Abstract
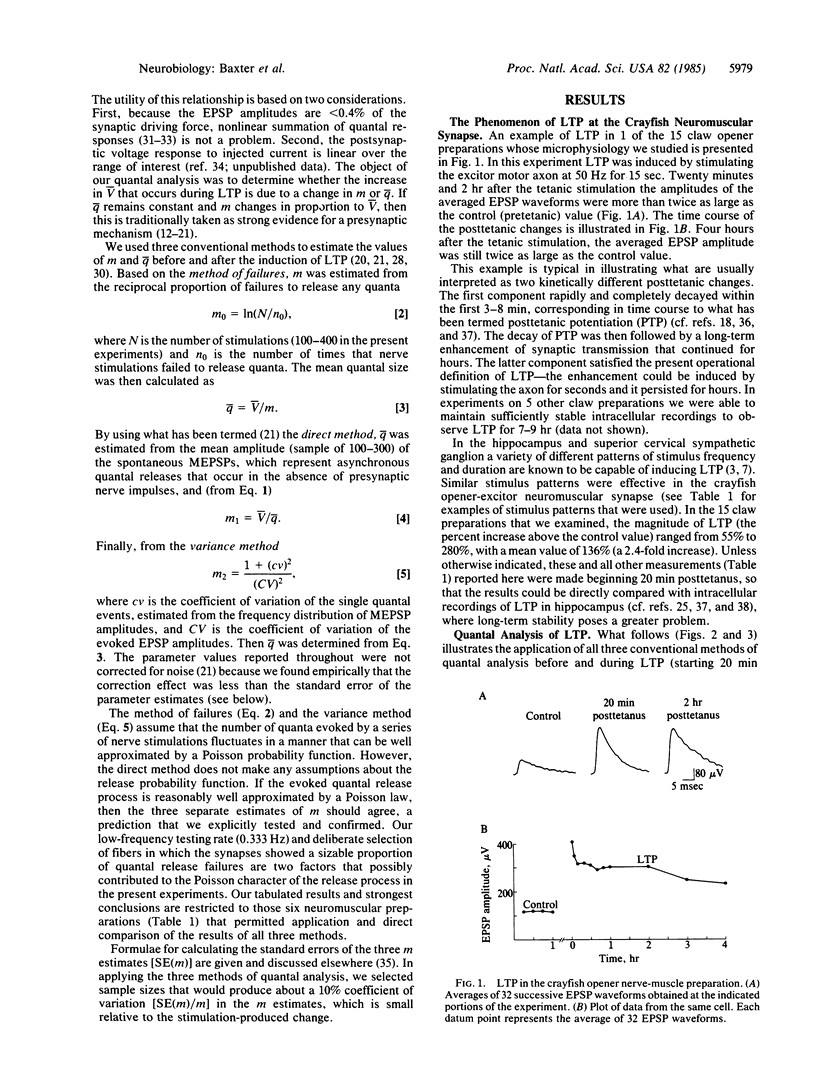
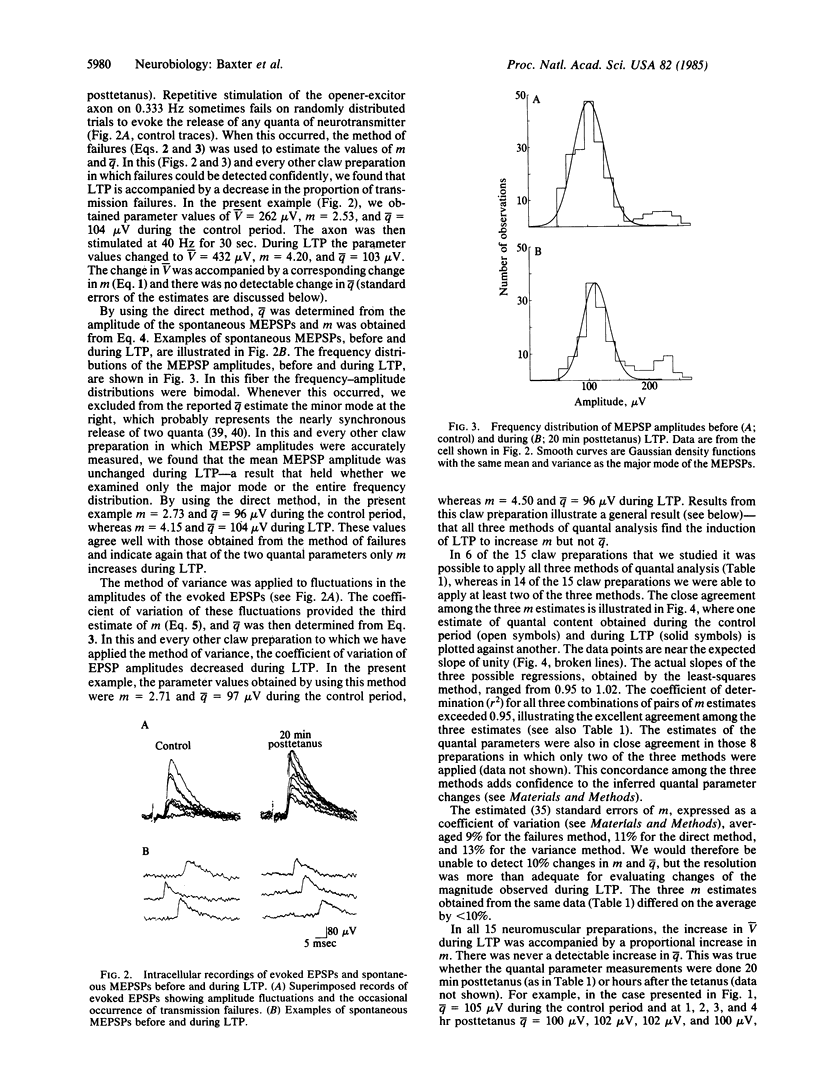
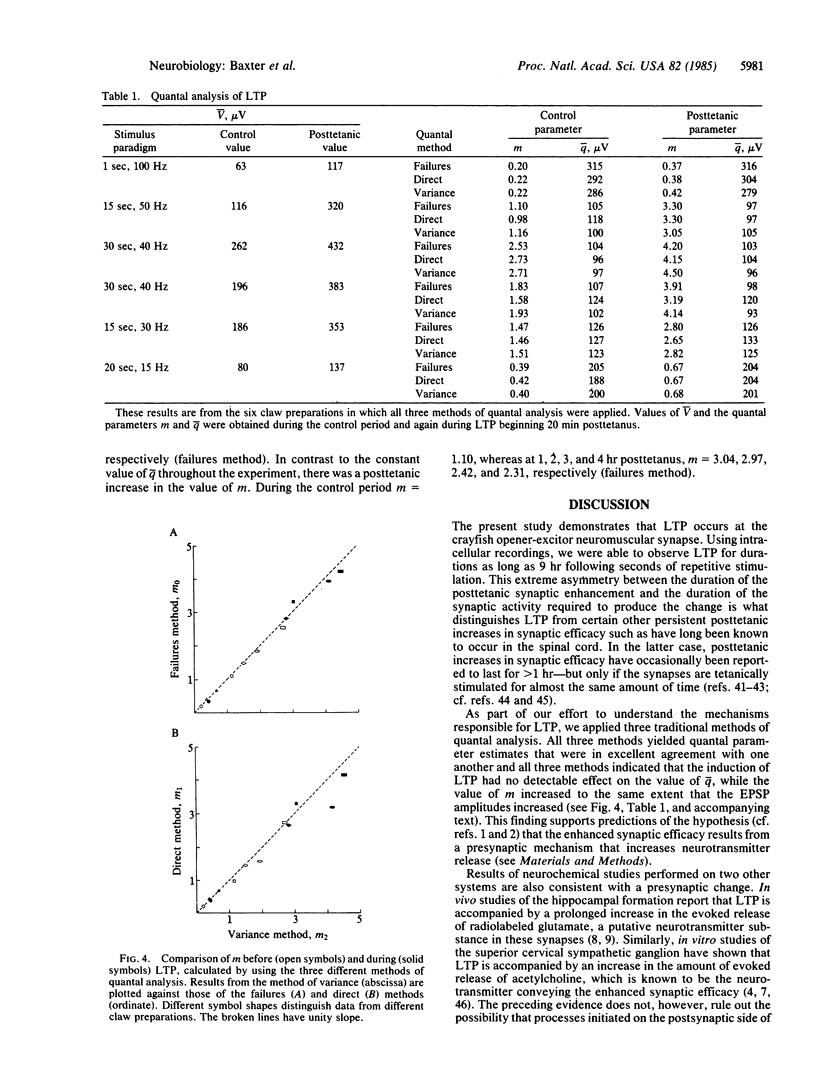
Intracellular recordings were used to demonstrate the occurrence and to analyze the microphysiology of long-term synaptic potentiation (LTP) in the crayfish opener neuromuscular synapse. Brief stimulation of the single excitor motor axon enhanced the amplitudes of subsequent postsynaptic potentials for several hours. Three methods of quantal analysis were used to evaluate the mechanism responsible for LTP. The results of all three methods supported predictions of the hypothesis that LTP results from a presynaptic mechanism that increases the average of neurotransmitter quanta evoked by nerve impulses in the excitor axon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrionuevo G., Brown T. H. Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick F. B., Conroy R. T. Optimal tetanic conditioning of heteronymous monosynaptic reflexes. J Physiol. 1965 Sep;180(1):134–146. [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D. Differentiation of nerve terminals in the crayfish opener muscle and its functional significance. J Gen Physiol. 1968 Jun;51(6):731–758. doi: 10.1085/jgp.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term potentiation of synaptic acetylcholine release in the superior cervical ganglion of the rat. J Physiol. 1985 Jun;363:181–190. doi: 10.1113/jphysiol.1985.sp015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., McAfee D. A. Long-term synaptic potentiation in the superior cervical ganglion. Science. 1982 Mar 12;215(4538):1411–1413. doi: 10.1126/science.6278593. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Perkel D. H., Feldman M. W. Evoked neurotransmitter release: statistical effects of nonuniformity and nonstationarity. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2913–2917. doi: 10.1073/pnas.73.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:530–542. doi: 10.1113/jphysiol.1961.sp006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Spencer W. A. Cellular neurophysiological approaches in the study of learning. Physiol Rev. 1968 Jan;48(1):65–134. doi: 10.1152/physrev.1968.48.1.65. [DOI] [PubMed] [Google Scholar]
- Lynch G., Kessler M., Halpain S., Baudry M. Biochemical effects of high-frequency synaptic activity studied with in vitro slices. Fed Proc. 1983 Sep;42(12):2886–2890. [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Martin A. R. The effect of membrane capacitance on non-linear summation of synaptic potentials. J Theor Biol. 1976 Jun;59(1):179–187. doi: 10.1016/s0022-5193(76)80031-8. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The statistics of transmitter release at chemical synapses. Int Rev Physiol. 1978;17:49–117. [PubMed] [Google Scholar]
- Sherman R. G., Atwood H. L. Synaptic facilitation: long-term neuromuscular facilitation in crustaceans. Science. 1971 Mar 26;171(3977):1248–1250. doi: 10.1126/science.171.3977.1248. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Malthe-Sørenssen D. Increased resting and evoked release of transmitter following repetitive electrical tetanization in hippocampus: a biochemical correlate to long-lasting synaptic potentiation. Brain Res. 1981 Mar 16;208(2):436–441. doi: 10.1016/0006-8993(81)90573-4. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. A comment on Martin's relation. Biophys J. 1976 Aug;16(8):891–895. doi: 10.1016/S0006-3495(76)85739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenarchuk L. E., Atwood H. L. Long-term synaptic facilitation with minimal calcium entry. Brain Res. 1975 Dec 12;100(1):205–208. doi: 10.1016/0006-8993(75)90261-9. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N. Transmitter release from insect excitatory motor nerve terminals. J Physiol. 1972 Dec;227(2):527–551. doi: 10.1113/jphysiol.1972.sp010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci. 1985 Mar;5(3):662–672. doi: 10.1523/JNEUROSCI.05-03-00662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L., Horn J. P., McAfee D. A., Yarowsky P. J. Facilitation, augmentation, and potentiation of synaptic transmission at the superior cervical ganglion of the rabbit. J Gen Physiol. 1980 Aug;76(2):213–231. doi: 10.1085/jgp.76.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972 Sep;35(5):621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]


