Abstract
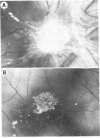
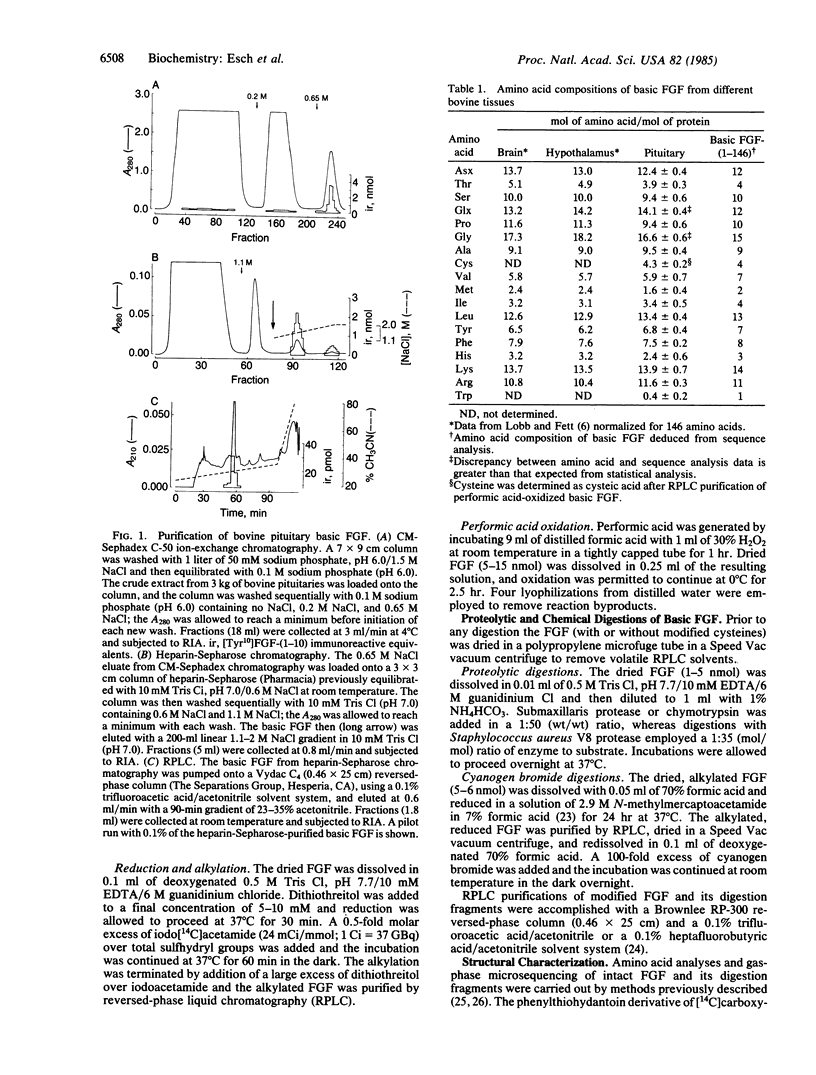
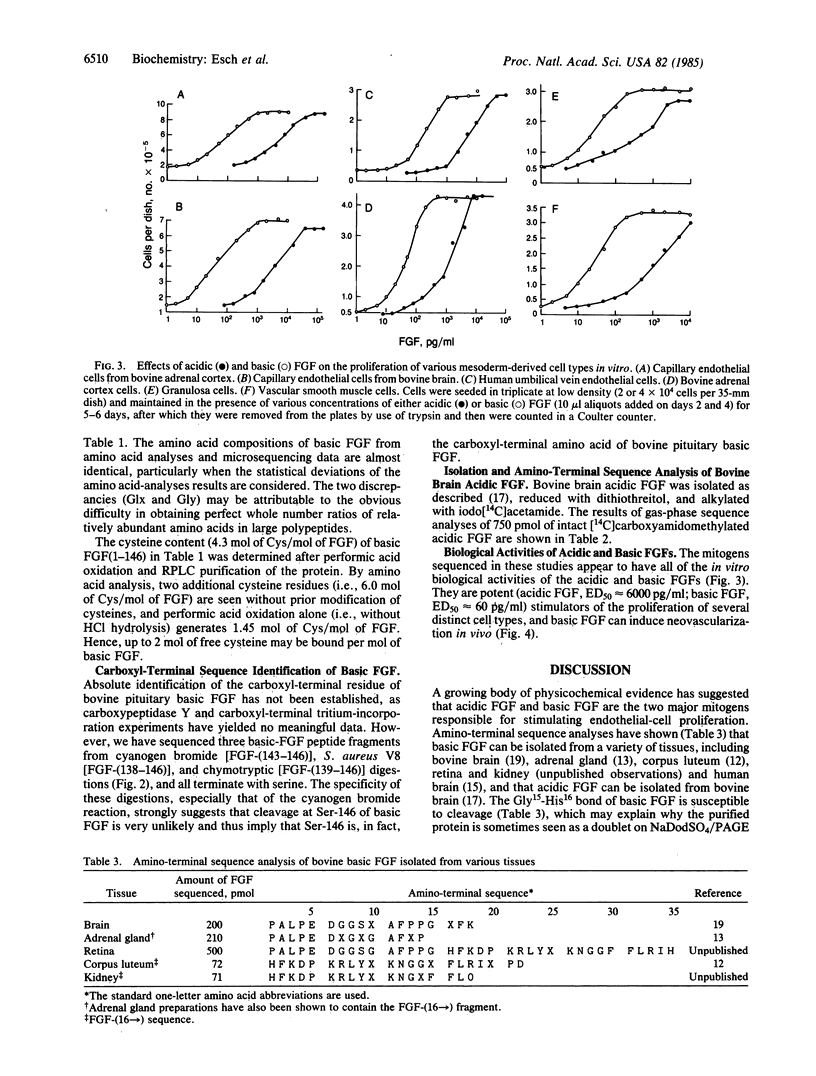
The two major mitogenic polypeptides for endothelial cells have been purified to homogeneity. The complete primary structure of bovine pituitary basic fibroblast growth factor (FGF) and the amino-terminal amino acid sequence of bovine brain acidic FGF have been established by gas-phase sequence analyses. Homogeneous preparations of these polypeptides are potent mitogens (basic FGF, ED50 approximately equal to 60 pg/ml; acidic FGF ED50 approximately equal to 6000 pg/ml) for many diverse cell types including capillary endothelial cells, vascular smooth muscle cells, and adrenocortical and granulosa cells; in vivo, basic FGF is a powerful angiogenic agent in the chick chorioallantoic membrane assay. The available protein sequence data demonstrate the existence of significant structural homology between the two polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A., Böhlen P., Ling N., Guillemin R. Radioimmunoassay for fibroblast growth factor (FGF): release by the bovine anterior pituitary in vitro. Regul Pept. 1985 Apr;10(4):309–317. doi: 10.1016/0167-0115(85)90043-6. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Baird A., Esch F., Ling N., Gospodarowicz D. Isolation and partial molecular characterization of pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5364–5368. doi: 10.1073/pnas.81.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Esch F., Baird A., Jones K. L., Gospodarowicz D. Human brain fibroblast growth factor. Isolation and partial chemical characterization. FEBS Lett. 1985 Jun 3;185(1):177–181. doi: 10.1016/0014-5793(85)80765-1. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Schroeder R. High-sensitivity amino acid analysis: methodology for the determination of amino acid compositions with less than 100 picomoles of peptides. Anal Biochem. 1982 Oct;126(1):144–152. doi: 10.1016/0003-2697(82)90120-8. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Glaser B. M., Brunson S. K., Fenselau A. H. Angiogenic activity from bovine retina: partial purification and characterization. Proc Natl Acad Sci U S A. 1981 May;78(5):3068–3072. doi: 10.1073/pnas.78.5.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore P. A., Klagsbrun M. Endothelial cell mitogens derived from retina and hypothalamus: biochemical and biological similarities. J Cell Biol. 1984 Oct;99(4 Pt 1):1545–1549. doi: 10.1083/jcb.99.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Ling N. C., Böhlen P. Microisolation of neuropeptides. Methods Enzymol. 1983;103:72–89. doi: 10.1016/s0076-6879(83)03007-4. [DOI] [PubMed] [Google Scholar]
- Esch F. S. Polypeptide microsequence analysis with the commercially available gas-phase sequencer. Anal Biochem. 1984 Jan;136(1):39–47. doi: 10.1016/0003-2697(84)90305-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lirette M. Bovine brain and pituitary fibroblast growth factors: comparison of their abilities to support the proliferation of human and bovine vascular endothelial cells. J Cell Biol. 1983 Dec;97(6):1677–1685. doi: 10.1083/jcb.97.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Massoglia S., Cheng J., Lui G. M., Böhlen P. Isolation of pituitary fibroblast growth factor by fast protein liquid chromatography (FPLC): partial chemical and biological characterization. J Cell Physiol. 1985 Feb;122(2):323–332. doi: 10.1002/jcp.1041220223. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. A computer program for predicting protein antigenic determinants. Mol Immunol. 1983 Apr;20(4):483–489. doi: 10.1016/0161-5890(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Li C. H. Reduction of sulfoxides in peptides and proteins. Methods Enzymol. 1983;91:549–559. doi: 10.1016/s0076-6879(83)91050-9. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Weinstein R. High and low molecular weight forms of endothelial cell growth factor. J Biol Chem. 1982 May 25;257(10):5333–5336. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Klagsbrun M. Purification of cartilage-derived growth factor by heparin affinity chromatography. J Biol Chem. 1985 Feb 25;260(4):2399–2403. [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Fitzpatrick S. Purification and characterization of acidic fibroblast growth factor from bovine brain. Proc Natl Acad Sci U S A. 1984 Jan;81(2):357–361. doi: 10.1073/pnas.81.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]