Abstract
PLAC8 motif-containing proteins form a large family and members can be found in fungi, algae, higher plants and animals. They include the PCR proteins of plants. The name giving PLAC8 domain was originally found in a protein residing in the spongiotrophoblast layer of the placenta of mammals. A further motif found in a large number of these proteins including several PCR proteins is the CCXXXXCPC or CLXXXXCPC motif. Despite their wide distribution our knowledge about the function of these proteins is very limited. For most of them two membranespanning α-helices are predicted, indicating that they are membrane associated or membrane intrinsic proteins. In plants PLAC8 motif-containing proteins have been described to be implicated in two very different functions. On one hand, it has been shown that they are involved in the determination of fruit size and cell number. On the other hand, two members of this family, AtPCR1 and AtPCR2 play an important role in transport of heavy metals such as cadmium or zinc. Transport experiments and approaches to model the 3_D structure of these proteins indicate that they could act as transporters for these divalent cations by forming homomultimers. In this minireview we discuss the present knowledge about this protein family and try to give an outlook on how to integrate the different proposed functions into a common picture about the role of PLAC8 motif-containing proteins.
Keywords: fruit size, heavy metal, mechanosensitive, PLAC8, transport
INTRODUCTION
Plants, as all living organisms, depend on heavy metals such as iron, zinc and copper as nutrients (Krämer and Clemens, 2006). These elements are cofactors of a multitude of enzymes and transcription factors and required for their activity. It has been estimated that in Arabidopsis thaliana about 1230 proteins bind to or transport zinc (Krämer and Clemens, 2006; Wintz et al., 2003). The concentration of these heavy metals has to be well adjusted within a cell. Deficiency of heavy metals leads to impaired plant growth, chlorotic phenotypes and early senescence. In contrast, if the metals are present in high amounts in the environment, they can be potentially toxic and impair plant growth and development (Marschner, 1995). Besides the essential heavy metals, non-essential heavy metals such as cadmium and lead are present in the environment. Cd2+, which has massively increased in the environment since the beginning of industrialization, is highly toxic and is one of the major pollutants worldwide. The toxicity of Cd2+ is due to its high reactivity with sulfhydryl groups, which leads to an inactivation of enzymes, and its interaction with binding sites of micronutrients, where Cd2+ may displace e.g. Zn2+ (Clemens, 2001; Cobbett and Goldsbrough, 2002). Only in a marine diatom cadmium has been described to act as an essential micronutrient (Lane et al., 2005). Non-essential heavy metals are taken up by transporters that cannot discriminate efficiently between an essential and a non-essential heavy metal. For instance IRT1, the main iron uptake transporter localized at the root plasma membrane, is also the main entry point for cadmium (Korshunova et al., 1999). Once taken up by the root, heavy metals are transported to the vascular tissue, loaded to the xylem and translocated to the shoot, where they have to be taken up in the different shoot organs. Also in these steps too, transporters that cannot completely discriminate between essential and purely toxic heavy metals are implicated in the different transport processes (Clemens et al., 2002; Hussain et al., 2004).
In order to reduce the toxicity of an excess of essential heavy metals and of non-essential heavy metals, plants have evolved mechanisms to cope with heavy metal excess. Detoxifying mechanisms are mainly based on the export of heavy metals from metabolically active sites to compartments with a low metabolic activity such as the apoplast or the vacuole, combined with the complexation of the heavy metals in these compartments. Well-described chelating agents are metallothioneins, glutathione, phytochelatins, organic acids and amino acids (Cobbett and Goldsbrough, 2002; Hall, 2002). At the root level, toxic heavy metals may be exported to the soil. Different types of transporters have been shown to be involved in heavy metal resistance at the root level but also in the shoot. AtPCR1 and AtPCR2, members of the PCR protein family in Arabidopsis thaliana, extrude Cd or Zn from the cells contributing to the cellular detoxification of these heavy metals (Song et al., 2004; 2010). CAXs and some MTP transporters catalyze the transport of heavy metals across the vacuolar membrane and are essential for vacuolar sequestration. Increasing the expression of the tonoplast-localized MTP1 can enhance Zn resistance (Blaudez et al., 2003; Gustin et al., 2009; van der Zaal et al., 1999). In yeast, ATP-binding cassette (ABC) transporters sequester heavy metals into vacuoles. ScYCF1 is an ABC transporter of Saccharomyces cerevisae that contributes to Cd(II) resistance by pumping glutathione-conjugated cadmium(II) into the vacuole (Li et al., 1997), and SpHMT1, a half-size ABC transporter from Schizosaccharomyces pombe, contributes to cadmium resistance by transporting phytochelatin-Cd complexes into the vacuole (Ortiz et al., 1995).
Finding factors responsible for heavy metal detoxification will allow understanding how plants can survive when being exposed to high amounts of heavy metals and will impact on agriculture as well as human nutrition. As mentioned above, excess of heavy metals can impair plant growth, hence improving tolerance against heavy metals might increase plant fitness and productivity. Humans are at the end of the food chain, it is therefore important that the primary source of food, plants, are not contaminated with toxic amounts of heavy metals. Understanding heavy metal detoxification mechanisms may therefore allow searching for plants or generating plants, which take up less heavy metals or translocate less heavy metals to the edible parts of a plant.
Identification and properties of PCRs
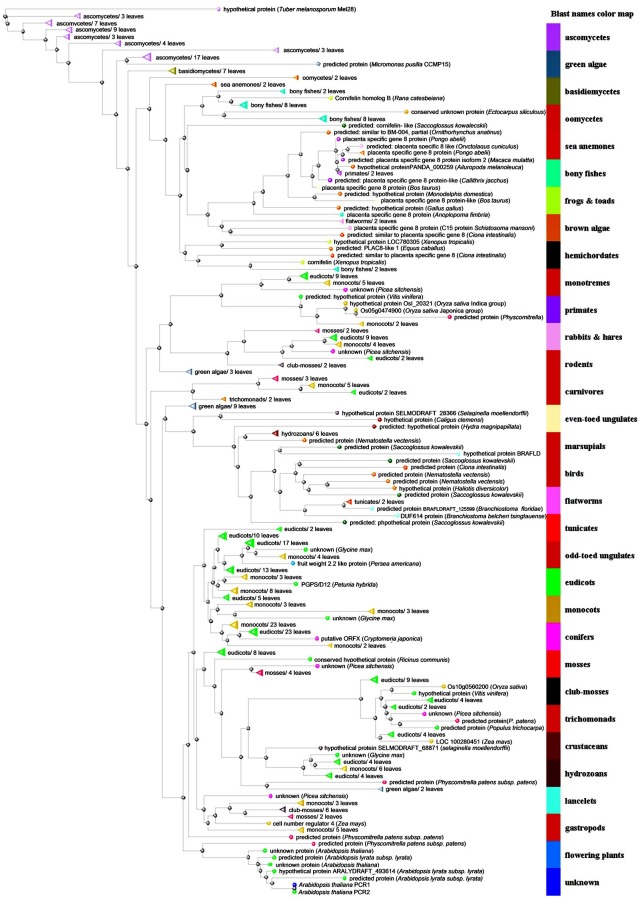
In a screen using the cadmium sensitive yeast strain ycf1, Song et al. (2004) identified a new protein conferring cadmium tolerance to these yeast cells. They called this gene Arabidopsis thaliana Plant Cadmium Resistance 1(AtPCR1). AtPCR1 is a small membrane protein, which according to ConPred (Arai et al., 2004) is constituted by two membrane-spanning α-helices. Several other prediction programs predict only one membranespanning domain for AtPCR1, but since the same programs predict two α-helices for the closest homologues AtPCR2 and AtPCR3, it is most likely that AtPCR1, AtPCR2 and AtPCR3 are membrane proteins constituted by two membrane-spanning α-helices. The members of the PCR protein family contain the common PLAC8 domain, which was originally found in the PCR homologue of the spongiotrophoblast layer of the placenta of mammals (Galaviz-Hernandez et al., 2003). However, so far the function of this domain is unknown. PCR genes belong to a huge gene family and a large number of members can be found in fungi, algae, higher plants and animals. These proteins can be divided into several clades (Fig. 1) and, interestingly, their functions appear to be quite diverse. On one side this class of proteins has been associated with heavy metal transport and resistance, on the other hand with fruit and plant size.
Fig. 1. Phylogenetic analysis of Arabidopsis PCRs and PLAC8 motif-containing proteins from other taxa.
In tomato the fruit weight 2.2 (fw2.2) has been identified as a quantitative trait locus, which is responsible for about 30% of the fruit size (Alpert and Tanksley, 1996; Frary et al., 2000). When ORFX, the corresponding gene, was introduced into a cultivar producing large fruits, tomato fruit size decreased. ORFX is expressed early during flower development and controls carpel cell number. Very recently, it has been shown that a close homologue of ORFX from maize exhibits a similar function. When ectopically expressed in maize, CNR1 decreased the overall plant size. In contrast, plant size increased when CNR1 was suppressed by RNAi (Guo et al., 2010). Another member of the PLAC8 domain protein, family, MCA1, either constitutes or is part of the regulatory pathway for a mechanosensitive calcium influx channel (Nakagawa et al., 2007). In the green alga Chlamydomonas, AGG2 and AGG3, flagellar membrane proteins, belonging to the plant cluster with PLAC8 domain and having 31% identity and 53% similarity with Arabidopsis PCR1, mediate the orientation of cell movement to light (Iomini et al., 2006).
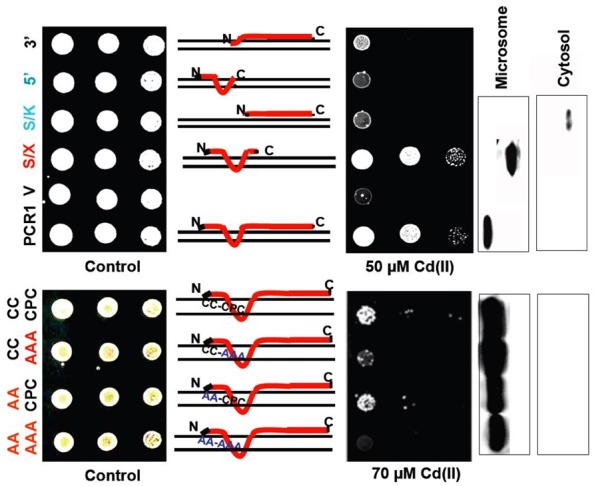
PLAC8 motif-containing proteins range in size from 108 to 557 amino acids, with the majority being relatively short and smaller than 200 amino acids. The animal proteins range from 108 to 144 amino acids and the fungal members from 148 to 157 (Guo et al., 2010). The proteins are rather Cys and Pro rich and the proportion of these amino acids accounts usually for approximately 15% of the total amino acids. Proteins with larger sizes are usually derived from hydrophilic N-terminal extensions, but in some cases a C-terminal extension can also be observed. AtPCR1 and AtPCR2 exhibit 80% identity at the amino acid level and 36% identity to OsPCR1 of rice. Expression of five out of twelve Arabidopsis thaliana PCRs and the closest AtPCR1 homologue of rice in yeast, revealed that AtPCR1, AtPCR2 and OsPCR1 conferred strong cadmium resistance, AtPCR9 and AtPCR10 an intermediate one while AtPCR8 did not at all restore cadmium tolerance in this yeast mutant (Song et al., 2004). Interestingly cadmium resistance could be conferred by expressing only the N-terminal hydrophobic part of PCR1, which contains a conserved CCXXXXCPC motif. This motif is not only found in AtPCR1-3 but also in OsPCR1 and in AtPCR9 and 11. Interestingly, this motif is predicated to reside in the transmembrane region. A similar motif, CLXXXXCPC, is present also in the maize CNR1 and FW2.2 homologues. When expressed in yeast, mutational analysis of AtPCR1 indicates that the CysCys motif at the beginning of the first membrane spanning α-helix is less important to confer cadmium resistance compared to the CPC motif situated later in this α-helix (Song et al., 2004). However, interestingly, a residual cadmium tolerance was also observed when CPC was mutated to AAA, only the complete exchange of all cysteins resulted in cadmium sensitivity comparable to the empty vector control (Fig. 2). AtPCR8 is the only Arabidopsis PCR tested so far that contains an aberrant CPC (VPC) and misses the CC motif. This correlates with its incapacity to confer cadmium resistance when expressed in the ycf1 yeast strain. The fact that AtPCR9 and AtPCR10 are much less efficient in rendering yeast cells cadmium tolerant indicates that additional properties are required to act efficiently in cadmium detoxification. The maize CNR2 has a CCXXXXCPC motif, while ZmCNR1 as well as the tomato LeORFX and 2 contain the CLXXXXCPC motif.
Fig. 2. Identification of domains and amino acids of AtPCR1 required to confer cadmium resistance when expressed in the cadmium sensitive yeast strain ycf1 (Song et al., 2004).
It will be interesting to investigate whether expression of these genes in ycf1 results in cadmium resistance. In case it does, the question will arise, whether fruit and plant size depend on the transport of a heavy metal or whether these proteins exhibit a dual function. In contrast, MCA1 contains a divergent motif, CFXXXXFPC, which may be an additional hint that an intact CPC motif is required for heavy metal transport. MCA1 has a long amino-terminal domain. This domain bears an EF-hand-like motif and is similar to the Amino-terminal domain of Rice Putative protein Kinase (ARPK) domain found in many putative protein kinases from rice.
The role of PCRs in heavy metal and calcium transport in plants
As mentioned above, AtPCR1 and AtPCR2 confer cadmium resistance when expressed in yeast. Using GFP fusion proteins both have been localized to the plasma membrane (Song et al., 2004; 2010). Plants overexpressing AtPCR1 under the control of the 35S promoter showed enhanced cadmium resistance and AtPCR1 and AtPCR2 co-silencing plants exhibited impaired cadmium tolerance (Song et al., 2004). Microarray results on the tissue-specific expression of AtPCR1 and 2 are not reliable, since the sequence used for the microarray analysis present in the public databases does not distinguish between the two. Promoter-reporter gene constructs revealed that AtPCR1 is expressed in stems, leaves, flowers and siliques but not in roots (Song et al., 2010). In young leaves it is exclusively expressed in trichomes, while in older leaves, AtPCR1 is expressed in all cells, although the strongest signal is still present in trichomes. In contrast, AtPCR2 is strongly expressed in roots. A weaker but still pronounced expression can be found in veins of leaves, stems, flowers, pollen and siliques. At the root level, AtPCR2 exhibits a complex expression pattern. It is expressed in the root tip and in the vasculature of very young rootlets. In the root hair zone, AtPCR2 is expressed in epidermal cells. As explained below, this expression pattern has a specific implication on the function of AtPCR2.
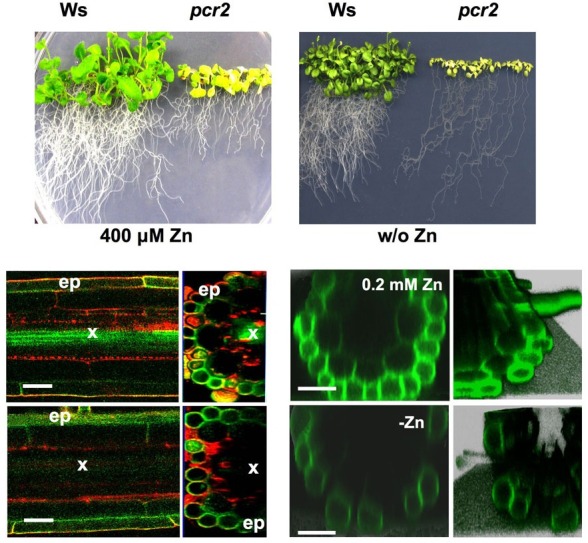
In order to identify which essential heavy metals could be taken up by plants through AtPCR2, Song et al. (2010) exposed Atpcr2 loss-of-function plants to different essential and non-essential heavy metals. In the presence of zinc, copper and cadmium, the growth of mutant plants was strongly impaired. A weaker effect could also be observed for iron. This result suggested that AtPCR2 is implicated in zinc tolerance. Further studies showed that the root-to-shoot ratio was altered in the atpcr2 knock out mutant. Loss-of-function mutants for AtPCR2 transferred less zinc to the shoot but more zinc was retained in the roots and showed impaired-growth under the zinc deficient conditions. This result can be explained by the dual localization of AtPCR2 in the root. AtPCR2 localized in the vascular tissue is responsible for the radial transport of zinc to the xylem, while AtPCR2 localized in the epidermal cells is responsible to reduce zinc accumulation if it is present at potentially toxic levels in the medium surrounding the root (Fig. 3). In accordance with this hypothesis, wild-type plants transformed with AtPCR2 driven by its own promoter were more tolerant to both levels of zinc that are toxic for wild-type as well as in the presence of sparingly amounts of zinc. Experiments with Zinpyr- 1, a fluorescent dye allowing the determination of zinc within a plant, also confirmed this hypothesis, since plants grown under zinc-deficient conditions exhibited a strongly reduced fluorescence in the vascular tissue close to the root tip (Fig. 3).
Fig. 3. AtPCR2 is required under zinc excess and limiting conditions. Phenotypes of the AtPCR2 loss-of-function mutant (pcr2) grown under excess zinc (A) and zinc limiting conditions (B). The dual role is due to the expression of AtPCR2 in the xylem in the root tip (C) and in the epidermis (ep) in the root hair zone (D). Under zinc excessive conditions AtPCR2-GFP is present in high amounts in the epidermis (E) and plays an important role in zinc detoxification, while under zinc limiting conditions AtPCR2-GFP is present only at very low levels in the epidermis (F). ep, root epidermis; x, xylem.
In a large-scale approach to identify new genes that could confer tolerance against oxidative stress, Luhua et al. (2008) overexpressed 41 candidate genes in Arabidopsis thaliana, among them AtPCR2 and AtPCR8. In contrast to controls, AtPCR2-overexpressing plants exhibited a slightly enhanced tolerance in the presence of t-butyl hydroperoxide and peroxide, two compounds producing oxidative stress, but they did not show a difference when osmotic or salt stress was applied. Interestingly, AtPCR2 was induced by a factor of two in plants that were mutated in an apolipoprotein D orthologue; a loss-offunction mutation, which resulted in plants very sensitive to drops in temperature and in paraquat treatment (Charron et al., 2008).
In contrast to the PCRs, MCA1 is not a heavy metal transporter but is involved in Ca2+ influx in response to mechanical signals (Nakagawa et al., 2007). It was identified in a screen using the yeast mutant mid1, which lacks a stretch-activated calcium channel. It was shown in Arabidopsis thaliana that MCA1 increased cytosolic calcium concentration in response to plasma membrane distortion. Growth of MCA1 overexpressing plants was impaired under high calcium levels. Loss-of-function mutants failed to penetrate in solid medium with a higher agar concentration (1.6%), indicating that MCA1 is an important factor for root growth in soil.
Possible mechanism of PCRs
Due to its small size and the presence of only two membranespanning α-helices, the question arose, whether PCRs indeed could act as transporters or channels for heavy metals. An alternative possibility would be that PCRs are not transporters themselves but could act as modulators of transporters or as proteins delivering heavy metals to other transporters. Although a possible role of PCRs as modulators or metal delivering proteins cannot be excluded yet, there are several arguments against this hypothesis: i) yeast cells do not have PCRs, nevertheless PCRs are functional in this organism and it is unlikely that PCRs would interact with yeast proteins (Song et al., 2004; 2010); ii) It has been shown that AtPCR forms homooligomers and may thus be able to form functional transporters as shown for the prokaryotic Mg2+ transporter which is constituted by five identical subunits, each one having also only two α-helices; iii) triple knock-out plants in which, the two other transporters besides AtPCR2 essential for zinc transfer to the shoot, the P-type ATPases AtHMA2 and AtHMA4, were deleted exhibited an additive phenotype (Song et al., 2010). If AtPCR2 would deliver zinc to one of the HMAs the phenotype would not be additive; iv) the CCXXXXCPC motif is predicted to reside within the transmembrane region and is likely involved in the binding of divalent cations. The possibility that AtPCR1 or AtPCR2 could act as chelators delivering heavy metals to other transporters is unlikely. Transfer of a heavy metal from the pore of the PCR to a pore of the transporter would require that the two pores temporary form supracomplexes.
Experiments using yeast cells and plant protoplasts indicated that AtPCR1 and AtPCR2 act as exporters. It has been shown that AtPCR1 expressing yeast cells take up less and that protoplasts isolated from antisense plants take up more cadmium, compared to control cells (Song et al., 2004). Similar observations were made for AtPCR2 expressing yeast cells, which also took up less zinc (Song et al., 2010). These results indicate that AtPCR1 and AtPCR2 act as heavy metal exporters.
Several cases have been described where homo-oligomers of membrane proteins containing only two membrane-spanning domains form functional potassium channels (Bryan and Aguilar- Bryan, 1999; Gazzarrini et al., 2009; Voelker et al., 2006). Furthermore, the bacterial magnesium transporter, CorA, has also only two hydrophobic α-helices (Payandesh and Pai, 2006). Experiments with AtPCR2 indicate that PCRs also can form oligomers. Song et al. (2010) transformed yeast with two AtPCR2 constructs, containing different tags. It could be shown by immunoblots that the protein bands exhibiting a molecular weight higher than AtPCR2 indeed corresponded to homo-oligomers. Whether PCRs can form hetero-oligomers is presently unknown. The results published so far do not allow to exactly determine the oligomerization state of AtPCR2, and hence to deduce the exact oligomerization state corresponding to the putative functional transporter.
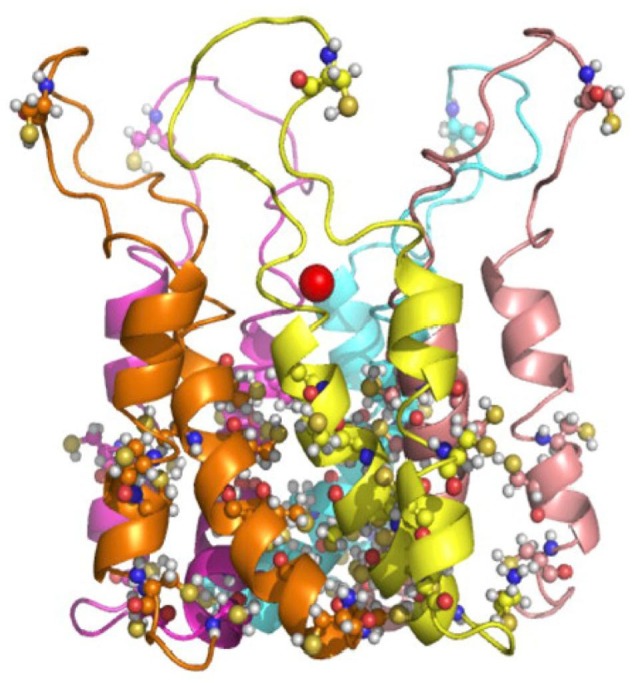
In order to get insight on the structure and function of AtPCR1, Guo et al. (2010) performed a modeling approach with CorA. As mentioned above, CorA is a Mg2+ transporter, which usually mediates Mg2+ uptake but in certain circumstances can also act as a Mg2+ exporter (Snavely et al., 1989). CorA has been crystallized and shown to form a homopentamer (Payandesh and Pai, 2006). Similar prokaryotic genes encode also Zn2+ efflux proteins (Worlock and Smith, 2002). Although these transporters are not phylogenetically related to PCRs, Guo et al. (2010) could create a model of AtPCR1 structure based on CorA. Since CorA and AtPCR1 are diverse proteins, this model is not highly reliable but shows the possibility that AtPCR1 could also form a pentamer to form a functional cation transporter or channel and indicates that a change of CC to CL in the CCXXXXCPC motif may affect substrate recognition (Fig. 4). Although all these results indicate that PCRs can form functional transporters, either transport experiments with proteoliposomes containing PCRs or structural studies are needed to confirm this hypothesis and to exclude that this class of proteins act as chaperons delivering divalent cations to a transporter.
Fig. 4. Modeling of the membrane spanning domains of AtPCR1 based on the structure of CorA. CorA does not show strong similarity to AtPCR1, but as AtPCR1 has only two membrane spanning α- helices. Structural analysis revealed that CorA forms homopentamers. Taken from Guo et al. (2010).
What could be the role of so far uncharacterized PCRs?
The Arabidopsis thaliana genome contains 12 PCR members. Rice also has 12 PCR genes, however, the distribution in the different clades is completely different and for instance there is no close homologue to the AtPCR1 clade in rice. The phylogenetic tree shown in Fig. 1 indicates that only limited overlap exists between PCRs encoded by dicotyledones and monocotyledons. AtPCR1, 2, 3, 9, 10 and 11 contain the CCXXXXCPC motif, which may be required for heavy metal or at least divalent cation transport. AtPCR7 has a ACXXXXCPC motif, while the remaining PCRs bear a XPC motif. In rice, seven PCRs contain the CCXXXXCPC motif and two a CL/FXXXXCPC motif. If the CCXXXXCPC motif indeed is an indicator for heavy metal or divalent cation transport, a targeted approach could reveal quite fast, which divalent cations are transported by the Arabidopsis or the rice PCRs. For AtPCR3, 7, and 9 microarray data are not available on Genevestigator (https://www.genevestigator.ethz. ch/) or ATTED (http://www.atted.bio.titech.ac.jp/). Due to its similarity to the well-described AtPCR1 and 2, AtPCR3 is expected to act as a heavy metal transporter. However, its expression is extremely low. In an attempt to clone AtPCR3 we realized that we could not detect transcripts in light-grown plants but only in seedlings grown in the dark, a result which corresponds to the fact that the only available AtPCR3 EST sequence comes from young seedlings grown in the dark. AtPCR10 is expressed at a quite low level overall in the plant, but highly in guard cells and the radicle. Furthermore, prediction of its cellular localization indicates it to localize to the chloroplast. If true, this could explain its weak effect on restoring heavy metal resistance to yeast (Song et al., 2004), since plastidic proteins are predominantly targeted to mitochondria in yeast. Heavy metal concentrations, such as Fe, Mg, Zn and Cu, have to be tightly controlled in chloroplasts as well and it will be interesting to investigate whether AtPCR10 is indeed involved in chloroplastic heavy metal homeostasis. AtPCR11, a close homologue of AtPCR3 and AtPCR10 is nearly exclusively expressed in pollen and growing pollen tubes. Due to this restricted expression it should be easy to see whether AtPCR11 plays an important role in pollen development and growth. AtPCR12 exhibits a similar expression pattern as AtPCR11, however, although being a close homologue to AtPCR9 it does not contain the CCXXXXCPC motif. This may indicate, that it does not transport divalent cations. This is probably also true for the Arabidopsis PCR4, 5, 6, 8. From this small clade only AtPCR8 is strongly expressed. Expression is highest in the radicle and in roots but pronounced expression is also observed in rosette leaves. In addition, AtPCR8 is strongly induced by pathogens such as Botrytis graminis or Pseudomonas syringae. Like AtPCR10 it is predicted to localize to the chloroplast. Correlation analysis with members of this clade did not reveal a putative function. We should, however, keep in mind that the mutational analysis of the CC motif of AtPCR1 resulted only in a slightly impaired cadmium tolerance when expressed in yeast. This observation indicates that although the CC and the CPC motifs maybe part of a substrate recognition site other structural elements are required to define the final substrate specificity.
CONCLUSIONS
PCRs belong to a large gene family, which is present in animals, fungi and plants. Plants contain by far the largest number of PCR-like proteins. Biochemical and modeling data suggest that PCRs can act as transporters, although they contain only two transmembrane domains. The function is supposed to be diverse, since members of this family have been shown to be important for the size of fruits and whole plants but also for heavy metal resistance and allocation. However, in the case of ORFX and the maize CNR1 and 2, the substrate for these membrane proteins has not been identified and it cannot be excluded that transport of a divalent cation at an early phase of development triggers a signal which affects cell numbers. This hypothesis is supported by the fact that ORFX as well as the maize CNRs contain the CPC motif required for heavy metal transport in AtPCR1 and 2. Since these transporters can be expressed in yeast it will be easy to test the possibility whether they are also cation transporters. In case they are, a more difficult task will be to find the link between cation transport and the regulation mechanisms defining the cell numbers. In contrast to the already described proteins, some PCR members do not contain the CPC domain. To identify their roles and substrates detailed analysis of the corresponding mutants will be a prerequisite. However, some of the members are closely related and may exhibit redundant functions. Therefore multiple knock-outs will probably be required to elucidate their function.
In summary, we are only at the beginning of understanding the functions of PCRs and more general PLAC8 domaincontaining membrane proteins. Further studies will reveal the mechanism of transport as well as their substrate specificities and show whether this class of membrane proteins has conserved common functions in the diverse organisms in which they are present.
References
- 1.Alpert K.B., Tanksley S.D. High-resolution mapping and isolation of a yeast artificial chromosome contig containing fw2.2: a major fruit weight quantitative trait locus in tomato. Proc. Natl. Acad. Sci. USA. (1996);93:15503–15507. doi: 10.1073/pnas.93.26.15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai M., Mitsuke H., Ikeda M., Xia J.X., Kikuchi T., Satake M., Shimizu T. ConPred II: a consensus prediction method for obtaining transmembrane topology models with high reliability. Nucleic Acids Res. (2004);32:W390–W393. doi: 10.1093/nar/gkh380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaudez D., Kohler A., Martin F., Sanders D., Chalot M. Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. Plant Cell. (2003);15:2911–2928. doi: 10.1105/tpc.017541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan J., Aguilar-Bryan L. Sulfonylurea receptors: ABC transporters that regulate ATP-sensitive K(+) channels. Biochim. Biophys. Acta. (1999);1461:285–303. doi: 10.1016/s0005-2736(99)00164-9. [DOI] [PubMed] [Google Scholar]
- 5.Charron J.B., Ouellet F., Houde M., Sarhan F. The plant apolipoprotein D ortholog protects Arabidopsis against oxidative stress. BMC Plant Biol. (2008);8:86. doi: 10.1186/1471-2229-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens S. Developing tools for phytoremediation: towards a molecular understanding of plant metal tolerance and accumulation. Int. J. Occup. Med. Environ. Health. (2001);14:235–239. [PubMed] [Google Scholar]
- 7.Clemens S., Palmgren M.G., Krämer U. A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci. (2002);7:309–315. doi: 10.1016/s1360-1385(02)02295-1. [DOI] [PubMed] [Google Scholar]
- 8.Cobbett C., Goldsbrough P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Ann. Rev. Plant Biol. (2002);53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 9.Davletova S., Rizhsky L., Liang H., Shengqiang Z., Oliver D.J., Coutu J., Shulaev V., Schlauch K., Mittler R. Cytosolic ascorbate peroxidase1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. (2005a);17:268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frary A., Nesbitt T.C., Grandillo S., Knaap E., Cong B., Liu J., Meller J., Elber R., Alpert K.B., Tanksley S.D. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science. (2000);289:85–88. doi: 10.1126/science.289.5476.85. [DOI] [PubMed] [Google Scholar]
- 11.Galaviz-Hernandez C., Stagg C., de Ridder G., Tanaka T.S., Ko M.S., Schlessinger D., Nagaraja R. Plac8 and Plac9, novel placental-enriched genes identified through microarray analysis. Gene. (2003);309:81–89. doi: 10.1016/s0378-1119(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 12.Gazzarrini S., Kang M., Abenavoli A., Romani G., Olivari C., Gaslini D., Ferrara G., van Etten J.L., Kreim M., et al. Chlorella virus ATCV-1 encodes a functional potassium channel of 82 amino acids. Biochem J. (2009);420:295–303. doi: 10.1042/BJ20090095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustin J.L., Loureiro M.E., Kim D., Na G., Tikhonova M., Salt D.E. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn hyperaccumulating plants. Plant J. (2009);57:1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- 14.Guo M., Rupe M.A., Dieter J.A., Zou J., Spielbauer D., Duncan K.E., Howard R.J., Hou Z., Simmons C.R. Cell Number Regulator1 affects plant and organ size in maize: implications for crop yield enhancement and heterosis. Plant Cell. (2010);22:1057–1073. doi: 10.1105/tpc.109.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall J.L. Cellular mechanisms for heavy metal detoxifycation and tolerance. J. Exp. Bot. (2002);53:1–11. [PubMed] [Google Scholar]
- 16.Hussain D., Haydon M.J., Wan Y., Wong E., Sherson S.M., Young J., Camakaris J., Harper J.F., Cobbett C.S. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell. (2004);16:1327–1339. doi: 10.1105/tpc.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iomini C., Li L., Mo W., Dutcher S.K., Piperno G. Two flagellar genes, AGG2 and AGG3, mediate orientation to light in Chlamydomonas. Curr. Biol. (2006);16:1147–1153. doi: 10.1016/j.cub.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Korshunova Y.O., Eide D., Clark W.G., Guerinot M.L., Pakrasi H.B. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. (1999);40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- 19.Krämer U., Clemens S. Functions and homeostasis of zinc, copper, and nickel in plants. In Molecular Biology of Metal Homeostasis and Detoxification from Microbes to Man, M.J. Tamás, and E. Martinoia, eds. Springer; Berlin, Germany: (2006). pp. 214–272. [Google Scholar]
- 20.Lane T.W., Saito M.A., George G.N., Pickering I.J., Prince R.C., Morel F.M. Biochemistry: a cadmium enzyme from a marine diatom. Nature. (2005);435:42. doi: 10.1038/435042a. [DOI] [PubMed] [Google Scholar]
- 21.Li Z.S., Lu Y.P., Zhen R.G., Szczypka M., Thiele D.J., Rea P.A. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. USA. (1997);94:42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luhua S., Ciftci-Yimaz S., Harpr J., Cushman J., Mittler R. Enhanced tolerance to oxidative stress in transgenic Arabidopsis plants expressing proteins of unknown function. Plant Physiol. (2008);148:280–292. doi: 10.1104/pp.108.124875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marschner H. Mineral Nutrition of Higher Plants. Academic Press; San Diego: (1995). [Google Scholar]
- 24.Nakagawa Y., Katagiri T., Shinozaki K., Qi Z., Tatsumi H., Furuichi T., Kishigami A., Sokabe M., Kojima I., Sato S., et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA. (2007);104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz D.F., Ruscitti T., McCue K.F., Ow D.W. Transport of metal-binding peptides by HMT1, a fission yeast ABCtype vacuolar membrane protein. J. Biol. Chem. (1995);270:4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- 26.Payandeh J., Pai E.F. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. EMBO J. (2006);25:3762–3773. doi: 10.1038/sj.emboj.7601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snavely M.D., Florer J.B., Miller C.G., Maguire M.E. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. (1989);171:4761–4766. doi: 10.1128/jb.171.9.4761-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song W.Y., Martinoia E., Lee J., Kim D., Kim D.Y., Vogt E., Shim D., Choi K.S., Hwang I., Lee Y. A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol. (2004);135:1027–1039. doi: 10.1104/pp.103.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song W.Y., Choi K.S., Kim D.Y., Geisler M., Park J., Vincenzetti V., Schellenberg M., Kim S.H.,, Lim Y.P., Noh E.W., et al. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell. (2010);22:2237–2252. doi: 10.1105/tpc.109.070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Zaal B.J., Neuteboom L.W., Pinas J.E., Chardonnens A.N., Schat H., Verkleij J.A.C., Hooykaas P.J.J. Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. (1999);119:1047–1056. doi: 10.1104/pp.119.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voelker C., Schmidt D., Mueller-Roeber B., Czempinski K. Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. (2006);48:296–306. doi: 10.1111/j.1365-313X.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- 32.Wintz H., Fox T., Wu Y.Y., Feng V., Chen W., Chang H.S., Zhu T., Vulpe C. Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J. Biol. Chem. (2003);278:47644–47653. doi: 10.1074/jbc.M309338200. [DOI] [PubMed] [Google Scholar]
- 33.Worlock A.J., Smith R.L. ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium. J. Bacteriol. (2002);184:4369–4373. doi: 10.1128/JB.184.16.4369-4373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]