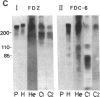
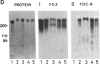
Abstract
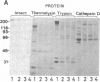
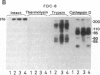
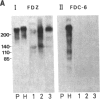
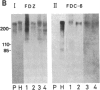
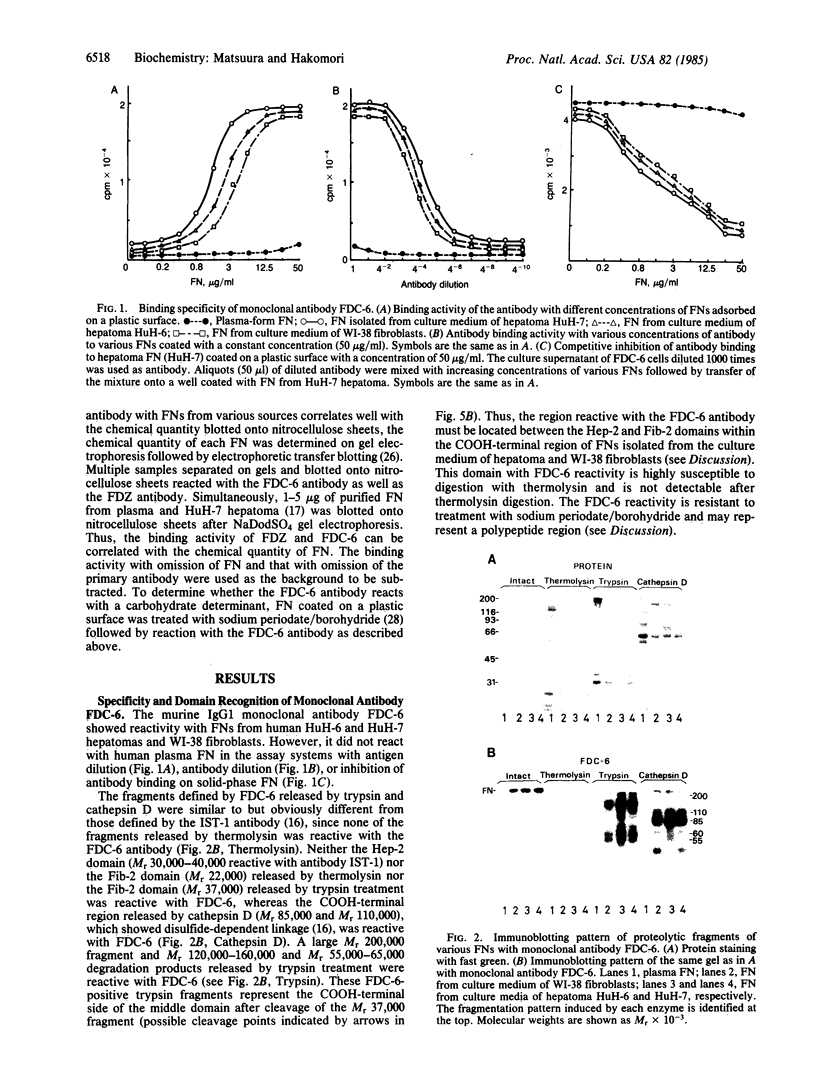
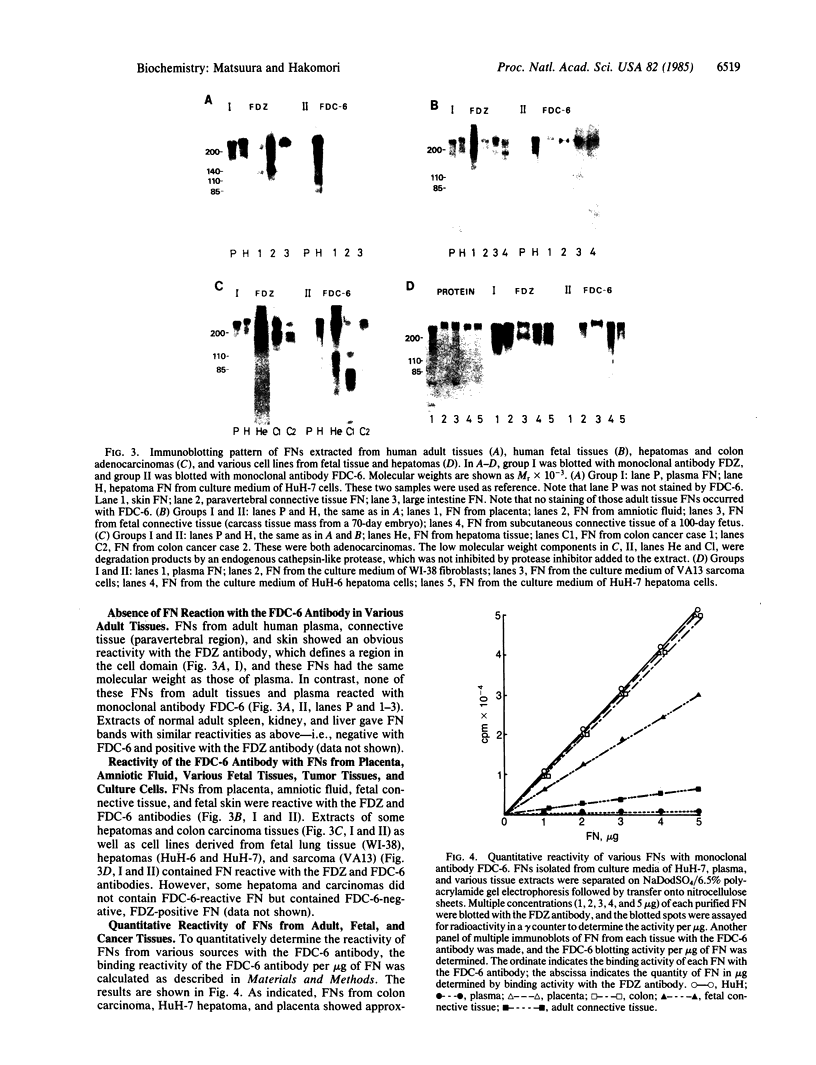
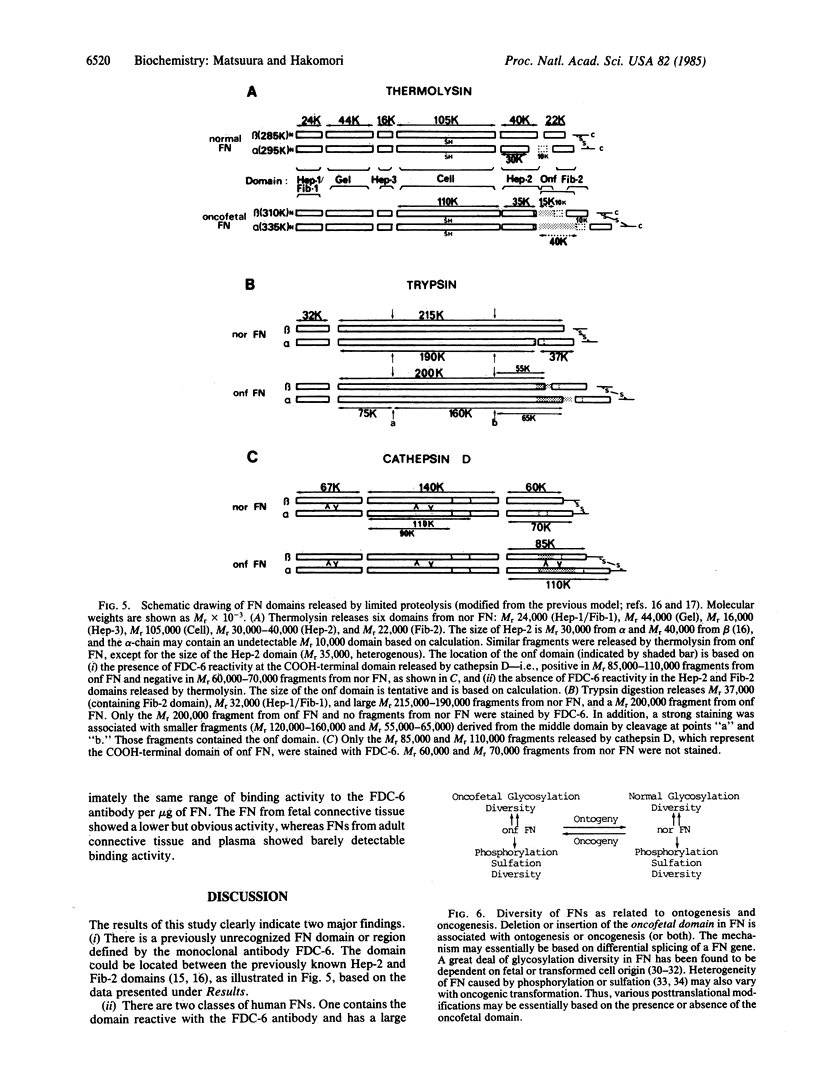
An IgG1 monoclonal antibody, FDC-6, was established, which defines a unique fibronectin (FN) domain, located between the "Hep-2" and the "Fib-2" domains, in the COOH-terminal region of FNs isolated from hepatoma, sarcoma, and fetal fibroblasts. A systematic study with this antibody indicates the presence of two classes of human FNs. (i) FN from fetal connective tissue, placenta, amniotic fluid, hepatoma, and colon carcinoma as well as cell lines from fetal tissues (WI-38), hepatomas (HuH-6 and HuH-7), and sarcoma (VA13) was characterized by the presence of the FDC-6-defined domain and by a high molecular weight (subunit Mr, 310,000-335,000). (ii) In contrast, FN from normal adult tissues and plasma was characterized by a lower molecular weight (subunit Mr, 285,000-295,000) and lack of reactivity with FDC-6 and is therefore devoid of the FDC-6-defined domain. The FDC-6-defined domain is therefore called the "oncofetal" domain, and FN containing this domain is hereby called "oncofetal FN" (onf FN). The onf FN is similar to the previously known "cellular-form" FN. FN from normal adult tissues and plasma, lacking the oncofetal domain, is hereby called "normal FN" (nor FN). The nor FN is similar to the previously known "plasma-form" FN. Development of FN from fetal to adult form is associated with loss of the oncofetal domain defined by the FDC-6 antibody, and oncogenic transformation is associated with activation in synthesis of the oncofetal domain defined by the FDC-6 antibody.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton B. T., Hynes R. O. A difference between plasma and cellular fibronectins located with monoclonal antibodies. Cell. 1981 Jul;25(1):133–141. doi: 10.1016/0092-8674(81)90237-3. [DOI] [PubMed] [Google Scholar]
- Cossu G., Warren L. Lactosaminoglycans and heparan sulfate are covalently bound to fibronectins synthesized by mouse stem teratocarcinoma cells. J Biol Chem. 1983 May 10;258(9):5603–5607. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Differences in domain structures between plasma and cellular fibronectins. J Biol Chem. 1981 Nov 10;256(21):11292–11300. [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem. 1983 Mar 10;258(5):3332–3340. [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: cell specific alternative mRNA splicing generates polypeptide chains differing in the number of internal repeats. Nucleic Acids Res. 1984 Jul 25;12(14):5853–5868. doi: 10.1093/nar/12.14.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: molecular cloning evidence for two mRNA species differing by an internal segment coding for a structural domain. EMBO J. 1984 Jan;3(1):221–226. doi: 10.1002/j.1460-2075.1984.tb01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu M. C., Lipmann F. Isolation of tyrosine-O-sulfate by Pronase hydrolysis from fibronectin secreted by Fujinami sarcoma virus-infected rat fibroblasts. Proc Natl Acad Sci U S A. 1985 Jan;82(1):34–37. doi: 10.1073/pnas.82.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Pearlstein E., Gold L. I., Garcia-Pardo A. Fibronectin: a review of its structure and biological activity. Mol Cell Biochem. 1980 Feb 8;29(2):103–128. doi: 10.1007/BF00220304. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Jalanko H., Comings D. E., Neville A. M., Raghavan D. Fibronectin from human germ-cell tumors resembles amniotic fluid fibronectin. Int J Cancer. 1981 Jun 15;27(6):763–767. doi: 10.1002/ijc.2910270606. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Paul J. I., Hynes R. O. On the origin of species of fibronectin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1424–1428. doi: 10.1073/pnas.82.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Fukuda M., Hakomori S. Domain structure of hamster plasma fibronectin. Isolation and characterization of four functionally distinct domains and their unequal distribution between two subunit polypeptides. J Biol Chem. 1981 Jun 25;256(12):6452–6462. [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Domain structure of human plasma fibronectin. Differences and similarities between human and hamster fibronectins. J Biol Chem. 1983 Mar 25;258(6):3967–3973. [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Functional domain structure of fibronectin. Proc Natl Acad Sci U S A. 1980 May;77(5):2661–2665. doi: 10.1073/pnas.77.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Patterson C. M., Ishigami F., Hakomori S. Monoclonal antibodies directed to two different domains of human plasma fibronectin: their specificities. FEBS Lett. 1982 Jun 7;142(2):243–246. doi: 10.1016/0014-5793(82)80144-0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Siri A., Zardi L., Hakomori S. Differences in domain structure between human fibronectins isolated from plasma and from culture supernatants of normal and transformed fibroblasts. Studies with domain-specific antibodies. J Biol Chem. 1985 Apr 25;260(8):5105–5114. [PubMed] [Google Scholar]
- Sekiguchi K., Siri A., Zardi L., Hakomori S. Differences in domain structure between pericellular matrix and plasma fibronectins as revealed by domain-specific antibodies combined with limited proteolysis and S-cyanylation: a preliminary note. Biochem Biophys Res Commun. 1983 Oct 31;116(2):534–540. doi: 10.1016/0006-291x(83)90556-9. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Hynes R. O. Plasma fibronectin is synthesized and secreted by hepatocytes. J Biol Chem. 1983 Apr 10;258(7):4641–4647. [PubMed] [Google Scholar]
- Tamkun J. W., Schwarzbauer J. E., Hynes R. O. A single rat fibronectin gene generates three different mRNAs by alternative splicing of a complex exon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5140–5144. doi: 10.1073/pnas.81.16.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng M. H., Rifkin D. B. Fibronectin from chicken embryo fibroblasts contains covalently bound phosphate. J Cell Biol. 1979 Mar;80(3):784–791. doi: 10.1083/jcb.80.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Siri A., Santi L., Accolla R. S. Somatic cell hybrids producing antibodies specific to human fibronectin. Int J Cancer. 1980 Mar 15;25(3):325–329. doi: 10.1002/ijc.2910250304. [DOI] [PubMed] [Google Scholar]
- Zhu B. C., Fisher S. F., Pande H., Calaycay J., Shively J. E., Laine R. A. Human placental (fetal) fibronectin: increased glycosylation and higher protease resistance than plasma fibronectin. Presence of polylactosamine glycopeptides and properties of a 44-kilodalton chymotryptic collagen-binding domain: difference from human plasma fibronectin. J Biol Chem. 1984 Mar 25;259(6):3962–3970. [PubMed] [Google Scholar]