Abstract
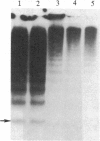
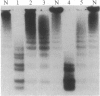
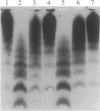
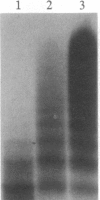
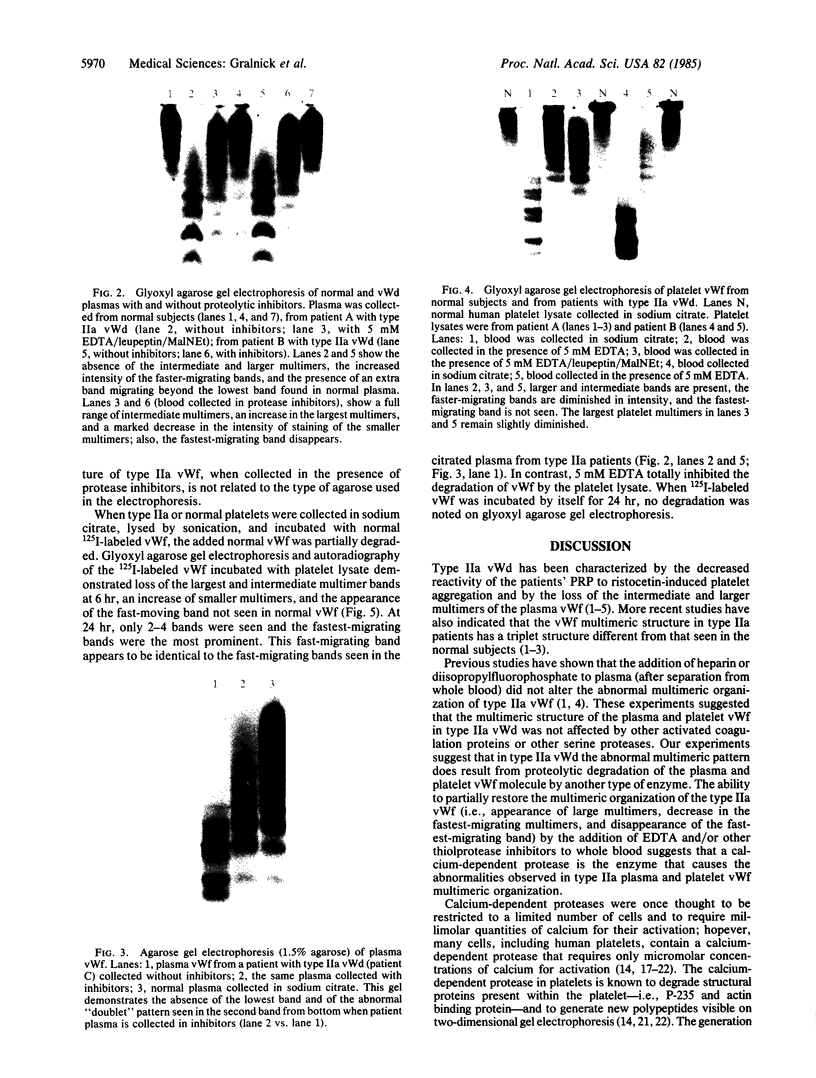
Type IIa von Willebrand's disease (vWd) has been characterized by the absence of the largest and a reduction in the intermediate-sized multimers of the plasma and platelet von Willebrand factor (vWf) and by the diminished response of the platelet-rich plasma of these patients to ristocetin. Other recently demonstrated abnormalities include the presence of an abnormal triplet structure of vWf. We have studied the plasma and platelets from three patients with this form of vWd and have found that both their plasma and platelets manifest the previously described abnormalities. Because of the heterogeneity of the multimeric structure of the vWf in these patients, we considered the possibility that postsynthetic events may have modified the vWf. When blood was collected in 5 mM EDTA or 5 mM EDTA/leupeptin/N-ethylmaleimide, the abnormal multimeric structure of the plasma and platelet vWf was partially normalized in that the intermediate and the largest vWf multimers were increased, the abnormal multimer structure was no longer as apparent, and the fastest migrating band (an abnormality seen only in the type IIa vWd plasma and platelets) disappeared. The enzymatic activity responsible for this degradation can be classified as a calcium-dependent protease. Studies of normal radiolabeled vWf incubated with platelet lysates from normal subjects and these patients revealed that the patients' platelets did not contain increased amounts of calcium-dependent protease activity as assessed by degradation of normal vWf. These data suggest that patients with type IIa vWd synthesize an abnormal vWf protein that is susceptible to in vitro proteolytic degradation and that proteolytic degradation can play a significant role in the phenotypic expression of vWd by modifying the plasma and platelet vWf multimeric structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corash L., Shafer B., Perlow M. Heterogeneity of human whole blood platelet subpopulations. II. Use of a subhuman primate model to analyze the relationship between density and platelet age. Blood. 1978 Oct;52(4):726–734. [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Enayat M. S., Hill F. G. Analysis of the complexity of the multimeric structure of factor VIII related antigen/von Willebrand protein using a modified electrophoretic technique. J Clin Pathol. 1983 Aug;36(8):915–919. doi: 10.1136/jcp.36.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Stimulus-induced activation of the calcium-dependent protease within platelets. Cell Motil. 1983;3(5-6):579–588. doi: 10.1002/cm.970030524. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Phillips D. R. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- Furlan M., Beck E. A. Cross-linking of human fibrinogen with glutaraldehyde and tetranitromethane. Thromb Res. 1975 Dec;7(6):827–838. doi: 10.1016/0049-3848(75)90086-9. [DOI] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., Morisato D. K. Effect of multimeric structure of the factor VIII/von Willebrand factor protein on binding to platelets. Blood. 1981 Aug;58(2):387–397. [PubMed] [Google Scholar]
- Hathaway D. R., Werth D. K., Haeberle J. R. Limited autolysis reduces the Ca2+ requirement of a smooth muscle Ca2+-activated protease. J Biol Chem. 1982 Aug 10;257(15):9072–9077. [PubMed] [Google Scholar]
- Kinoshita S., Harrison J., Lazerson J., Abildgaard C. F. A new variant of dominant type II von Willebrand's disease with aberrant multimeric pattern of factor VIII-related antigen (type IID). Blood. 1984 Jun;63(6):1369–1371. [PubMed] [Google Scholar]
- Kunicki T. J., Montgomery R. R., Schullek J. Cleavage of human von Willebrand factor by platelet calcium-activated protease. Blood. 1985 Feb;65(2):352–356. [PubMed] [Google Scholar]
- Kunicki T. J., Mosesson M. W., Pidard D. Cleavage of fibrinogen by human platelet calcium-activated protease. Thromb Res. 1984 Jul 15;35(2):169–182. doi: 10.1016/0049-3848(84)90212-3. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium. FEBS Lett. 1980 Jan 1;109(1):129–133. doi: 10.1016/0014-5793(80)81326-3. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Lombardi R., Federici A. B., Zimmerman T. S. Multimeric composition of factor VIII/von Willebrand factor following administration of DDAVP: implications for pathophysiology and therapy of von Willebrand's disease subtypes. Blood. 1982 Jun;59(6):1272–1278. [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Lombardi R., Federici A. B., Zimmerman T. S. Multimeric composition of factor VIII/von Willebrand factor following administration of DDAVP: implications for pathophysiology and therapy of von Willebrand's disease subtypes. Blood. 1982 Jun;59(6):1272–1278. [PubMed] [Google Scholar]
- Ruggeri Z. M., Nilsson I. M., Lombardi R., Holmberg L., Zimmerman T. S. Aberrant multimeric structure of von Willebrand factor in a new variant of von Willebrand's disease (type IIC). J Clin Invest. 1982 Nov;70(5):1124–1127. doi: 10.1172/JCI110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Pareti F. I., Mannucci P. M., Ciavarella N., Zimmerman T. S. Heightened interaction between platelets and factor VIII/von Willebrand factor in a new subtype of von Willebrand's disease. N Engl J Med. 1980 May 8;302(19):1047–1051. doi: 10.1056/NEJM198005083021902. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan Y., Simeon J., Caen J. P. Electrophoretic heterogeneity of normal factor VIII/Von Willebrand protein, and abnormal electrophoretic mobility in patients with Von Willebrand's disease. J Lab Clin Med. 1976 Feb;87(2):185–194. [PubMed] [Google Scholar]
- Takano E., Murachi T. Purification and some properties of human erythrocyte calpastatin. J Biochem. 1982 Dec;92(6):2021–2028. doi: 10.1093/oxfordjournals.jbchem.a134134. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Pietu G., Rabinowitz R., Girma J. P., Rogers J., Meyer D. Heterogeneous abnormalities in the multimeric structure, antigenic properties, and plasma-platelet content of factor VIII/von Willebrand factor in subtypes of classic (type I) and variant (type IIA) von Willebrand's disease. J Lab Clin Med. 1983 Mar;101(3):411–425. [PubMed] [Google Scholar]