Abstract
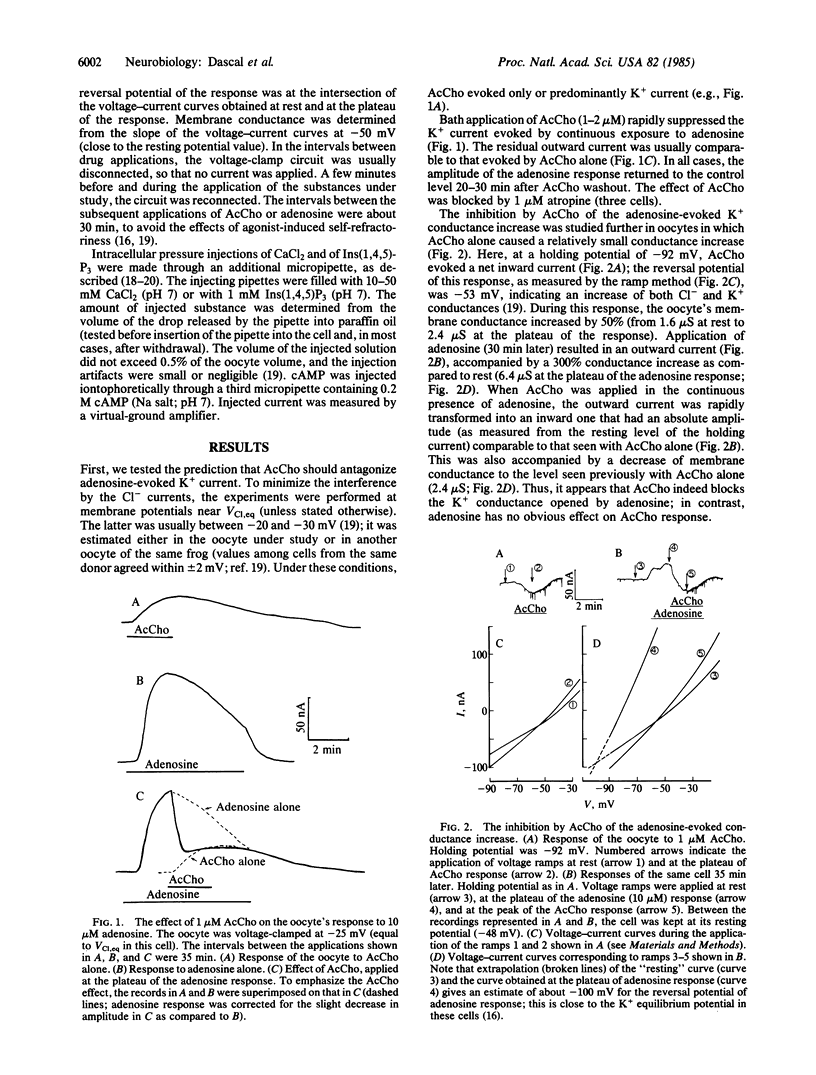
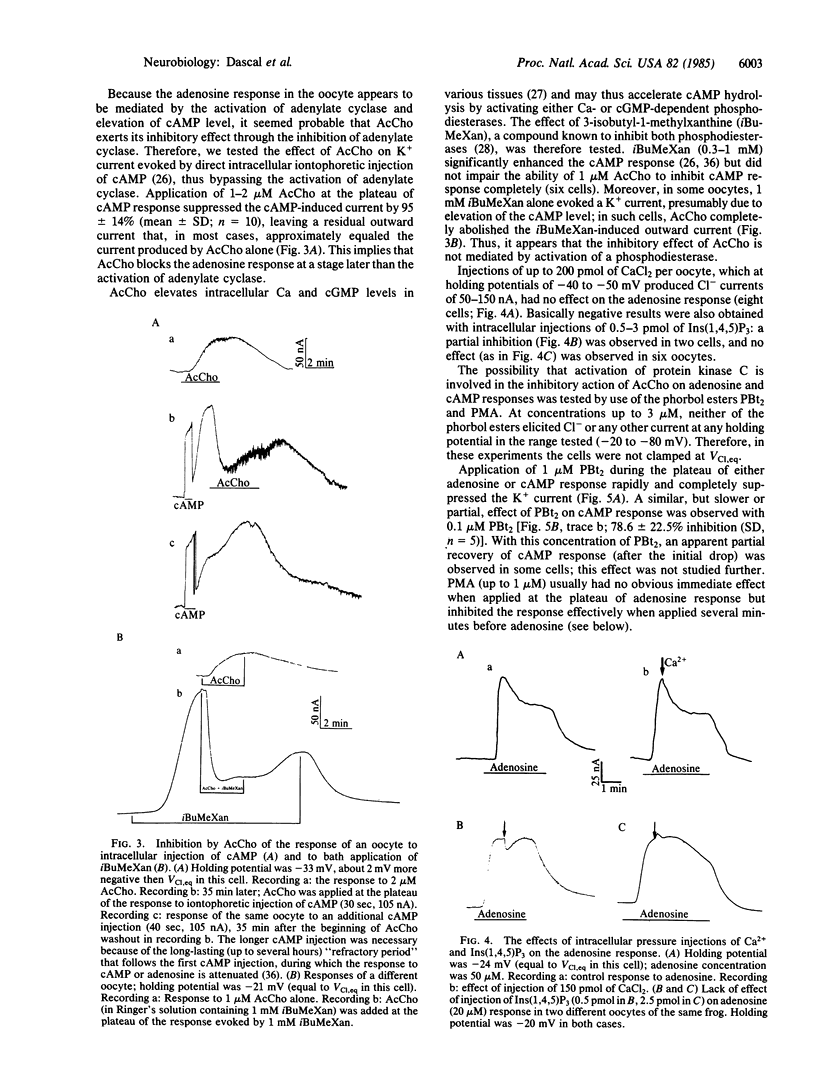
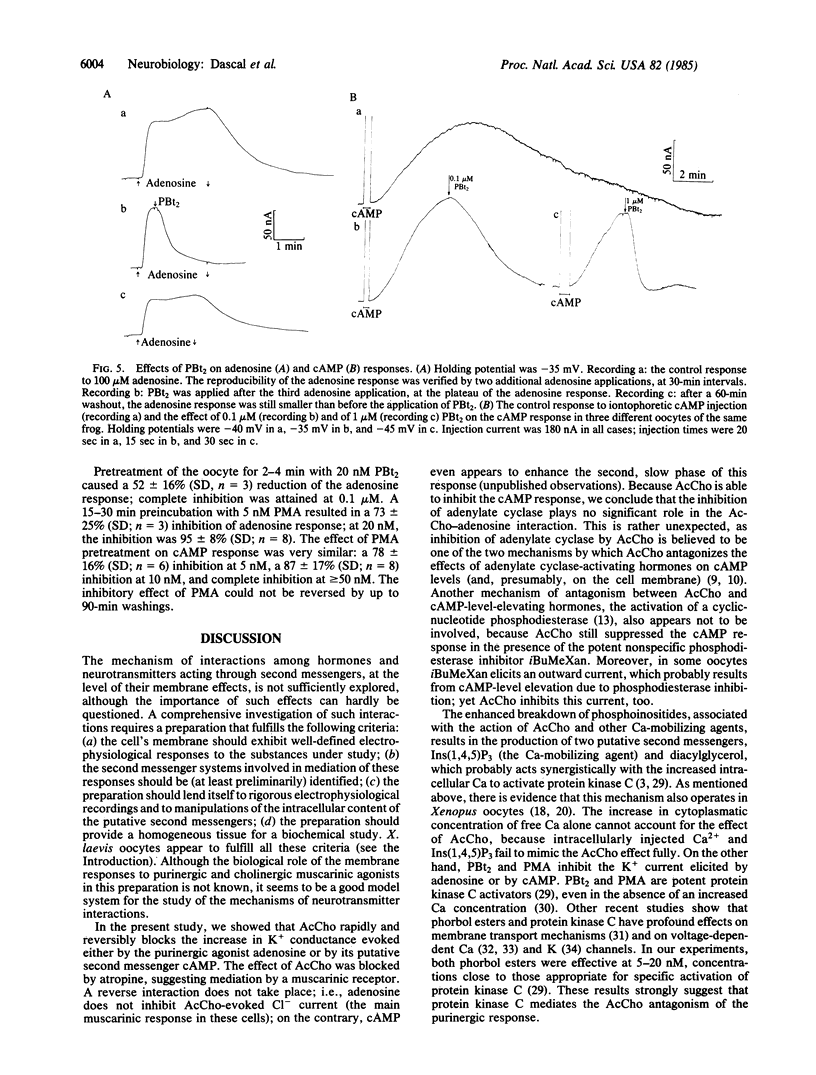
In Xenopus laevis oocytes, adenosine and other purinergic agonists induce a K+-conductance increase that is fully mimicked by intracellular application of cAMP. Acetylcholine suppresses the K+-conductance increase caused by adenosine, by the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine, or by intracellular injection of cAMP. This effect of acetylcholine is not mimicked by intracellular injection of Ca2+ or of the Ca-mobilizing agent inositol 1,4,5-trisphosphate. However, adenosine and cAMP responses are inhibited by 4 beta-phorbol 12,13-dibutyrate and 4 beta-phorbol 12-myristate 13-acetate. These results suggest that, in Xenopus oocytes, the muscarinic inhibition of purinergic and cAMP responses is mediated through the activation of the phospholipid-dependent, Ca-activated protein kinase (protein kinase C).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Hansen R. S., Harrison S. A., Hurwitz R. L., Martins T. J., Mumby M. C. Identification and properties of cyclic nucleotide phosphodiesterases. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):387–410. doi: 10.1016/0303-7207(82)90135-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cooper D. M. Bimodal regulation of adenylate cyclase. FEBS Lett. 1982 Feb 22;138(2):157–163. doi: 10.1016/0014-5793(82)80431-6. [DOI] [PubMed] [Google Scholar]
- Dascal N., Landau E. M., Lass Y. Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3',5'-cyclic monophosphate. J Physiol. 1984 Jul;352:551–574. doi: 10.1113/jphysiol.1984.sp015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M. Types of muscarinic response in Xenopus oocytes. Life Sci. 1980 Oct 13;27(15):1423–1428. doi: 10.1016/0024-3205(80)90407-5. [DOI] [PubMed] [Google Scholar]
- DeRiemer S. A., Strong J. A., Albert K. A., Greengard P., Kaczmarek L. K. Enhancement of calcium current in Aplysia neurones by phorbol ester and protein kinase C. Nature. 1985 Jan 24;313(6000):313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Mechanisms of slow postsynaptic potentials. Nature. 1981 Jun 18;291(5816):539–544. doi: 10.1038/291539a0. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Schatzman R. C., Turner R. S., Mazzei G. J. Phospholipid-sensitive Ca2+-dependent protein kinase: a major protein phosphorylation system. Mol Cell Endocrinol. 1984 May;35(2-3):65–73. doi: 10.1016/0303-7207(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Role of cyclic nucleotides in excitable cells. Annu Rev Physiol. 1980;42:629–641. doi: 10.1146/annurev.ph.42.030180.003213. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Cohen S., Lass Y. Adenosine-induced slow ionic currents in the Xenopus oocyte. Nature. 1982 Aug 5;298(5874):572–574. doi: 10.1038/298572a0. [DOI] [PubMed] [Google Scholar]
- Meeker R. B., Harden T. K. Muscarinic cholinergic receptor-mediated activation of phosphodiesterase. Mol Pharmacol. 1982 Sep;22(2):310–319. [PubMed] [Google Scholar]
- Miot F., Dumont J. E., Erneux C. The involvement of a calmodulin-dependent phosphodiesterase in the negative control of carbamylcholine on cyclic AMP levels in dog thyroid slices. FEBS Lett. 1983 Jan 24;151(2):273–276. doi: 10.1016/0014-5793(83)80085-4. [DOI] [PubMed] [Google Scholar]
- Miot F., Erneux C. Characterization of the soluble cyclic nucleotide phosphodiesterases in Xenopus laevis oocytes. Evidence for a calmodulin-dependent enzyme. Biochim Biophys Acta. 1982 Feb 18;701(2):253–259. doi: 10.1016/0167-4838(82)90121-2. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Nargeot J., Nerbonne J. M., Engels J., Lester H. A. Time course of the increase in the myocardial slow inward current after a photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2395–2399. doi: 10.1073/pnas.80.8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecek G. M., Honeyman T. W. The role of cyclic nucleotide phosphodiesterase in the inhibition of cyclic AMP accumulation by carbachol and phosphatidate. J Cyclic Nucleotide Res. 1982;8(6):395–408. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Pace C. S. Influence of a tumor-promoting phorbol ester on the electrical response of B-cells to glucose and glyburide. Mol Pharmacol. 1984 Sep;26(2):267–271. [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Richelson E., El-Fakahany E. The molecular basis of neurotransmission at the muscarinic receptor. Biochem Pharmacol. 1981 Nov 1;30(21):2887–2891. doi: 10.1016/0006-2952(81)90248-3. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L., Cooper D. M. Progesterone inhibition of Xenopus oocyte adenylate cyclase is not mediated via the Bordetella pertussis toxin substrate. Mol Pharmacol. 1984 Nov;26(3):526–531. [PubMed] [Google Scholar]


