for use in the event of a nuclear or radiological attack. Cellular responses to radiation begin almost instantaneously after exposure to radiation214, and radiosensitive organs such as the bone marrow and the gastrointestinal tract undergo a significant amount of cell death within the first 24 hours. Therefore, in order for mitigators to be effective when administered after this time point, they will probably need to promote the regeneration of surviving cells or prevent the life-threatening sequelae of cell loss after the first 24 hours.
The ability of stem cells and oligopotent progenitors to maintain tissue function by replacing damaged cells determines the onset and severity of the effects of radiation215, so strategies to mitigate normal tissue injury must increase the survival and functionality of these cells. Increasing evidence suggests that stem and progenitor cell populations respond differently to DNA damage than more differentiated cells216. Thus, mitigation strategies that alter the homeostasis of tissue-specific stem cells and progenitor cells could enhance their fitness and regenerative capacity.
Antioxidants
In addition to amifostine, several other antioxidants have been used to protect cells and tissues from radiation injury. The nitroxides are recycling anti-oxidants that protect cells exposed to oxidative stress217. A Phase I clinical trial in patients undergoing whole-brain radiotherapy suggested that one nitroxide, tempo, may be effective at preventing radiation-induced alopecia218. Antioxidant vitamins such as α-tocopherol and β-carotene have also been suggested to be effective at reducing xerostomia, mucositis, pulmonary fibrosis, cystitis and alopecia during radiation therapy219. However, antioxidant vitamins taken during radiation therapy have also been associated with decreased tum our control, which limits their clinical use220,221.
In addition to the rapid production o f ROS caused by radiation, cells can exhibit chronic increases in ROS levels following exposure to radiation222. There is evidence that elevated levels of ROS, which may be a by-product of inflammatory cytokines such as TGFβ, can lead to persistent tissue damage several months after irradiation223–225. Inducing superoxide dismutase (SOD) expression seems to protect against ROS-induced damage226. Indeed, SOD can mitigate radiation-induced fibrosis in a porcine model227 and in humans228. Similar results have been observed with a combination of tocopherol and pentoxifyl line (a vasodilator and anti-inflammatory drug), which suggests that this combination may work in part through reducing ROS229,230.
p53 modulation
In contrast to the radiosensitizing effect of inhibiting p53 in endothelial cells, p53 inhibition has been proposal as a mechanism for protecting other normal tissues such as haematopoietic cells against radiation injury185. The development of pifithrin-α, a small-molecule inhibitor of p53, has led to accumulating evidence that p53 inhibition can protect some normal tissues from radiation-induced apoptosis without affecting tumour radionsensitivity231. As p53 is induced by DNA damage in part via ribosomal protein L26 (RPL26)-mediated increases in TP53 niRNA translation252, oligonucleotides binding to TP53 mRNA to blunt p53 induction after radiation could potentially be used as therapeutics to block the p53-mediated response to radiation233.
Importantly, the p53-mediated response to DNA damage does not seem to contribute to tum our suppression234–236. Thus, transient inhibition of p53 during radiation therapy may protect some normal tissues from radiation without increasing the probability of radiation-induced tumorigenesis. Moreover, the transcriptional programmes involved in acute DNA damage responses and tumour suppression can be separated, which indicates that the acute p53 response can be targeted selectively without affecting tumour suppression235,236. Despite the evidence supporting the safe use of p53 inhibitors in animal models, to our knowledge pifithrin-α and other drugs targeting p53 have not yet been tested in the clinic.
Hormones, cytokines and cell signalling pathways
Several hormones and cytokines have been utilized to support cellular survival and promote the proliferation of normal tissue stem cells before, during and after radiation exposure. Care must be taken when using hormones and cytokines during cancer therapy because tumours commonly express the receptors for cytokines and growth factors. Melatonin is a hormone that increases the expression of antioxidant enzymes within cells237. Owing to its additional antitumour effects238, it has been tested as a neurological protector in patients with brain metastases. However, a Phase II clinical trial of melatonin during brain radiation therapy showed no difference in survival time or neurological function239.
Various growth factors have been utilized to directly affect stem cell survival and recovery. It has been established that G-CSF (granulocyte colony-stimulating factor) promotes bone marrow recovery and can reduce the lethality of total-body radiation exposure when administered to non-human primates 6 hours after exposure to radiation240. Notably, this approach was not effective when administered to mice 24 hours after radiation241. Keratinocyte growth factor (KGF) stimulates DNA repair, cell proliferation, survival, differentiation and a reduction in levels of ROS, making it potentially useful as a radiation mitigator242. KGF has been shown to prevent radiation-induced xerostomia243 and mucositis244 in animal models. A recombinant human KGF, palifermin, is currently in clinical trials for patients with head and neck cancer. Phase II clinical trials of palifermin in patients receiving hyperfractionated radiotherapy showed a lower incidence and shorter duration of mucositis245.
In addition to triggering microvascular collapse in tumours after radiation, ceramide-mediated endothelial cell apoptosis has been suggested to mediate acute radiation-induced gastrointestinal syndrome246. However, other groups have not observed similar levels of endothelial cell apoptosis and have suggested that intestinal crypt stem cells are the main target of radiation-induced gastrointestinal syndrome141,247 Basic fibroblast growth factor (FGF) inhibits endothelial cell apoptosis and protects the gastrointestinal tract from radiation injury246,248. In addition, a ceramide-targeting antibody to prevent ceramide platform formation in endothelial cells has been reported to protect against gastrointestinal syndrome249. Another group has shown that a peptide derived from the receptor binding domain of FGF2, given to mice 4 hours after a lethal dose of subtotal-body irradiation, increased survival by preventing gastrointestinal syndrome250. However, the mechanism of mitigation may not be through the inhibition of apoptosis, because radiation-induced apoptosis has already started to peak 4 hours after exposure to radiation246.
NF-κB signalling appears to be important for intestinal crypt survival following exposure to radiation251. It was shown that the Toll-like receptor 5 agonist CBLB502 can activate NF-κB and protect mice and rhesus monkeys from acute radiation syndrome when given before or after lethal total-body irradiation252.
Many of the late effects of radiation result from normal tissue fibrosis. Because TGFβ drives smooth muscle cell proliferation and collagen production in fibroblasts, which can lead to fibrosis253, TGFβ inhibition may protect or mitigate against late-developing tissue injury. Experimen is in animal models have shown that blockade of TGFβ signalling decreases fibrosis in the lung254,255 and intestine256 following exposure to radiation.
Anti-inflammatory drugs
The most efficacious drugs for mitigating toxicity to normal tissues are anti-inflammatory drugs. Many different inflammatory pathways have been targeted with the same goal of reducing the autoinflammatory responses and vascular damage that can lead to catastrophic late radiation effects. Directly inhibiting coagulation can block microvascular damage and reduce radiation-induced nephropathy257 as well, is small intestine toxicity258,259 and nervous system injury260 in animal models.
Activated protein C is a potent anticoagulant and cytoprotectant that inhibits blood clotting (through the proteolysis of factors V and VII), promotes fibrinolysis and exerts potent anti-inflammatory and cytoprotective effects on endothelial cells, neurons and innate immune cell populations261. It has shown considerable promise as a radiation mitigator. Systemic administration of activated protein C as late as 24 hours after exposure to radiation mitigated radiation-induced mortality in mice after total-body irradiation262.
Statins are HMG-CoA reductase inhibitors that were originally developed as lipid-lowering agents. However, they have also demonstrated potent anti-inflammatory and antithrom botic properties263. Statins have shown potential as mitigators of late radiation damage by decreasing lung fibrosis and increasing survival when started 8 weeks post-irradiation264. In addition, statins can ameliorate delayed radiation-induced damage in the intestine when started 2 weeks before radiation265. They also have potential in radiation oncology, as they are relatively safe and can mitigate radiation-induced enteropathy when given 14 days alter radiation without allecting the efficacy of radiation on tumours266.
The peroxisome proliferator-activated receptor (PPAR) inhibitors are another family of drugs that are currently in clinical use and may be useful in protecting against normal tissue injury after radiation therapy. PPAR inhibitors are currently used to treat diabetes but they can inhibit pro-inflammatory responses in several cell types267. One PPAR inhibitor, fenofibrate (Tricor; Abbott), was shown to improve neurogenesis in mice after whole-brain irradiation268, which indicates that PPAR inhibitors could decrease neurological side effects in patients receiving irradiation for brain tumours.
Angiotensin-converting enzyme inhibitors have been shown to mitigate lung269, renal270 and neurological injuries271 following irradiation, presumably by protecting the vasculature. However, the mechanism of action is unclear because there is no substantial evidence that the renin–angiotensin system is activated by radiation272.
Stem cell therapies
As damage to stem cells seems to be a major cause of normal tissue injury following exposure to radiation, stem cell therapy could potentially alleviate many of the adverse effects of radiation on normal tissues215. Tissue-specific stem cells have been identified in many tissues215, but very few stem cells have been demonstrated to reconstitute normal tissues when transplanted after radiation. Bone marrow transplantation has been well established as a mitigation strategy for haematopoietic syndrome and is recommended by the Strategic National Stockpile (SNS) Radiation Working Group if sufficient resources are available273. However, the salivary gland is the only solid tissue for which adult stem cell transplantation has been demonstrated to contribute to tissue function after radiation274.
Bone marrow-derived stem cells may contribute to the recovery of several organs outside the bone marrow275. These cells can be stimulated by G-CSF to reduce radiation damage in salivary glands276. Stromal stem cells in the bone marrow, referred to as mesenchymal stem cells, contribute to the haematopoietic stem cell niche and can differentiate into multiple lineages in vitro277. These cells may contribute to the fibrotic response of irradiated tissues278 but they may also help to repair radiation damage in the oesophagus278.
Finally, induced pluripotent stem cells can differentiate into all cell lineages in a living organism239 and have tremendous potential for regenerative medicine in situations of normal tissue damage. However, these cells have not been used successfully in the clinic.
Although a considerable amount of effort will be required to translate the use of stem cells to the clinic, the potential of these cells to repopulate and restore function within normal tissues makes them promising future therapeutics for mitigating radiation injury.
Conclusion
Tremendous progress has been made towards understanding the hallmarks of cancer. Likewise, elucidating the mechanisms through which tum ours evade radiation therapy has led to the development of a new generation of targeted radiosensitizers and radioprotectors that have the potential to selectively in crease the killing of tumour cells during radiation therapy. Although only a few of these approaches have been successfully translated to the clinic, promising approaches to improve local control with radiation therapy in the clinic include triggering synthetic lethality, targeting cancer stem cells and inhibiting multiple kinases that mediate the DNA damage response. Although tumours are heterogeneous and may contain regions of hypoxia or harbour tumour cells with mutations that promote resistance to radiation therapy, this heterogeneity offers opportunities to use drugs that target hypoxic cells or specific mutant proteins to sensitize tumours to radiation therapy. Furthermore, with a greater understanding of the mechanisms by which the microenvironment, and particularly the immune system, affects tumour cure by radiation therapy, there will be new opportunities for improving tumour eradication by manipulating the tumour microenvironment with targeted drugs that block tumour recurrence, by inhibiting angiogenesis or by triggering the immune response to eliminate surviving cancer cells. As radiation therapy is utilized to treat over half of all patients with cancer, translating our growing understanding of cancer and radiation biology into effective radiosensitizers of tumours or radioprotectors of normal tissues has the potential to improve outcomes for many patients with cancer.
Box 1 | Using radiation to treat tumours.
Radiation therapy is ore of the most commonly used anticancer therapies in the clinic. Although radiation can be used to palliate cancer symptoms such as pain, the majority of patients are treated with the intent to cure their tumour. Radiation therapy uses high-energy ionizing radiation to kill cancer cells by damaging their DNA. As normal cells are also affected by radiation, radiation oncologists deliver radiation to the tumour area while limiting the dose of radiation to surrounding normal tissues as much as possible. For many cancers, such as breast cancer, radiation is used before or after surgery to increase the probability of local control. For a subset of these patients, adjuvant radiation therapy increases the probability of a cure. For other cancers, such as squamous cell carcinoma of the head and neck, definitive radiation therapy is used to achieve local control. The addition of concurrent chemotherapy or biologically targeted agents such as the epidermal growth factor receptor (EGFR) inhibitor cetuximab (Erbitux; Bristol-Myers Squibb/Lilly) can increase local control.
Before delivering radiation therapy, radiation oncologists develop an individualized treatment plan for each patient. This process begins with a simulation where the patient is immobilized in the treatment position and a computed tomography scan is acquired for treatment planning. As small changes in body position can affect the total dose delivered to the tumour and normal tissues, skin marks, moulds and other devices are used during simulation to ensure that the patientis in the same position for daily treatment. Following simulation, the radiation on cologist uses all of the imaging and clinical data available to determine the area that needs to be treated, the total dose that will be delivered and how much radiation can be safely delivered to surrounding normal tissues. Based on these constraints, the radiation on cology team uses detailed computer modelling to design the treatment plan for the patient.
Acknowledgements
The authors thank the US National Institutes of Health and the Duke Cancer Institute for long-term financial support.
Glossary
- Hoematopoietic
syndrme Acute radiation toxicity caused by bone marrow failure that occurs within a month after whole-body exposure to radiation.
Footnotes
Competing interests statement The authors declare no competing financial interests.
References
- 1.Intensity Modulated Radiation Therapy Collaborative Working Group Intensity-modulated radiotherapy: current status and issues of interest. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 2.Lo SS, et al. Stereotactic body radiation therapy: a novel treatment modality. Nature Rev. Clin. Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]; This manuscript reviews prospective clinical trials-and current clinical use of stereotactic body radiation therapy.
- 3.Timmerman R, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006;24:4835–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 4.Forquer JA, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity In apical tumor sites. Radiother. Oncol. 2009;93:408–413. doi: 10.1016/j.radonc.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int. J. Radiat Oncol. Biol. Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 6.Andolino DL, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Madsen BL, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:1099–1105. doi: 10.1016/j.ijrobp.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 8.Svedman C, et al. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–1955. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 10.Durante M, Loeffler JS. Charged particles in radiation oncology. Nature Rev. Clin. Oncol. 2010;7:37–43. doi: 10.1038/nrclinonc.2009.183. [DOI] [PubMed] [Google Scholar]; This article reviews potential benefits and disadvantages of particle therapy and describes contemporary clinical outcomes following charged particle therapy.
- 11.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 2007;25:953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 12.Greco C, Wolden S. Current status of radiotherapy with proton and light ion beams. Cancer. 2007;109:1227–1238. doi: 10.1002/cncr.22542. [DOI] [PubMed] [Google Scholar]
- 13.Sheets NC, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoskin PJ, Bownes P. Innovative technologies in radiation therapy: brachytherapy. Semin. Radiat. Oncol. 2006;16:209–217. doi: 10.1016/j.semradonc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Bhide SA, Nutting CM. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson LA, Jaffray DA. Advances in image-guided radiation therapy. J. Clin. Oncol. 2007;25:938–946. doi: 10.1200/JCO.2006.09.9515. [DOI] [PubMed] [Google Scholar]
- 17.Elshaikh M, Ljungman M, Ten Haken R, Lichter AS. Advances in radiation oncology. Annu. Rev. Med. 2006;57:19–31. doi: 10.1146/annurev.med.57.121304.131431. [DOI] [PubMed] [Google Scholar]
- 18.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nature Rev. Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 19.Seiwart TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm — general principles. Nature Clin. Practice. Oncol. 2007;4:86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura Y. Rationale for chemoradiotherapy. Int. J. Clin. Oncol. 2004;9:414–420. doi: 10.1007/s10147-004-0443-z. [DOI] [PubMed] [Google Scholar]
- 21.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature Rev. Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7:492–508. doi: 10.1634/theoncologist.7-6-492. [DOI] [PubMed] [Google Scholar]
- 23.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 24.Chapman JD. Hypoxic sensitizers — implications for radiation therapy. N. Engl. J. Med. 1979;301:1429–1432. doi: 10.1056/NEJM197912273012606. [DOI] [PubMed] [Google Scholar]
- 25.Overgaard J. Hypoxic radiosensitization: adored and ignored. J. Clin. Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]; This article is a systematic review of randomized clinical trials providing evidence that modifying tumor hypoxia improves outcomes with radiotherapy.
- 26.Bennett MH, Feldmeier J, Smee R, Milross C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst. Rev. 2012;4:CD005007. doi: 10.1002/14651858.CD005007.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henke M, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 28.Arcasoy MO, et al. Erythropoietin and erythropoietin receptor expression in Head and neck cancer: relationship to tumor hypoxia. Clin. Cancer Res. 2005;11:20–27. [PubMed] [Google Scholar]
- 29.Overgaard J, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother. Oncol. 1998;46:135–146. doi: 10.1016/s0167-8140(97)00220-x. [DOI] [PubMed] [Google Scholar]
- 30.Lee DJ, et al. Results of an RTOC phase III trial (RTOG 85–27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 1995;32:567–576. doi: 10.1016/0360-3016(95)00150-W. [DOI] [PubMed] [Google Scholar]
- 31.Brown JM, Koong A. Therapeutic advantage of hypoxic cells in tumors: a theoretical study. J. Natl Cancer Inst. 1991;83:178–185. doi: 10.1093/jnci/83.3.178. [DOI] [PubMed] [Google Scholar]
- 32.Rischin D, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J. Clin. Oncol. 2010;28:2989–2995. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 33.Le Q-T, et al. Prognostic and predictive significance of plasma HGF and IL-8 in a phase III trial of chemoradiation with or without tirapazamine in locoregionally advanced head and neck cancer. Clin. Cancer Res. 2012;18:1798–1807. doi: 10.1158/1078-0432.CCR-11-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Niu C, Chan N, Shen B, Chen X. Tumor hypoxia imaging. Mol. Imag. Biol. 2011;13:399–410. doi: 10.1007/s11307-010-0420-z. [DOI] [PubMed] [Google Scholar]
- 35.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 36.Yuhas JM. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980;40:1519–1524. [PubMed] [Google Scholar]
- 37.Calabro-Jones PM, Fahey RC, Smoluk GD, Ward JF. Alkaline phosphatase promotes radioprotection and accumulation of WR-1065 in V79-171 cells incubated in medium containing WR-2721. Int. J. Radiat. Biol. Rclat. Stud. Phys. Chem. Med. 1985;47:23–27. doi: 10.1080/09553008514550041. [DOI] [PubMed] [Google Scholar]
- 38.Yuhas JM, Storer JB. Differential chemoprotection of normal and malignant tissues. J. Natl Cancer Inst. 1969;42:331–335. [PubMed] [Google Scholar]
- 39.Buentzel J, et al. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: a randomized placebo-controlled Phase III study Int. J. Radiat. Oncol. Biol. Phys. 2006;64:684–691. doi: 10.1016/j.ijrobp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Fournel P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoradque-Groupe Français de Pneumo-Cancérologie NPC 95–01 Study. J. Clin. Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 41.Lorusso D, et al. Phase III multicenter randomized trial of amifostine as cytoprotectant in first-line chemotherapy in ovarian cancer patients. Ann. Oncol. 2003;14:1086–1093. doi: 10.1093/annonc/mdg301. [DOI] [PubMed] [Google Scholar]
- 42.Hensley ML, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]; This article provides clinical guidelines for the use of radiation protectors in combination with radiation therapy.
- 43.Glover D, Glick JH, Weiler C, Fox K, Guerry D. WR-2721 and high-dose cisplatin: an active combination in the treatment of metastatic melanoma. J. Clin. Oncol. 1987;5:574–578. doi: 10.1200/JCO.1987.5.4.574. [DOI] [PubMed] [Google Scholar]
- 44.Rubin JS, et al. Audiological findings in a Phase I protocol investigating the effect of WR 2721, high-dose cisplatin and radiation therapy in patients with locally advanced cervical carcinoma. J. Laryngoi. Otol. 1995;109:744–747. doi: 10.1017/s0022215100131202. [DOI] [PubMed] [Google Scholar]
- 45.Mollman JE, Glover DJ, Hogan WM, Furman RE. Cisplatin neuropathy. Risk factors, prognosis, and protection by WR-2721. Cancer. 1988;61:2192–2195. doi: 10.1002/1097-0142(19880601)61:11<2192::aid-cncr2820611110>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 46.Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nature Rev. Mol. Cell Biol. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- 47.Schwachofer JH, Hoogenhout J, Kal HB, Koedam J, van Wezel HP. Radiosensitivity of different human turner lines grown as xenografts determined from growth delay and survival data. In Vivo. 1990;4:253–257. [PubMed] [Google Scholar]
- 48.Gerweck LE, Zaidi ST, Zietman A. Multivariate determinants of radiocurability. I: Prediction of single fraction tumor control doses. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:57–66. doi: 10.1016/0360-3016(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 49.Singh M, Muiriel CL, Johnson L. Genetically engineered mouse models: closing the gap between preclinical data and trial outcomes. Cancer Res. 2012;72:2695–2700. doi: 10.1158/0008-5472.CAN-11-2786. [DOI] [PubMed] [Google Scholar]
- 50.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Karar J, Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol. Ther. 2009;8:1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roses RE, Xu M, Koski GK, Czerniecki BJ. Radiation therapy and Toll-like receptor signaling: implications for the treatment of cancer. Oncogene. 2008;27:200–207. doi: 10.1038/sj.onc.1210909. [DOI] [PubMed] [Google Scholar]
- 53.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 54.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharpless NE, Depinlio RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature Rev. Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 56.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graves EE, et al. Hypoxia in models of lung cancer: implications for targeted therapeutics. Clin. Cancer Res. 2010;16:4843–4852. doi: 10.1158/1078-0432.CCR-10-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maity A, Koumenis C. Location, location, location-makes all the difference for hypoxia in lung tumors. Clin. Cancer Res. 2010;16:4685–4687. doi: 10.1158/1078-0432.CCR-10-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh M, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nature Biotech. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 60.Chen Z, et al. A murine lung cancer co-clmical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 59 and 60 illustrate the potential of genetically engineered mouse models to predict the outcomes of human clinical trials of cancer therapies.
- 61.Suit H, Skates S, Taghian A, Okunieff P, Efird JT. Clinical implications of heterogeneity of tumor response to radiation therapy. Radio ther. Oncol. 1992;25:251–260. doi: 10.1016/0167-8140(92)90244-o. [DOI] [PubMed] [Google Scholar]
- 62.Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391–1399. [PubMed] [Google Scholar]
- 63.Jiang H, et al. The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev. 2009;23:1895–1909. doi: 10.1101/gad.1815309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choudhury A, et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010;70:7017–7026. doi: 10.1158/0008-5472.CAN-10-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Navin N, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schepers AG, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]; References 66–68 illustrate that a subset of tumour cells can contribute to tumour growth and regrowth following anticancer therapy.
- 69.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 70.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nature Rev. Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 71.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 72.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cells. 2010;28:639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jang Y-Y, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 75.Moncharmont C, et al. Targeting a cornerstone of radiation resistance: cancer stem cell. Cancer Lett. 2012;322:139–147. doi: 10.1016/j.canlet.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 76.Kaelin WG. The concept of synthetic lethality, in the context of anticancer therapy. Nature Rev. Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 77.Núñez MI, McMillan TJ, Valenzuela MT, Ruiz de Almodóvar JM, Pedraza V. Relationship between DNA damage, rejoining and cell killing by radiation in mammalian cells. Radio ther. Oncol. 1996;39:155–165. doi: 10.1016/0167-8140(96)01732-x. [DOI] [PubMed] [Google Scholar]
- 78.Jorgensen TJ. Enhancing radio sensitivity: targeting the DNA repair pathways. Cancer Biol. Ther. 2009;8:665–670. doi: 10.4161/cbt.8.8.8304. [DOI] [PubMed] [Google Scholar]
- 79.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nature Rev. Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 80.Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nature Rev. Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 81.Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin. Cancer Res. 2007;13:1383–1388. doi: 10.1158/1078-0432.CCR-06-2260. [DOI] [PubMed] [Google Scholar]
- 82.Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 2002;8:571–576. doi: 10.1016/s1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- 83.De Soto JA, et al. The inhibition and treatment of breast cancer with poly (ADP-ribose) polymerase (PARP-1) inhibitors. Int. J. Biol. Sci. 2006;2:179–185. doi: 10.7150/ijbs.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 85.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]; References 84 and 85 demonstrate that BRCA-mutant breast cancer cells are sensitivs to PARP inhibition.
- 86.McCabe N, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 87.Löser DA, et al. Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol. Cancer Ther. 2010;9:1775–1787. doi: 10.1158/1535-7163.MCT-09-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bryant HE, Helleday T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006;34:1685–1691. doi: 10.1093/nar/gkl108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kennedy RD, et al. Fanconi anemia pathway-deficient tumor cells are hypersensitive to inhibition of ataxia telangiectasia mutated. J. clin. Invest. 2007;117:1440–1449. doi: 10.1172/JCI31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rainey MD, Charlton ME, Stanton RV, Kastan MB. Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res. 2008;68:7466–7474. doi: 10.1158/0008-5472.CAN-08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hickson I, et al. Identification and characterization of a novel and specific inhibitor of the ataxiateIangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 93.Westphal C, II, et al. Loss of ATM radiosensitfzes multiple p53 null tissues. Cancer Res. 1998;58:5637–5639. [PubMed] [Google Scholar]
- 94.Rasom A, et al. Characterization of glioma stem cells through multiple stem cell markers and their specific sensitization to double-strand break-inducing agents by pharmacological inhibition of ataxia telangiectasia mutated protein. Brain Pathol. 2012;22:677–688. doi: 10.1111/j.1750-3639.2012.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Cenes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 96.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shechter D, Costanzo V, Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nature Cell Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- 98.Toledo LI, et al. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nature Struct. Mol. Biol. 2011;18:721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fokas E, et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012;3:e441. doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maira SM, et al. Identification and characterization of NVP-BEZ235. a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 101.Konstantinidou C, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–7652. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukherjee B, et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and DNA-PKCs-mediated DNA damage responses. Neoplasia. 2012;14:34–43. doi: 10.1593/neo.111512. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that the dualPI3K/mTOR inhibitor NVP-BEZ235 also blocks ATM and DNA-PKCS.
- 103.Cupta AK, et al. The Ras radiation resistance pathway. Cancer Res. 2001;61:4278–4282. [PubMed] [Google Scholar]
- 104.Fokas E, et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Res. 2012;72:239–248. doi: 10.1158/0008-5472.CAN-11-2263. [DOI] [PubMed] [Google Scholar]
- 105.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 106.Dent P, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat. Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 107.Golding SE, et al. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–1053. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 108.Kriegs M, et al. The epidermal growth factor receptor modulates DNA double-strand break repair by regulating non-homologous end-joining. DNA Repair. 2010;9:889–897. doi: 10.1016/j.dnarep.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 109.Andarawewa KL, Paupert J, Pal A, Barcellos-Hoff MH. New rationales for using TGFbeta inhibitors in radiotherapy. Int. J. Radiat. Biol. 2007;83:803–811. doi: 10.1080/09553000701711063. [DOI] [PubMed] [Google Scholar]
- 110.Engelman JA. Targeting PI3K signalling in cancer: opportumtles, challenges and limitations. Nature Rev. Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 111.Gupta AK, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin. Cancer Res. 2002;8:885–892. [PubMed] [Google Scholar]
- 112.Hambardzumyan D, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim I-A, et al. Selective inhibition of Ras. phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer Res. 2005;65:7902–7910. doi: 10.1158/0008-5472.CAN-05-0513. [DOI] [PubMed] [Google Scholar]
- 114.Bonner JA, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 115.Bonner JA, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 116.Eberhard DA, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancel treated with chemotherapy alone and in combination with erlotinib. J. Clin. Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 117.Karapetis CS, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 118.Valerie K, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol. Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 119.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31:363–372. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 120.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nature Rev. Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 121.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 122.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 123.Gandhi L, et al. Phase I study of Navitoclax (ABT-263). a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J. Clin. Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilson WH, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Opferman JT, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 126.Opferman JT, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 127.Skvara H, et al. Mcl-1 blocks radiation-induced apoptosis and inhibits clonogenic cell death. Anticancer Res. 2005;25:2697–2703. [PubMed] [Google Scholar]
- 128.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 129.Moretti L, Li B, Kim KW, Chen H, Lu B. AT-101, a pan-Bcl-2 inhibitor, leads to radiosensitization of non-small cell lung cancer. J. Thorac. Oncol. 2010;5:680–687. doi: 10.1097/JTO.0b013e3181d6e08e. [DOI] [PubMed] [Google Scholar]
- 130.Zerp SF, et al. AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis. Radiat. Oncol. 2009;4:47. doi: 10.1186/1748-717X-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hudson SG, et al. Microarray determination of Bcl-2 family protein inhibition sensitivity in breast cancer cells. Exp. Biol. Med. 2013;238:248–256. doi: 10.1177/1535370212474582. [DOI] [PubMed] [Google Scholar]
- 132.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 133.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shangary S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc. Natl Acad. Sci. USA. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 136.Xu B, O'Donnell A, Kim S-T, Kastan MB. Phosphorylation of serine 1387 in Brca 1 is specifically required for the Atm-mediated S-phase checkpoint after ionizing irradiation. Cancer Res. 2002;62:4588–4591. [PubMed] [Google Scholar]
- 137.Xu B, Kim S-T, Lim D-S, Kastan MB. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 2002;22:1049–1059. doi: 10.1128/MCB.22.4.1049-1059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bunz F, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 139.Waldman T, et al. Cell-cycle arrest versus cell death in cancer therapy. Nature Med. 1997;3:1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- 140.Wouters BG, Giacda AJ, Denko NC, Brown JM. Loss of p21 Waf 1/Cip 1 sensitizes tumors to radiation by an apoptosis-independent mechanism. Cancer Res. 1997;57:4703–4706. [PubMed] [Google Scholar]; References 139 and 140 illustrate that clonogenic survival in vitro does not always predict radiation sensitivity in vivo.
- 141.Kirsch DG, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Komarova EA, et al. Dual effect of p53 on radiation sensitivity in vivo. p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]; This articles demonstrates the complex and tissue -dependent role of p53 in acute radiation injury.
- 143.Leibowitz BJ, et al. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol. Cancer Res. 2011;9:616–625. doi: 10.1158/1541-7786.MCR-11-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee CL, et al. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci. Signal. 2012;5:ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rowley R, Hudson J, Young PG. The wee 1 protein kinase is required for radiation-induced mitotic delay. Nature. 1992;356:353–355. doi: 10.1038/356353a0. [DOI] [PubMed] [Google Scholar]
- 146.Sarcar B, et al. Targeting radiation-induced G(2) checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines. Mol. Cancer Ther. 2011;10:2405–2414. doi: 10.1158/1535-7163.MCT-11-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Y, et al. Radiosensitization of p53 mutant cells by PDO 166285, a novel G(2) checkpoint abrogator. Cancer Res. 2001;61:8211–8217. [PubMed] [Google Scholar]
- 148.Caretti V, et al. WEE 1 kinase inhibition enhances the radiation response of diffuse intrinsic pontine gliomas. Mol. Cancer Ther. 2013;12:141–150. doi: 10.1158/1535-7163.MCT-12-0735. [DOI] [PubMed] [Google Scholar]
- 149.De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJ, Wordinger T. WEE1 kinase targeting combined with DNA damaging cancer therapy catalyzes mitotic catastrophe. Clin. Cancer ReS. 2011;17:4200–4207. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 150.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase 1 targeting in the treatment of cancer. Br. J. Cancer. 2008;98:523–528. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mitchell JB, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor AZD7762. Clin. Cancer Res. 2010;16:2076–2084. doi: 10.1158/1078-0432.CCR-09-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morgan MA, et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 Checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 2010;70:4972–4981. doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nature Rev. Cancer. 2007;7:495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 154.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 155.Kozin SV, Duda DG, Munn LL, Jain RK. Neovascularization after irradiation: what is the source of newly formed vessels in recurring tumors? J. Natl Cancer Inst. 2012;104:899–905. doi: 10.1093/jnci/djs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 157.Garcia-Barros M, et al. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010;70:8179–8186. doi: 10.1158/0008-5472.CAN-10-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Truman JP, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS ONE. 2010;5:e12310. doi: 10.1371/journal.pone.0012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin X, Fuks Z, Kolesnick R. Ceramide mediates radiation-induced death of endothelium. Crit. Care Med. 2000;28:N87–N93. doi: 10.1097/00003246-200004001-00010. [DOI] [PubMed] [Google Scholar]
- 160.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 161.Budach W, Taghian A, Freeman J, Gioioso D, Suit HD. Impact of stromal sensitivity on radiation response of tumors. J. Natl Cancer Inst. 1993;85:988–993. doi: 10.1093/jnci/85.12.988. [DOI] [PubMed] [Google Scholar]; This article provides evidence that the sensitivity of stromal cells to radiation does not affect tumour cure following radiation, therapy.
- 162.Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006;66:8352–8355. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- 163.Ogawa K, et al. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007;67:4016–4021. doi: 10.1158/0008-5472.CAN-06-4498. [DOI] [PubMed] [Google Scholar]
- 164.Ahn GO, Brown JM. Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle. 2009;8:970–976. doi: 10.4161/cc.8.7.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ahn GO, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis. 2009;12:159–164. doi: 10.1007/s10456-009-9135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc. Ncitl Acad. Sci. USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 168.Lyden D, et al. Impaired recruitment of bone-marraw-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 169.Purhonen S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc. Natl Acad. Sci. USA. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kozin SV, Duda DG, Munn LL, Jain RK. Is vasculogenesis crucial for the regrowth of irradiated tumours? Nature Rev. Cancer. 2011;11:532. doi: 10.1038/nrc2007-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is a comprehensive review on vascular damage and regrowth following radiation therapy.
- 171.Kozin SV, et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kioi M, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after iiradiation in mice. J. Clin. Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumorvasculogenesis but not for angiogenesis: role of bone marrow-derived myeIomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ahn GO, et al. Inhibition of Mac-1 (CD 11 b/CD 18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl Acad. Sci. USA. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tseng D, Vasquez-Medrano DA, Brown JM. Targeting SDF-1/CXCR4 to inhibit tumour vascuiature for treatment of glioblastomas. Br. J. Cancer. 2011;104:1805–1809. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu SK, et al. Delta-like ligand 4-Notch blockade and tumor radiation response. J. Natl Cancer Inst. 2011;103:1778–1798. doi: 10.1093/jnci/djr419. [DOI] [PubMed] [Google Scholar]
- 177.Lee CG, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 178.Dings RP, et al. Scheduling of radiation with angiogenesis inhibitors a ng in ex and avastin improves therapeutic outcome via vessel normalization. Clin. Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Teng L-S, et al. Advances in combination of antiangiogenic agents targeting VEGF-binding and conventional chemotherapy and radiation for cancer treatment. JCMA. 2010;73:281–288. doi: 10.1016/S1726-4901(10)70062-9. [DOI] [PubMed] [Google Scholar]
- 180.Willett CG, et al. Direct evidence that the VEGF-specific: antibody bevacizumab has antivascular effects in human rectal cancer. Nature Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Willett CG, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lai A, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Drappatz J, et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:85–90. doi: 10.1016/j.ijrobp.2009.07.1741. [DOI] [PubMed] [Google Scholar]
- 184.Stiewe T. The p53 family in differentiation and tumorigenesis. Nature Rev. cancer. 2007;7:165–168. doi: 10.1038/nrc2072. [DOI] [PubMed] [Google Scholar]
- 185.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nature Rev. Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 186.Komarova EA, Christov K, Faerman AI, Gudkov AV. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791–3798. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 187.MacCallum DE, et al. The p53 response to ionising radiation in adult and developing murine tissues. Oncogene. 1996;13:2575–2587. [PubMed] [Google Scholar]
- 188.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 189.Yu H, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115:3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brash R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nature Rev. Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 191.Burdelya LG, et al. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. 2006;66:9356–9361. doi: 10.1158/0008-5472.CAN-06-1223. [DOI] [PubMed] [Google Scholar]
- 192.Gough MJ, Crittenden MR. Combination approaches to immunotherapy: the radiotherapy example. Immunotherapy. 2009;1:1025–1037. doi: 10.2217/imt.09.64. [DOI] [PubMed] [Google Scholar]
- 193.DuPage M, et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression ol tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dunn GP, Bruce AT, Ikeda K, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 2002;3:991–999. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 196.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 197.Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br. J. Raclioi. 1973;46:220–222. doi: 10.1259/0007-1285-46-543-220. [DOI] [PubMed] [Google Scholar]
- 198.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br. J. Radiol. 1975;48:863–866. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 199.Nobler MP. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology. 1969;93:410–412. doi: 10.1148/93.2.410. [DOI] [PubMed] [Google Scholar]
- 200.Ohba K, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Postow MA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl. J. Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Demaria S, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]; This article provides evidence that local radiation therapy can activate the immune system to fight distant metastatic disease.
- 203.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor α mRNA after cellular exposure to ionizing radiation. Proc. Natl Acad. Sci. USA. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hong JH, et al. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int. J. Radiat. Biol. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 205.Nemoto K, et al. Expression of IL-1β mRNA in mice after whole body X-irradiation. J. Radiat. Res. 1995;36:125–133. doi: 10.1269/jrr.36.125. [DOI] [PubMed] [Google Scholar]
- 206.Hareyama M, et al. Effect of radiation on the expression of carcinoembiyonic antigen of human gastric adenocarcinoma cells. Cancer. 1991;67:2269–2274. doi: 10.1002/1097-0142(19910501)67:9<2269::aid-cncr2820670910>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 207.Hauser SH, Calorini L, Wazer DE, Gattoni-Celli S. Radiation-enhanced expression of major histocompatibility complex class I antigen H-2Db in B 16 melanoma cells. Cancer Res. 1993;53:1952–1955. [PubMed] [Google Scholar]
- 208.Nesslinger NJ, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin. Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 209.Schaue D, Xie MW, Ratikan JA, McBride WH. Regulatory T cells in radiotherapeutic responses. Front. Oncol. 2012;2:90. doi: 10.3389/fonc.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 211.Kachikwu EL, et al. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eltzschig HK, Eckle T. Ischemia and reperfusion — from mechanism to translation. Nature Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stone HB, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop. December 3-4, 2003. Radiat. Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 214.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 215.Coppes RP, van der Goot A, Lombaert IM. Stem cell therapy to reduce radiation-induced normal tissue damage. Semin. Radiat. Oncol. 2009;19:112–121. doi: 10.1016/j.semradonc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 216.Blanpain C, Mohrin M, Sotiropoulou PA, Passegue E. DMA-damage response rn tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 217.Mitchell JB, et al. Inhibition of oxygen-dependent radiation induced damage by the nitroxide superoxide dismutase mimic. tempol. Arch. Bioehem. Biophys. 1991;289:62–70. doi: 10.1016/0003-9861(91)90442-l. [DOI] [PubMed] [Google Scholar]
- 218.Metz JM, et al. A phase I study of topical tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin. Cancer Res. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- 219.Citrin D, et al. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bairati I, et al. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J. Clin. Oncol. 2005;23:5805–5813. doi: 10.1200/JCO.2005.05.514. [DOI] [PubMed] [Google Scholar]
- 221.Meyer F, et al. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int. J. Cancer. 2008;122:1679–1683. doi: 10.1002/ijc.23200. [DOI] [PubMed] [Google Scholar]
- 222.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a reviaw. Int. J. Radiat. Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 223.Jack CI, et al. Indicators of free radical activity in patients developing radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 1996;34:149–154. doi: 10.1016/0360-3016(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 224.Kang SK, et al. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int. J. Radicit. Oncoi. Biol. Phys. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 225.Robbins ME, Zhao W, Davis C, Toyokuni S, Bonsib SM. Radiation-induced kidney iniury; a role for chronic oxidative stress? Micron. 2002;33:133–141. doi: 10.1016/s0968-4328(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 226.Carpenter M, et al. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther. 2005;12:685–693. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 227.Lefaix JL, et al. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int. J. Radat. Oncol. Biol. Phys. 1996;35:305–312. doi: 10.1016/0360-3016(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 228.Delanian S, et al. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother. Oncol. 1994;32:12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]; This study provides support for SOD to mitigate radiation-induced fibrosis in patients.
- 229.Delanian S, Balla-Mekias S, Lefaix JL. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J. Clin. Oncol. 1999;17:3283–3290. doi: 10.1200/JCO.1999.17.10.3283. [DOI] [PubMed] [Google Scholar]
- 230.Lefaix JL, et al. Striking regression of subcutaneous til rosis induced by high doses of gamma rays using a combination of pentoxifylline and α-tocopherol: an experimental study. Int. J. Radiat. Oncoi. Biol. Phys. 1999;43:839–847. doi: 10.1016/s0360-3016(98)00419-2. [DOI] [PubMed] [Google Scholar]
- 231.Komarov PG, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 232.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 233.Chen J, Kastan MB. 5′-3′-UTR interactions regulate p53 mRNA translation and provide a target for modulating p53 induction after DNA damage. Genes Dev. 2010;24:2146–2156. doi: 10.1101/gad.1968910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]; This article indicates that an acute p53 response to DNA damage is not necessary for p53-mediated tumour suppression.
- 235.Li T, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Brady CA, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145:571–583. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Okatani Y, Wakatsukl A, Shinohara K, Kaneda C, Fukaya T. Melatonin stimulates glutathione peroxidase activity in human chorion. J. Pineal Res. 2001;30:199–205. doi: 10.1034/j.1600-079x.2001.300402.x. [DOI] [PubMed] [Google Scholar]
- 238.Akagi T, et al. Chronopharmacology of melatonin in mice to maximize the antitumor effect and minimize the rhythm disturbance effect. J. Pharmacol. Exp. Ther. 2004;308:378–384. doi: 10.1124/jpet.103.055657. [DOI] [PubMed] [Google Scholar]
- 239.Berk L, et al. Randomized phase II trial of high-dose melatonin and radiation therapy for RPA class 2 patients with brain metastases (RTOG 0119) Int. J. Radiat Oncol. Biol. Phys. 2007;68:852–857. doi: 10.1016/j.ijrobp.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bertho J-M, et al. Comparison of autologous cell therapy and granulocyte-colony stimulating factor (G-CSF) injection versus G-CSF injection alone for the treatment of acute radiation syndrome in a non-human primate model. Int. J. Radiat Oncol. Biol. Phys. 2005;63:911–920. doi: 10.1016/j.ijrobp.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 241.Uckun FM, Souza L, Waddick KG, Wick M, Song CW. In vivo radioprotective effects of recombinant human granulocyte colony-stimulating factor in lethally irradiated mice. Blood. 1990;75:638–645. [PubMed] [Google Scholar]
- 242.Finch PW, Rubin JS. Keratinocyte growth factor fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv. Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 243.Lombaert IM, et al. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008;26:2595–2601. doi: 10.1634/stemcells.2007-1034. [DOI] [PubMed] [Google Scholar]
- 244.Farrell CL, et al. Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and Irradiated mice. Int. J. Radiat. Biol. 1999;75:609–620. doi: 10.1080/095530099140258. [DOI] [PubMed] [Google Scholar]
- 245.Brizel DM, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J. Clin. Oncol. 2008;26:2489–2496. doi: 10.1200/JCO.2007.13.7349. [DOI] [PubMed] [Google Scholar]
- 246.Paris F, et al. Endothelial apoptosis as the primary lesion Initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 247.Schuller BW, et al. No significant endothelial apoptosis in the radiation-induced gastrointestinal syndrome. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:205–210. doi: 10.1016/j.ijrobp.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 248.Fuks Z, et al. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994;54:2582–2590. [PubMed] [Google Scholar]
- 249.Rotolo J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome In mice. J. Clin. Invest. 2012;122:1786–1790. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang L, et al. Mitigation effect of an FGF-2 peptide on acute gastrointestinal syndrome after high-dose ionizing radiation. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:261–268. doi: 10.1016/j.ijrobp.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang V, et al. Activation of nuclear factor κB in vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Cancer Res. 2004;64:6240–6246. doi: 10.1158/0008-5472.CAN-04-0591. [DOI] [PubMed] [Google Scholar]
- 252.Burdelya LG, et al. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Martin M, Lefaix J, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic taiget? Int. J. Radiat. Oncol. Biol. Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 254.Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-β antibody 1D 11 ameliorates normal tissue damage caused by high-dose radiation. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:876–881. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 255.Anscher MS, et al. Small molecular inhibitor of transforming growth factor-β protects against development of radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 256.Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer-Jensen M. Recombinant soluble transforming growth factor β type II receptor ameliorates radiation enteropathy in mice. Castro enterology. 2000;119:1286–1296. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- 257.Verheij M, Stewart F, Oussoren Y, Weening J, Dewit L. Amelioration of radiation nephropathy by acetylsalicylic acid. Int. J. Radiat. Biol. 1995;67:587–596. doi: 10.1080/09553009514550701. [DOI] [PubMed] [Google Scholar]
- 258.Wang J, et al. Hirudin ameliorates Intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J. Thromb. Haemost. 2004;2:2027–2035. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 259.Wang J, et al. Short-term Inhibition of ADP-induced platelet aggregation by clopidogrel ameliorates radiation-induced toxicity in rat small intestine. Thromb. Haemostasis. 2002;87:122–128. [PubMed] [Google Scholar]
- 260.Glantz MJ, et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020–2027. doi: 10.1212/wnl.44.11.2020. [DOI] [PubMed] [Google Scholar]
- 261.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 262.Geiger H, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nature Med. 2012;18:1123–1129. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates mitigation of acute radiation injury when the thrombomodulin-activated protein C pathway is targeted 24 hours after exposure to radiation.
- 263.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nature Rev. Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 264.Williams JP, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat. Res. 2004;161:560–567. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 265.Wang J, et al. Simvastatin ameliorates radiation enteropathy development after localized, fractionated irradiation by a protein C-independent mechanism. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1483–1490. doi: 10.1016/j.ijrobp.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Haydont V, et al. Successlnl mitigation of delayed Intestinal radiation injury using pravastatin is not associated with acute injury improvement or tumor protection. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1471–1482. doi: 10.1016/j.ijrobp.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 267.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nature Rev. Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 268.Ramanan S, et al. The PPARa agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Molteni A, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-β (TGF-β) and α-actomyosin (α SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr. Pharm. Design. 2007;13:1307–1316. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 270.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and All receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr. Pharm. Design. 2003;9:737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 271.Ryu S, Kolozsvary A, Jenrow KA, Brown SL, Kim JH. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J. Neuro-Oncol. 2007;82:119–124. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 272.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin. Radiat. Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 273.Waselenko JK, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Internal Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 274.Lombaert IM, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article illustrates that stem cell transplantation can contribute to the function of solid organs after radiation.
- 275.Vieyra D,S, Jackson KA, Goodell MA. Plasticity and tissue regenerative potential of bone marrow-derived cells. Stem Cell Rev. 2005;1:65–69. doi: 10.1385/SCR:1:1:065. [DOI] [PubMed] [Google Scholar]
- 276.Lombaert IM, et al. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin. Cancer Res. 2006;12:1804–1812. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- 277.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 278.Epperly MW, et al. Bone marrow origin of cells with capacity for homing and differentiation to esophageal squamous epithelium. Radiat. Res. 2004;162:233–240. doi: 10.1667/rr3224. [DOI] [PubMed] [Google Scholar]
- 279.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]