Abstract
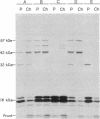
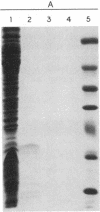
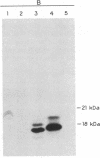
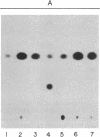
Pulse-chase experiments with [3H]tetradecanoic acid and ATP showed that the bioluminescence-related 32-kDa acyltransferase from Vibrio harveyi can specifically catalyze the deacylation of a 3H-labeled 18-kDa protein observed in extracts of this bacterium. The 18-kDa protein has been partially purified and its physical and chemical properties strongly indicate that it is fatty acyl-acyl carrier protein (acyl-ACP). Both this V. harveyi [3H]acylprotein and [3H]palmitoyl-ACP from Escherichia coli were substrates in vitro for either the V. harveyi 32-kDa acyltransferase or the analogous enzyme (“34K”) from Photobacterium phosphoreum. TLC analysis indicated that the hexane-soluble product of the reaction is fatty acid. Phosphate ions and, to a lesser extent, organic alcohols stimulated the rate of acyl-protein cleavage. No significant cleavage of either E. coli or V. harveyi tetradecanoyl-ACP was observed in extracts of these bacteria unless the 32-kDa or 34K acyltransferase was present. Since these enzymes are believed to be responsible for the supply of fatty acids for reduction to form the aldehyde substrate of luciferase, the above results suggest that long-chain acyl-ACP is the source of fatty acids for bioluminescence.
Keywords: tetradecanoic acid, acyltransferase, Vibrio harveyi, Photobacterium phosphoreum
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byers D., Meighen E. Purification and characterization of a bioluminescence-related fatty acyl esterase from Vibrio harveyi. J Biol Chem. 1985 Jun 10;260(11):6938–6944. [PubMed] [Google Scholar]
- Byers D., Meighen E. Vibrio harveyi aldehyde dehydrogenase. Partial reversal of aldehyde oxidation and its possible role in the reduction of fatty acids for the bioluminescence reaction. J Biol Chem. 1984 Jun 10;259(11):7109–7114. [PubMed] [Google Scholar]
- Carey L. M., Rodriguez A., Meighen E. Generation of fatty acids by an acyl esterase in the bioluminescent system of Photobacterium phosphoreum. J Biol Chem. 1984 Aug 25;259(16):10216–10221. [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Nealson K. H. Bacterial bioluminescence. Annu Rev Microbiol. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- Jaworski J. G., Stumpf P. K. Fat metabolism in higher plants. Enzymatic preparation of E. coli stearyl-acyl carrier protein. Arch Biochem Biophys. 1974 May;162(1):166–173. doi: 10.1016/0003-9861(74)90115-5. [DOI] [PubMed] [Google Scholar]
- Knudsen J., Clark S., Dils R. Purification and some properties of a medium-chain acyl-thioester hydrolase from lactating-rabbit mammary gland which terminates chain elongation in fatty acid synthesis. Biochem J. 1976 Dec 15;160(3):683–691. doi: 10.1042/bj1600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Smith S. Purification and properties of a thioesterase from lactating rat mammary gland which modifies the product specificity of fatty acid synthetase. J Biol Chem. 1978 Mar 10;253(5):1393–1401. [PubMed] [Google Scholar]
- Riendeau D., Meighen E. Evidence for a fatty acid reductase catalyzing the synthesis of aldehydes for the bacterial bioluminescent reaction. Resolution from luciferase and dependence on fatty acids. J Biol Chem. 1979 Aug 25;254(16):7488–7490. [PubMed] [Google Scholar]
- Riendeau D., Rodriguez A., Meighen E. Resolution of the fatty acid reductase from Photobacterium phosphoreum into acyl protein synthetase and acyl-CoA reductase activities. Evidence for an enzyme complex. J Biol Chem. 1982 Jun 25;257(12):6908–6915. [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl-acyl carrier protein synthetase from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):163–168. doi: 10.1016/0076-6879(81)71023-1. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr, Armitage I. M. Molecular properties of acyl carrier protein derivatives. J Biol Chem. 1981 Mar 25;256(6):2669–2674. [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Re-evaluation of the solution structure of acyl carrier protein. J Biol Chem. 1979 Oct 10;254(19):9778–9785. [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Regulation of phospholipid synthesis in Escherichia coli. Composition of the acyl-acyl carrier protein pool in vivo. J Biol Chem. 1982 Sep 25;257(18):10759–10765. [PubMed] [Google Scholar]
- Rodriguez A., Riendeau D., Meighen E. Purification of the acyl coenzyme A reductase component from a complex responsible for the reduction of fatty acids in bioluminescent bacteria. Properties and acyltransferase activity. J Biol Chem. 1983 Apr 25;258(8):5233–5237. [PubMed] [Google Scholar]
- Rogers L., Kolattukudy P. E., deRenobales M. Purification and characterization of S-acyl fatty acid synthase thioester hydrolase which modifies the product specificity of fatty acid synthase in the uropygial gland of mallard. J Biol Chem. 1982 Jan 25;257(2):880–886. [PubMed] [Google Scholar]
- Rosenfeld I. S., D'Agnolo G., Vagelos P. R. Identification and quantitation of acyl thioesters by thin-layer chromatography of hydroxamic acid derivatives. Anal Biochem. 1975 Mar;64(1):221–228. doi: 10.1016/0003-2697(75)90422-4. [DOI] [PubMed] [Google Scholar]
- Ryan R. O., de Renobales M., Dillwith J. W., Heisler C. R., Blomquist G. J. Biosynthesis of myristate in an aphid: involvement of a specific acylthioesterase. Arch Biochem Biophys. 1982 Jan;213(1):26–36. doi: 10.1016/0003-9861(82)90435-0. [DOI] [PubMed] [Google Scholar]
- Spencer A. K., Greenspan A. D., Cronan J. E., Jr Thioesterases I and II of Escherichia coli. Hydrolysis of native acyl-acyl carrier protein thioesters. J Biol Chem. 1978 Sep 10;253(17):5922–5926. [PubMed] [Google Scholar]
- Ulitzur S., Hastings J. W. Evidence for tetradecanal as the natural aldehyde in bacterial bioluminescence. Proc Natl Acad Sci U S A. 1979 Jan;76(1):265–267. doi: 10.1073/pnas.76.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Hastings J. W. Myristic acid stimulation of bacterial bioluminescence in "aldehyde" mutants. Proc Natl Acad Sci U S A. 1978 Jan;75(1):266–269. doi: 10.1073/pnas.75.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L. A., Byers D. M., Meighen E. A. In vivo and in vitro acylation of polypeptides in Vibrio harveyi: identification of proteins involved in aldehyde production for bioluminescence. J Bacteriol. 1984 Aug;159(2):720–724. doi: 10.1128/jb.159.2.720-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]