Abstract
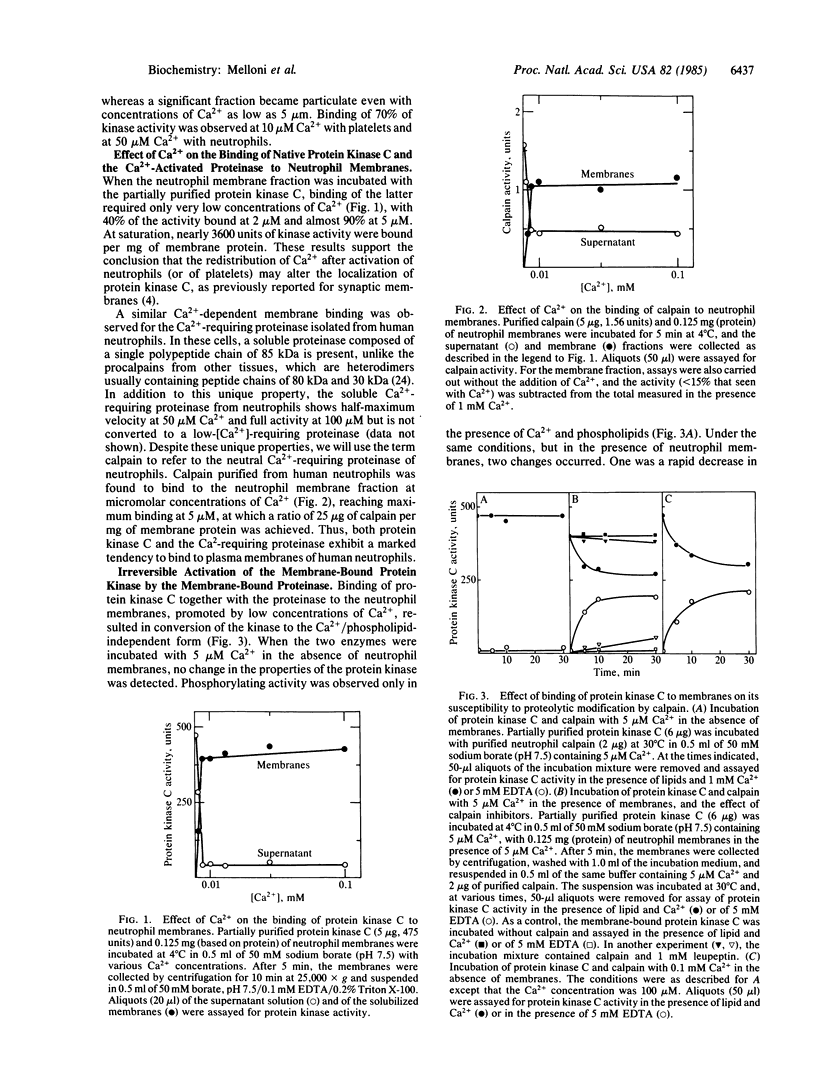
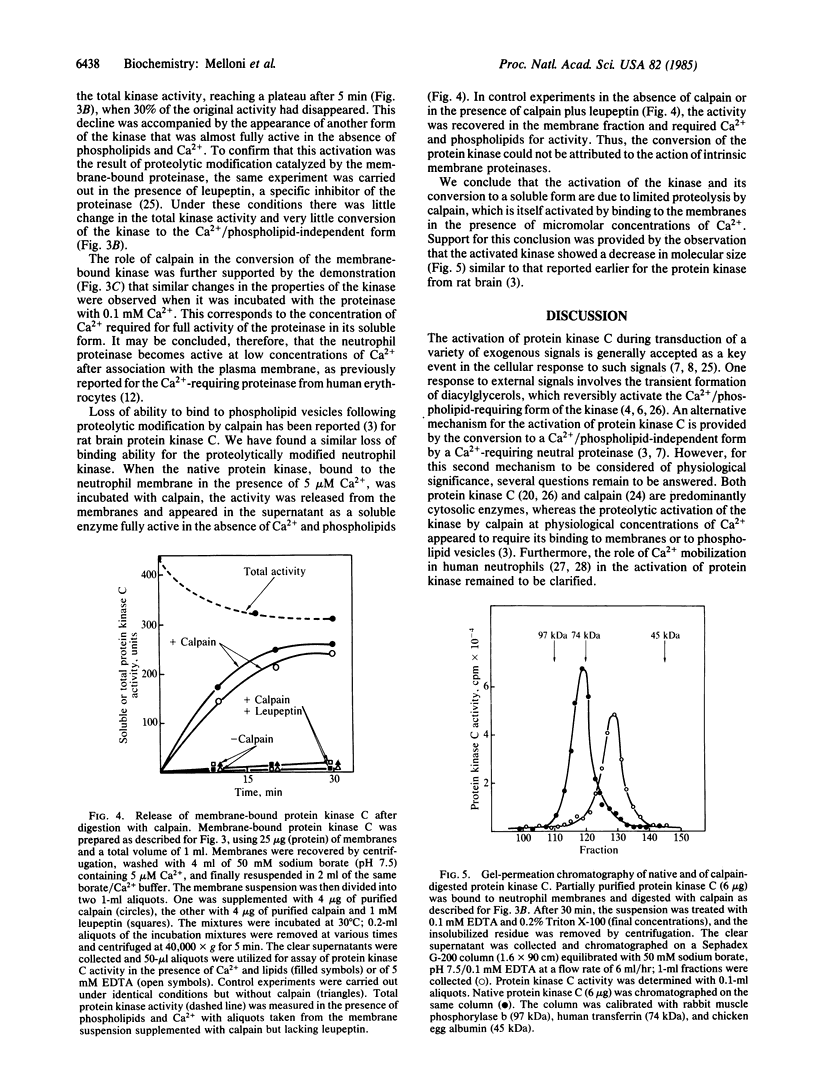
In the presence of micromolar concentrations of Ca2+, both protein kinase C and a cytosolic Ca2+-requiring neutral proteinase of human neutrophils become associated with the neutrophil membrane. Binding to the membrane results in activation of the proteinase, which then catalyzes limited proteolysis of the kinase to produce a form that is fully active in the absence of Ca2+ and phospholipid. This irreversibly activated protein kinase is released from the membrane and may thus have access, in the intact cell, to intracellular protein substrates. In the absence of the proteinase, Ca2+ promotes the binding of protein kinase C, but conversion to the Ca2+/phospholipid-independent form does not occur and the kinase remains associated with the membrane fraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Majerus P. W. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Appelbaum B. D., Vogler W. R., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase and its substrates in human neutrophils. Biochem Biophys Res Commun. 1983 Mar 29;111(3):847–853. doi: 10.1016/0006-291x(83)91376-1. [DOI] [PubMed] [Google Scholar]
- Hoffstein S. T. Ultrastructural demonstration of calcium loss from local regions of the plasma membrane of surface-stimulated human granulocytes. J Immunol. 1979 Sep;123(3):1395–1402. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Jergil B., Sommarin M. Determination of Ca2+- and phospholipid-dependent protein kinase in rat liver membranes. Biochim Biophys Acta. 1983 Jul 5;758(1):10–16. doi: 10.1016/0304-4165(83)90003-x. [DOI] [PubMed] [Google Scholar]
- Katoh N., Kuo J. F. Subcellular distribution of phospholipid-sensitive calcium-dependent protein kinase in guinea pig heart, spleen and cerebral cortex, and inhibition of the enzyme by Triton X-100. Biochem Biophys Res Commun. 1982 May 31;106(2):590–595. doi: 10.1016/0006-291x(82)91151-2. [DOI] [PubMed] [Google Scholar]
- Katoh N., Wrenn R. W., Wise B. C., Shoji M., Kuo J. F. Substrate proteins for calmodulin-sensitive and phospholipid-sensitive Ca2+-dependent protein kinases in heart, and inhibition of their phosphorylation by palmitoylcarnitine. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4813–4817. doi: 10.1073/pnas.78.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Melloni E., Salamino F., Sparatore B., Michetti M., Pontremoli S. Ca2+-dependent neutral proteinase from human erythrocytes: activation by Ca2+ ions and substrate and regulation by the endogenous inhibitor. Biochem Int. 1984 Apr;8(4):477–489. [PubMed] [Google Scholar]
- Mottola C., Romeo D. Calcium movement and membrane potential changes in the early phase of neutrophil activation by phorbol myristate acetate: a study with ion-selective electrodes. J Cell Biol. 1982 Apr;93(1):129–134. doi: 10.1083/jcb.93.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch G. H., Franco R., Evans R. M., Rosenfeld M. G. Polypeptide hormone regulation of gene expression. Thyrotropin-releasing hormone rapidly stimulates both transcription of the prolactin gene and the phosphorylation of a specific nuclear protein. J Biol Chem. 1983 Dec 25;258(24):15329–15335. [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Borgeat P., White J. R., Sha'afi R. I. Phorbol esters inhibit the fMet-Leu-Phe- and leukotriene B4-stimulated calcium mobilization and enzyme secretion in rabbit neutrophils. J Biol Chem. 1985 Feb 25;260(4):2125–2131. [PubMed] [Google Scholar]
- Nishizuka Y., Takai Y., Kishimoto A., Kikkawa U., Kaibuchi K. Phospholipid turnover in hormone action. Recent Prog Horm Res. 1984;40:301–345. doi: 10.1016/b978-0-12-571140-1.50012-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Petroski R. J., Naccache P. H., Becker E. L., Sha'afi R. I. Effect of the chemotactic factor formyl methionyl- leucyl-phenylalanine and cytochalasin B on the cellular levels of calcium in rabbit neutrophils. FEBS Lett. 1979 Apr 1;100(1):161–165. doi: 10.1016/0014-5793(79)81155-2. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Sparatore B., Salamino F., Michetti M., Sacco O., Horecker B. L. Binding to erythrocyte membrane is the physiological mechanism for activation of Ca2+-dependent neutral proteinase. Biochem Biophys Res Commun. 1985 Apr 16;128(1):331–338. doi: 10.1016/0006-291x(85)91683-3. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Sha'afi R. I., White J. R., Molski T. F., Shefcyk J., Volpi M., Naccache P. H., Feinstein M. B. Phorbol 12-myristate 13-acetate activates rabbit neutrophils without an apparent rise in the level of intracellular free calcium. Biochem Biophys Res Commun. 1983 Jul 29;114(2):638–645. doi: 10.1016/0006-291x(83)90828-8. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977 Nov 10;252(21):7603–7609. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y., Tamura A., Fujii T. A role of membranes in the activation of a new multifunctional protein kinase system. J Biochem. 1979 Aug;86(2):575–578. doi: 10.1093/oxfordjournals.jbchem.a132557. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Modulation of Ca2+-activated, phospholipid-dependent protein kinase in platelets treated with a tumor-promoting phorbol ester. Biochem Biophys Res Commun. 1984 Jul 18;122(1):158–164. doi: 10.1016/0006-291x(84)90453-4. [DOI] [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Platelet Ca2+-activated, phospholipid-dependent protein kinase: evidence for proteolytic activation of the enzyme in cells treated with phospholipase C1. Biochem Biophys Res Commun. 1984 Feb 14;118(3):835–841. doi: 10.1016/0006-291x(84)91470-0. [DOI] [PubMed] [Google Scholar]
- WINTERHALTER K. H., HUEHNS E. R. PREPARATIONS, PROPERTIES, AND SPECIFIC RECOMBINATION OF ALPHA-BETA-GLOBIN SUBUNITS. J Biol Chem. 1964 Nov;239:3699–3705. [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983 May 31;113(1):44–50. doi: 10.1016/0006-291x(83)90429-1. [DOI] [PubMed] [Google Scholar]
- Wrenn R. W. Phospholipid-sensitive calcium-dependent protein kinase and its endogenous substrate proteins in rat pancreatic acinar cells. Life Sci. 1983 May 16;32(20):2385–2392. doi: 10.1016/0024-3205(83)90770-1. [DOI] [PubMed] [Google Scholar]


