Abstract
To date, genome-wide association studies (GWAS) permit a comprehensive scan of the genome in an unbiased manner, with high sensitivity, and thereby have the potential to identify candidate genes for the prevalence or development of multifactorial diseases such as bronchial asthma. However, most studies have only managed to explain a small additional percentage of hereditability estimates, and often fail to show consistent results among studies despite large sample sizes. Epistasis is defined as the interaction between multiple different genes affecting phenotypes. By applying epistatic analysis to clinical genetic research, we can analyze interactions among more than 2 molecules (genes) considering the whole system of the human body, illuminating dynamic molecular mechanisms. An increasing number of genetic studies have investigated epistatic effects on the risk for development of asthma. The present review highlights a concept of epistasis to overcome traditional genetic studies in humans and provides an update of evidence on epistatic effects on asthma. Furthermore, we review concerns regarding recent trends in epistatic analyses from the perspective of clinical physicians. These concerns include biological plausibility of genes identified by computational statistics, and definition of the diagnostic label of ‘physician-diagnosed asthma’. In terms of these issues, further application of epistatic analysis will prompt identification of susceptibility of diseases and lead to the development of a new generation of pharmacological strategies to treat asthma.
Keywords: clinical application, epistasis, genetic, asthma - physiopathology, disease susceptibility
Background
To date, the rapid evolution of genetic technology provides opportunities for genome-wide association studies (GWAS) to identify genes related to multifactorial diseases such as bronchial asthma [1–4]. One of the advantages of GWAS is that they permit a comprehensive scan of the genome in an unbiased manner and thereby have the potential to identify novel genetic factors for the development of related diseases. In addition, GWAS have high sensitivity, which allows detection of signals localized to small regions of the chromosome containing only a single or a few genes, enabling us to identify the targeted genes. However, most GWAS have only managed to explain a small additional percentage of hereditability estimates, and exhibit poor reproducibility [5,6]. Several complementary strategies have been adopted to overcome these study limitations, including large-scale recruitment of cases and controls. In fact, it is known that an increase in sample size by collection of data from multiple studies, such as a meta-analysis, generates additional statistical power to detect smaller odds ratios [7,8]. Nevertheless, these large-scale studies often fail to show consistent results. In addition, from the point of view of clinical specialists, large-scale recruitment of samples leads to difficulty in ensuring uniformity of the phenotype among individuals, as is the case with asthma. An increasing number of reports clearly demonstrate that asthma is a complex disease made up of a number of disease variants (often referred to as asthma ‘endotypes’) with different underlying pathophysiology [9–11].
Thus, the design of genetic studies is now being reviewed from newly emerged perspectives such as systems biology using network models in cellular signaling systems [12]. As one of these research trends, the concept of gene-gene interactions, referred to as epistasis, has recently gained attention in genetic studies [13,14]. Epistasis is defined as the interaction, mainly statistical, between 2 different factors (gene-gene or protein-protein) affecting phenotypes. By applying an epistatic analysis to clinical genetic research, we can analyze interactions among more than 2 molecules (genes) considering the whole system of the human body, illuminating the reality of dynamic molecular mechanisms. Furthermore, it might provide a potential breakthrough to address the current limitations of ordinary GWAS.
The purpose of the present review was to introduce the concept of epistasis and to summarize previous articles using this concept in asthma research. Furthermore, we aim to discuss the limits and potential for clinical application of epistatic analysis developed in biology, mainly based on mathematics and statistics. Such practical application will help us investigate the pathophysiology of common respiratory diseases such as asthma.
Gene-gene Interactions (Epistasis)
Epistasis is a promising concept among various investigations of the association of genetic predisposition with phenotypes [15]. Contrary to Mendel’s classical law of heredity, in which each gene locus is supposed to show an independent effect on a single phenotype without any interactions between genes, it has been proven that 2 different gene loci can affect the same phenotype [16–18]. The action between 2-loci was classically defined as ‘epistasis’ [19]. Although the definitions and interpretations of this term are somewhat inconsistent in the literature [20], the term is now used: 1) when 2 or more loci interact to create a new phenotype, and 2) when an allele at 1 locus masks or modifies the effects of alleles at 1 or more other loci [15]. The phenotypic effect of a specific gene mutation entirely depends on the overall genetic background affected by many other gene mutations [19,21]. Furthermore, the combined effects of more than 2 loci on phenotypes are not simply additive, but are either synergistic or antagonistic [22].
While the new concepts of epistasis and multiple gene approaches continue to evolve further [23], studies of epistasis still tend to place importance on mathematically rigorous analyses, often without taking biological plausibility into account. However, studies of epistasis are more in line with a recent trend of molecular interactions in ‘systems biology’ [24]. According to systems biology, dynamic networks consisting of numerous interactions among intracellular molecules modulate cellular activities including growth, development, and physiological actions [25,26]. For example, many receptors, including G-coupled protein receptor (GPCR), can modify the function of other receptors through dimer formation or intracellular signal cross-talking [27,28]. Recently, such molecular systems provided the basis for new strategies for epistatic analysis [29–31]. A growing number of studies based on the observation of epistatic interaction have been conducted in various clinical settings, such as essential hypertension [32–34], diabetic nephropathy [35], psychiatric disorders [36,37], and nicotine dependence [38]. These studies show examples of epistatic interactions in human diseases, and findings can be interpreted on the basis of systems biology. This research trend will undoubtedly extend into the research area of respiratory medicine. An increasing number of asthma articles have been published using epistatic analyses (Table 1, see the next section).
Table 1.
Major original literatures of epistatic analysis in diagnosis of asthma or asthma-related phenotypes in humans.
| Authors | Gene combination | Outcome of epistatic effects |
|---|---|---|
| [Howard TD, et al., 2002] | IL-4Rα and IL-13 | Physician-diagnosed asthma |
| [Adjers K, et al., 2004] | IL-1α and IL-4Rα | Risk of atopy assessed by positive skin prick test in non-asthmatic patients |
| [Lee SG, et al., 2004] | IL-4Rα and IL-4 | Physician-diagnosed asthma |
| [Adjers K, et al., 2005] | TLR4 and IL-4 | Risk of asthma in females |
| [Barnes KC, et al., 2006] | AOAH and CD14 | Physician-diagnosed asthma |
| [Hizawa N, et al., 2006] | PAI-1 and FCER1β | Physician-diagnosed asthma |
| [Kim HB, et al., 2006] | IL-13Rα1 and IL-13 | Higher total IgE in children with atopic asthma |
| [Millstein J, et al., 2006] | NQO1, MPO and CAT | Risk of asthma |
| [Battle NC, et al., 2007] | IL-4Rα and IL-13 | Baseline FEV1 in patients with asthma |
| [Orsmark-Pietras C, et al., 2008] | NPSR1 and TNC | Atopic sensitization assessed by serum IgE levels or doctor’s diagnosis of asthma |
| [Kim SH, et al., 2009] | IL-10 and TGF-β1 | Aspirin-intolerant asthma |
| [Bottema RW, et al., JACI 2010] | FOXP3 and TGFβR2 | Milk-specific IgE levels in serum |
| [Bottema RW, et al., ERJ 2010] | CD86 and VTCN1/CD274 and LILRA4 | Total IgE |
| [De Lobel L, et al., 2010] | NPSR1 and DPP10 | Subjects who ever had asthma |
| [Yang KD, et al., 2010] | IL-13, IL-17A, and redox genes | High levels of cord blood IgE |
| [Wu X, et al., 2010] | IL-4, IL-13, IL-4Rα, STAT6, and CD14 | Risk of asthma |
| [Ungvari I, et al., 2012] | PTGER2, PRPF19 and FRMD6 | Physician-diagnosed asthma |
| [Choi WA, et al., 2012] | CTLA4 and IL-13/IL-13Rα1 and IL-13 | Increased total serum IgE levels |
| [Yoshikawa T, et al., 2012] | EGFR/PAR-1 | Asymptomatic AHR |
| [Acevedo N, et al., 2013] | RORA and NPSR-1 | Physician-diagnosed asthma |
AHR – airway hyperresponsiveness; AOAH – acyloxyacyl hydroxylase; CAT – catalase; CTLA4 – cytotoxic T-lymphocyte antigen 4; DPP10 – dipeptidyl peptidase 10; EGFR – epidermal growth factor receptor; FCER1β – β-chain of the high-affinity receptor for IgE; FOXP3 – forkhead box protein 3; FRMD6 – FERM domain containing 6; IgE – immunoglobulin E; IL – interleukin; IL-4Rα – interleukin-4 receptor α; IL-13Rα1 – interleukin-13 receptor α1; LILRA4 – leukocyte immunoglobulin-like receptor subfamily A-4; MPO – myeloperoxidase; NPSR-1 – neuropeptide-S receptor 1; NQO-1 – nicotinamide adenine dinucleotide (phosphate) reduced: quinone oxidoreductase; PAI-1 – plasminogen activator inhibitor 1; PAR-1 – protease-activated receptor-1; PTGER2 – prostaglandin-E2 receptor; PRPF19 – pre-mRNA-processing factor 19; RORA – retinoic acid receptor-related orphan receptor α; STAT6 – signal transducer and activator of transcription 6; TGFβ1 – transforming growth factor β1; TGFβR2 – TGF-β receptor-2; TLR4 – Toll-like receptor-4; TNC – tenascin C; VTCN1 – V-set domain containing T-cell activation inhibitor 1.
Epistatic Analyses in Asthma Research
Interleukin (IL)-4- and IL-13-related genes
Asthma is characterized by variable airflow obstruction and airway hyperresponsiveness (AHR), particularly in response to bronchial provocation with methacholine and histamine [39]. Airway inflammation plays a crucial role in the mechanisms responsible for asthma diathesis [40]. An increasing number of studies on epistasis in asthma research have focused on interactions among cytokines and cytokine-associated receptors, particularly IL-4 and IL-13. For example, an earlier report in a Dutch population demonstrated that epistatic interaction between IL4-Rα (S478P) and IL-13 (−1111 C/T) markedly increases an individual’s susceptibility to asthma [41]. A synergic gene-gene effect was reported between the IL-4 (−590C) and the IL-4Rα (Arg551 allele), which significantly increases susceptibility to asthma in Korean children, as diagnosed by physicians [42]. In addition, the same Korean research group reported that the IL-13 (A-1512C or C-1112T) and IL-13 receptor (IL-13R) α-1 polymorphisms (A+1398G) may interact to enhance total immunoglobulin E (IgE) production [43]. In a study investigating African Americans with asthma, ethnicity-specific gene-gene interaction between the IL-13 (A-646G) and IL-4R genes (A+4679G) were shown to have a significant effect on baseline lung function (forced expiratory volume in 1 second [FEV1]) [44]. A candidate gene study in a Finnish population demonstrated an epistatic effect between a pair of T-cell cytokine genes encoding IL-1α and IL-4Rα on the risk for atopy [45]. In a study focused on females in the same Finnish population [46], a significant epistatic interaction was observed between Toll-like receptor 4 (TLR-4) and IL-4. More recently, some studies have investigated epistatic effects of a pair of genes including IL-4 and IL-13, based on known asthma-related genes whose epistatic effects were not previously investigated [47–49].
T cell and transforming growth factor (TGF)-β-related genes
Besides IL-4 and IL-13, regulatory T (Treg) cells have been recently identified to play a key role in balancing immune responses to maintain or acquire tolerance against allergens [50]. Some studies investigated the epistatic effects of genes involved in the development and function of Treg cells, such as IL-2Rα, TLR-2, TGF-β-1, TGF-β receptor (TGF-βR)-2, IL-10, and Forkhead box protein 3 (FOXP3) on asthma-related phenotypes. A large-scale study consisting of 3 Dutch birth cohorts demonstrated an epistatic effect between TGF-βR-2 and FOXP3 polymorphisms on milk-specific IgE serum levels in boys and girls [51]. Furthermore, the same research group examined epistatic effects among genes involved in T cell and antigen-presenting cell co-stimulatory genes, and logistic regression analyses revealed 2-locus interactions of CD86 with the V-set domain containing T-cell activation inhibitor 1 (VTCN1) and CD274 with leukocyte immunoglobulin-like receptor subfamily A-4 (LILRA4) on serum IgE levels [52]. In addition, in a study of the other role of TGF-β and IL-10 in allergic diseases, genes of TGF-β-1 (−509C/T) and IL-10 (−1082A/G) were reported to be associated with aspirin-intolerant asthma by gene-gene interactions [53].
Innate immunity-related genes
The interplay between innate and adaptive immunity plays a role in the development of allergic diseases. For example, environmental exposure of bacterial lipopolysaccharide (LPS) affects the risk for allergy and asthma [54 55]. LPS recognition is mediated by a signaling pathway consisting of CD14, MD-2, and TLR-4 [56]. In particular, CD14 has been identified as a candidate gene affecting serum IgE levels and asthma [57–60]. A leukocyte enzyme, acyloxyacyl hydroxylase (AOAH), is also an important host defense molecule, as deacylation of LPS by AOAH decreases stimulation of cells through the signaling complex of CD14-MD2-TLR-4 [61]. A previous study investigating gene-gene interactions between AOAH marker rs2727831 and CD14 (−260) C>T genotype demonstrated that the presence of the TT genotype at either loci (but not both) reduced the risk for asthma (odds ratios <1) and conferred lower total IgE levels compared with those in the reference group. However, the presence of the TT genotype at both loci increased the risk for asthma (odds ratios >1), suggesting the presence of a qualitative interaction in a population with African ancestry [62].
Oxidative stress-related genes
Catalase (CAT) and myeloperoxidase (MPO) are enzymes known to play a role in the etiology of various respiratory conditions related to oxidative stress, such as asthma [63–65]. A novel strategy of focused interaction testing framework (FITF) identified a significant multilocus epistatic effect between the nicotinamide adenine dinucleotide (phosphate) reduced: quinone oxidoreductase gene (NQO1), MPO, and CAT on the risk for asthma, and these results have been replicated among children in various ethnic groups [66].
Airway remodeling-related genes
Many structural and functional alterations (remodeling) of residual cells and extracellular matrix, as well as airway inflammation, play crucial roles in the mechanisms responsible for asthma diathesis [67,68]. The plasminogen activator system is one of the mechanisms that regulate the tissue remodeling process, such as endogenous fibrosis and extracellular matrix (ECM) proteolysis [69]. In addition, the plasminogen activator inhibitor 1 (PAI-1) gene is expressed in activated mast cells after minute amounts of allergen and high-affinity receptor for IgE (FcɛRI) cross-link on these cells [70]. A previous study showed synergistic effects of the PAI-1 4G/5G genotype and the β chain of FcɛRI (FCER1B) −109C/T or FCER1B −654C/T on the presence of asthma in a Japanese population [71].
Few epistatic analyses have focused on AHR per se in asthma. Although AHR is one of the essential characteristics in symptomatic patients with asthma, many studies show that some atopic young adults with no symptoms of asthma also exhibit AHR (referred to as asymptomatic AHR) [72–74]. Previous findings have revealed the important roles of epidermal growth factor receptor (EGFR) [75–77] and protease-activated receptor (PAR)-1 [78–81] in mediating a variety of aspects in asthma, including airway remodeling. Moreover, in vitro studies previously revealed that EGFR activation mediates PAR-1 mitogenic signaling through intracellular signal cross-talking [82]. Epistatic analyses revealed an integrated effect of EGFR and PAR-1 on the development of asymptomatic AHR with atopy [83]. In addition, lower baseline FEV1 (% predicted) was observed in subjects with AHR compared with those without AHR.
Other little-known genes in asthma
Although many of the studies described above investigated epistatic interactions among genes as defined by well-studied biologic pathways, recent genetic studies tend to use traditional linkage studies or GWAS findings. For example, retinoid acid receptor-related orphan receptor α (RORA) was identified as the susceptibility gene for asthma in a previous GWAS [84]. A recent study showed that 7 single-nucleotide polymorphisms (SNP) on the gene encoding RORA were associated with physician-diagnosed childhood asthma, and that the risk effect of one of the SNPs was dependent on the SNP on the gene of neuropeptide S receptor 1 (NPSR-1) [85]. RORA is a transcription factor in the nuclear hormone-receptor superfamily and binds to the specific binding site of hormone response elements (RORE) in DNA. Regarding NPSR-1, other studies also demonstrated epistatic effects of NPSR-1 with tenascin C (TNC) [86] and dipeptidyl peptidase 10 (DPP10) on the risk for asthma [87]. Another study selected various candidate genes on 11q13 and 14q22 asthma susceptibility genome regions on the basis of past linkage analyses [88]. The study used Bayesian network-based Bayesian multilevel analysis of relevance (BN-BMLA), which provides detailed characterization of the relevance of factors, such as joint significance, the type of dependency, and multi-target aspects. The study showed the possibility of epistatic interactions among FERM domain containing 6 (FRMD6), prostaglandin-E2 receptor (PTGER2), and pre-mRNA-processing factor 19 (PRPF19).
Considerations About Clinical Application of Epistatic Analyses in Asthma Research – Limits and Potentials
Many years have passed since the proposal of theoretical epistasis and we can now actually examine the effects of epistatic interactions on the development of diseases such as asthma in the human body by using advanced genetic experiments and mathematical approaches. An increasing number of clinical studies have selected more than 2 candidate genes on the basis of biological pathways already known by previous in vitro experiments. Furthermore, recent studies have selected novel type of genes from the gene lists of GWAS. However, some issues should be considered when establishing the actual clinical application of epistatic analyses through the eyes of clinical doctors.
First, we should consider whether there is ample biological plausibility of the findings made by epistatic analyses. The advantage of the examination of epistasis between genes is that we can bring out a truly concentrated effect of molecular interactions along the putative pathophysiological pathway. However, recent mathematical tools have revealed some epistatic interactions between novel genes as a risk of physician-diagnosed asthma in an unbiased manner [85–88]. These genes and their epistatic relationships appear to lack biological plausibility compared with those defined by well-studied biologic pathways. Furthermore, these findings cannot be used to draw any conclusions about whether the epistatic effects are primary or secondary causes in the disease process. In addition, it is often difficult for clinical researchers without mathematical knowledge to understand the abstruse computational techniques used in epistatic analyses. Collectively, most clinical researchers do not know how to evaluate the biological plausibility or clinical value of the results of these analyses.
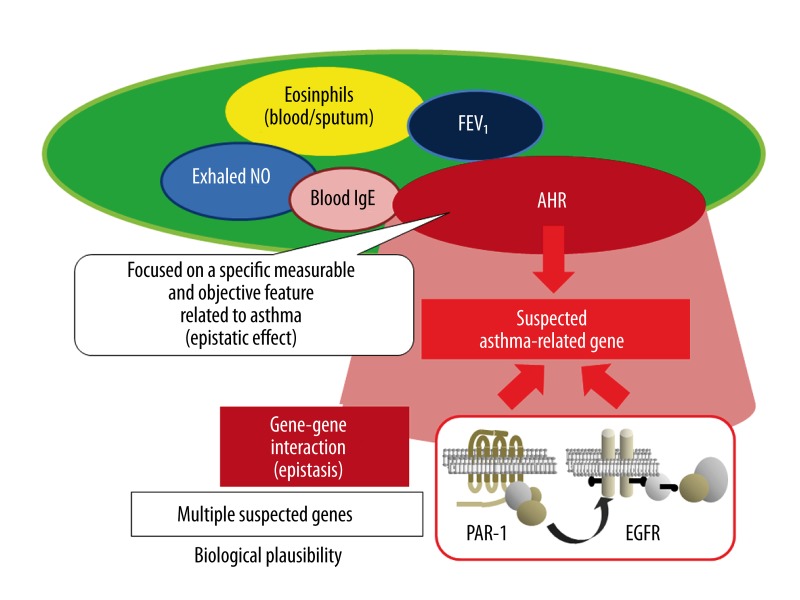
Second, and more importantly, most studies investigating epistatic interactions attach little importance to the definition of disease or phenotype. Even if the gene experimental techniques or computational genetics are likely superior to other classical ones, the poor definition of the target phenotype or disease definitely degrades the quality of the study findings, and thus doctors cannot apply the evidence to clinical practice. The epistatic effects are often represented by the presence or absence of the diagnostic label of ‘disease’ like ‘physician-diagnosed asthma’. However, from the perspective of clinical professionals, the symptoms and signs of asthma are multifaceted and vary among patients. In this sense, such a definition of the epistatic effects (ie, presence or absence of disease) is inappropriate because the definition depends largely on the clinical decisions by individual physicians, particularly in a large-scale study, even when diagnostic criteria were consistently set in the genetic study. Accordingly, we need to determine a clear and objective definition of the epistatic effects so that all researchers can make the same judgment based on the presence or absence of the epistatic effects (Figure 1). To that end, it is ideal to focus on quantitatively measurable parameters representing disease components along the presumable pathway between disease and genotype, thus eliminating ambiguity of the definition of epistatic effects. In this sense, total IgE levels in blood, FEV1, and AHR can be objectively measured and thus are appropriate for clear definitions of the epistatic effects [89]. Our recent study design is an example of this approach [83]. Such quantitatively measurable parameters provide other advantages for genetic studies. In general, a patient exhibits a subset of quantitative characteristics of asthma, such as eosinophilic or neutrophilic inflammation, lower baseline FEV1, and AHR, adding a level of complexity. In contrast, some individuals have only a single salient feature, as seen in young adults with asymptomatic AHR, who are likely to be suitable for genetic studies because confounding features can be minimized. In this context, a genetic approach using quantitatively measurable parameters is well suited for the identification of risk factors in individuals who are likely to have disease prior to the complete development of significant symptoms and signs. Furthermore, it seems worthy to combine this style of genetic approach with prospective research to follow individuals at risk for disease. However, we need to be aware that findings obtained from this approach are unlikely to capture the overall variety of disease features, because this approach limits findings to a specific feature of the disease at some structural or functional level in a single study. To show clinical relevance, we will need to accumulate evidence from similar approaches with different objective parameters and different combinations of molecules.
Figure 1.
Clinical application of gene-gene interactions (epistasis) focused on measurable and objective features. Epistasis is defined as the interaction between two different factors (gene-gene) affecting phenotypes. It seems reasonable for clinical application of epistatic analyses to focus on a specific measurable and objective feature among plausible functional or pathophysiological mechanisms. Please see the section entitled ‘Considerations about clinical application of epistatic analyses in asthma research – limits and potentials’.
Conclusions
Phenotypes and genotypes are the most basic concepts in clinical science as well as in genetics and evolutionary biology, and phenotypes usually connect to multiple genes through complex and entangled networks. To unravel these networks and to investigate true causal gene-phenotype couplings, we should take advantage of epistatic analyses, which offers a clear definition of disease features defined by measurable parameters along plausible functional or pathophysiological mechanisms. This type of study design facilitates identification of clinically relevant factors that are the core goal of clinical genetic studies. Future advances in this style of research will prompt identification of susceptibility of diseases in individuals at risk and will help develop of a new generation of pharmaceutical strategies to treat common respiratory diseases such as asthma.
Acknowledgments
The authors thank Forte Science Communications for editorial help with the manuscript.
Footnotes
Declaration of interest
No conflict of interest.
Source of support: This study was supported by a Grants-in-Aid for Scientific Research (KAKENHI: 20590902) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) in Japan
References
- 1.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavlopoulos GA, Oulas A, Iacucci E, et al. Unraveling genomic variation from next generation sequencing data. BioData Min. 2013;6:13. doi: 10.1186/1756-0381-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson TA, Manolio TA. How to interpret a genome-wide association study. JAMA. 2008;299:1335–44. doi: 10.1001/jama.299.11.1335. [DOI] [PubMed] [Google Scholar]
- 4.Tamari M, Tanaka S, Hirota T. Genome-wide association studies of allergic diseases. Allergol Int. 2013;62:21–28. doi: 10.2332/allergolint.13-RAI-0539. [DOI] [PubMed] [Google Scholar]
- 5.Cookson WO, Moffatt MF. Genetics of complex airway disease. Proc Am Thorac Soc. 2011;8:149–53. doi: 10.1513/pats.201101-003MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers AJ, Raby BA, Lasky-Su JA, et al. Assessing the reproducibility of asthma candidate gene associations, using genome-wide data. Am J Respir Crit Care Med. 2009;179:1084–90. doi: 10.1164/rccm.200812-1860OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakaoka H, Inoue I. Meta-analysis of genetic association studies: methodologies, between-study heterogeneity and winner’s curse. J Hum Genet. 2009;54:615–23. doi: 10.1038/jhg.2009.95. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy MI, Abecasis GR, Cardon LR, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–69. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 9.Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–60. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012;67:835–46. doi: 10.1111/j.1398-9995.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhong H, Yang X, Kaplan LM, et al. Integrating pathway analysis and genetics of gene expression for genome-wide association studies. Am J Hum Genet. 2010;86:581–91. doi: 10.1016/j.ajhg.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Maxwell S, Feng T, et al. Gene, pathway and network frameworks to identify epistatic interactions of single nucleotide polymorphisms derived from GWAS data. BMC Syst Biol. 2012;6(Suppl 3):S15. doi: 10.1186/1752-0509-6-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Kwon MS, Park T. Network graph analysis of gene-gene interactions in genome-wide association study data. Genomics Inform. 2012;10:256–62. doi: 10.5808/GI.2012.10.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epistasis: Gene Interaction and Phenotype Effects. http://www.nature.com/scitable/topicpage/epistasis-gene-interaction-and-phenotype-effects-460.
- 16.Bateson W. Experiments with poultry. Rep Evol Comm Roy Soc. 1902;1:87–124. [Google Scholar]
- 17.Bateson W, Saunders ER, Punnett RC. Experimental studies in the physiology of heredity. Reports to the Evolution Committee of Royal Society. 1904;2:1–154. [Google Scholar]
- 18.Bateson W. Mendel’s Principles of Heredity. Cambridge: Cambridge University Press; 1909. [Google Scholar]
- 19.Phillips PC. Epistasis-the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet. 2008;9:855–67. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordell HJ. Epistasis: what it means, what it doesn’t mean, and statistical methods to detect it in humans. Hum Mol Genet. 2002;11:2463–68. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- 21.Lehner B. Molecular mechanisms of epistasis within and between genes. Trends Genet. 2011;27:323–31. doi: 10.1016/j.tig.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Phillips PC. The language of gene interaction. Genetics. 1998;149:1167–71. doi: 10.1093/genetics/149.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, Li S, Cui Y. Genetic association studies: an information content perspective. Curr Genomics. 2012;13:566–73. doi: 10.2174/138920212803251382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan X, Yang C, Yu W. Comments on ‘An empirical comparison of several recent epistatic interaction detection methods’. Bioinformatics. 2012;28:145–46. doi: 10.1093/bioinformatics/btr596. [DOI] [PubMed] [Google Scholar]
- 25.Gibson TJ. Cell regulation: determined to signal discrete cooperation. Trends Biochem Sci. 2009;34:471–82. doi: 10.1016/j.tibs.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Vert G, Chory J. Crosstalk in cellular signaling: background noise or the real thing? Dev Cell. 2011;21:985–91. doi: 10.1016/j.devcel.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- 28.Schulte G, Levy FO. Novel aspects of G-protein-coupled receptor signalling – different ways to achieve specificity. Acta Physiol. 2007;190:33–38. doi: 10.1111/j.1365-201X.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 29.Kasarskis A, Yang X, Schadt E. Integrative genomics strategies to elucidate the complexity of drug response. Pharmacogenomics. 2011;12:1695–715. doi: 10.2217/pgs.11.115. [DOI] [PubMed] [Google Scholar]
- 30.Jain P, Vig S, Datta M, et al. Systems biology approach reveals genome to phenome correlation in type 2 diabetes. PLoS One. 2013;8:e53522. doi: 10.1371/journal.pone.0053522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma L, Clark AG, Keinan A. Gene-based testing of interactions in association studies of quantitative traits. PLoS Genet. 2013;9:e1003321. doi: 10.1371/journal.pgen.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams SM, Ritchie MD, Phillips JA, III, et al. Multilocus analysis of hypertension: a hierarchical approach. Hum Hered. 2004;57:28–38. doi: 10.1159/000077387. [DOI] [PubMed] [Google Scholar]
- 33.Rana BK, Insel PA, Payne SH, et al. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 34.Ndiaye NC, Said el S, Stathopoulou MG, et al. Epistatic study reveals two genetic interactions in blood pressure regulation. BMC Med Genet. 2013;14:2. doi: 10.1186/1471-2350-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh CH, Liang KH, Hung YJ, et al. Analysis of epistasis for diabetic nephropathy among type 2 diabetic patients. Hum Mol Genet. 2006;15:2701–8. doi: 10.1093/hmg/ddl203. [DOI] [PubMed] [Google Scholar]
- 36.Nicodemus KK, Kolachana BS, Vakkalanka R, et al. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet. 2007;120:889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- 37.Talkowski ME, Kirov G, Bamne M, et al. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17:747–58. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou XY, Chen GB, Yan L, et al. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. 2007;80:1125–37. doi: 10.1086/518312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 40.Beasley R, Roche WR, Roberts JA, et al. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989;139:806–17. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- 41.Howard TD, Koppelman GH, Xu J, et al. Gene-gene interaction in asthma: IL4RA and IL13 in a Dutch population with asthma. Am J Hum Genet. 2002;70:230–36. doi: 10.1086/338242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SG, Kim BS, Kim JH, et al. Gene-gene interaction between interleukin-4 and interleukin-4 receptor alpha in Korean children with asthma. Clin Exp Allergy. 2004;34:1202–8. doi: 10.1111/j.1365-2222.2004.02015.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim HB, Lee YC, Lee SY, et al. Gene-gene interaction between IL-13 and IL-13Ralpha1 is associated with total IgE in Korean children with atopic asthma. J Hum Genet. 2006;51:1055–62. doi: 10.1007/s10038-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 44.Battle NC, Choudhry S, Tsai HJ, et al. Ethnicity-specific gene-gene interaction between IL-13 and IL-4Ralpha among African Americans with asthma. Am J Respir Crit Care Med. 2007;175:881–87. doi: 10.1164/rccm.200607-992OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adjers K, Pessi T, Karjalainen J, et al. Epistatic effect of IL1A and IL4RA genes on the risk of atopy. J Allergy Clin Immunol. 2004;113:445–47. doi: 10.1016/j.jaci.2003.12.582. [DOI] [PubMed] [Google Scholar]
- 46.Adjers K, Karjalainen J, Pessi T, et al. Epistatic effect of TLR4 and IL4 genes on the risk of asthma in females. Int Arch Allergy Immunol. 2005;138:251–56. doi: 10.1159/000088726. [DOI] [PubMed] [Google Scholar]
- 47.Yang KD, Chang JC, Chuang H, et al. Gene-gene and gene-environment interactions on IgE production in prenatal stage. Allergy. 2010;65:731–39. doi: 10.1111/j.1398-9995.2009.02260.x. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Li Y, Chen Q, et al. Association and gene-gene interactions of eight common single-nucleotide polymorphisms with pediatric asthma in middle china. J Asthma. 2010;47:238–44. doi: 10.3109/02770900903509099. [DOI] [PubMed] [Google Scholar]
- 49.Choi WA, Kang MJ, Kim YJ, et al. Gene-gene interactions between candidate gene polymorphisms are associated with total IgE levels in Korean children with asthma. J Asthma. 2012;49:243–52. doi: 10.3109/02770903.2012.660294. [DOI] [PubMed] [Google Scholar]
- 50.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 51.Bottema RW, Kerkhof M, Reijmerink NE, et al. Gene-gene interaction in regulatory T-cell function in atopy and asthma development in childhood. J Allergy Clin Immunol. 2010;126:338–46. doi: 10.1016/j.jaci.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Bottema RW, Postma DS, Reijmerink NE, et al. Interaction of T-cell and antigen presenting cell co-stimulatory genes in childhood IgE. Eur Respir J. 2010;35:54–63. doi: 10.1183/09031936.00018909. [DOI] [PubMed] [Google Scholar]
- 53.Kim SH, Yang EM, Lee HN, et al. Combined effect of IL-10 and TGF-beta1 promoter polymorphisms as a risk factor for aspirin-intolerant asthma and rhinosinusitis. Allergy. 2009;64:1221–25. doi: 10.1111/j.1398-9995.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- 54.Braun-Fahrländer C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 55.Liu AH. Endotoxin exposure in allergy and asthma: reconciling a paradox. J Allergy Clin Immunol. 2002;109:379–92. doi: 10.1067/mai.2002.122157. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 57.Koppelman GH, Reijmerink NE, Colin Stine O, et al. Association of a promoter polymorphism of the CD14 gene and atopy. Am J Respir Crit Care Med. 2001;163:965–69. doi: 10.1164/ajrccm.163.4.2004164. [DOI] [PubMed] [Google Scholar]
- 58.Bucková D, Hollá LI, Schüller M, et al. Two CD14 promoter polymorphisms and atopic phenotypes in Czech patients with IgE-mediated allergy. Allergy. 2003;58:1023–26. doi: 10.1034/j.1398-9995.2003.00271.x. [DOI] [PubMed] [Google Scholar]
- 59.Woo JG, Assa’ad A, Heizer AB, et al. The -159 C→T polymorphism of CD14 is associated with nonatopic asthma and food allergy. J Allergy Clin Immunol. 2003;112:438–44. doi: 10.1067/mai.2003.1634. [DOI] [PubMed] [Google Scholar]
- 60.Martin AC, Laing IA, Khoo SK, et al. Acute asthma in children: Relationships among CD14 and CC16 genotypes, plasma levels, and severity. Am J Respir Crit Care Med. 2006;173:617–22. doi: 10.1164/rccm.200509-1367OC. [DOI] [PubMed] [Google Scholar]
- 61.Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–5. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 62.Barnes KC, Grant A, Gao P, et al. Polymorphisms in the novel gene acyloxyacyl hydroxylase (AOAH) are associated with asthma and associated phenotypes. J Allergy Clin Immunol. 2006;118:70–77. doi: 10.1016/j.jaci.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 63.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–39. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 64.Asikainen TM, Raivio KO, Saksela M, et al. Expression and developmental profile of antioxidant enzymes in human lung and liver. Am J Respir Cell Mol Biol. 1998;19:942–49. doi: 10.1165/ajrcmb.19.6.3248. [DOI] [PubMed] [Google Scholar]
- 65.Nadif R, Kleeberger SR, Kauffmann F. Polymorphisms in manganese superoxide dismutase and catalase genes: functional study in Hong Kong Chinese asthma patients. Clin Exp Allergy. 2006;36:1104–5. doi: 10.1111/j.1365-2222.2006.02545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millstein J, Conti DV, Gilliland FD, et al. A testing framework for identifying susceptibility genes in the presence of epistasis. Am J Hum Genet. 2006;78:15–27. doi: 10.1086/498850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambert RK, Wiggs BR, Kuwano K, et al. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol. 1993;74:2771–81. doi: 10.1152/jappl.1993.74.6.2771. [DOI] [PubMed] [Google Scholar]
- 68.Bramley AM, Thomson RJ, Roberts CR, et al. Hypothesis: excessive bronchoconstriction in asthma is due to decreased airway elastance. Eur Respir J. 1994;7:337–41. doi: 10.1183/09031936.94.07020337. [DOI] [PubMed] [Google Scholar]
- 69.Kucharewicz I, Kowal K, Buczko W, Bodzenta-Łukaszyk A. The plasmin system in airway remodeling. Thromb Res. 2003;112:1–7. doi: 10.1016/j.thromres.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Cho SH, Tam SW, Demissie-Sanders S, Filler SA, Oh CK. Production of plasminogen activator inhibitor-1 by human mast cells and its possible role in asthma. J Immunol. 2000;165:3154–61. doi: 10.4049/jimmunol.165.6.3154. [DOI] [PubMed] [Google Scholar]
- 71.Hizawa N, Maeda Y, Konno S, et al. Genetic polymorphisms at FCER1B and PAI-1 and asthma susceptibility. Clin Exp Allergy. 2006;36:872–76. doi: 10.1111/j.1365-2222.2006.02413.x. [DOI] [PubMed] [Google Scholar]
- 72.Jansen DF, Timens W, Kraan J, et al. (A) symptomatic bronchial hyper-responsiveness and asthma. Respir Med. 1997;91:121–34. doi: 10.1016/s0954-6111(97)90048-2. [DOI] [PubMed] [Google Scholar]
- 73.Hopp RJ, Townley RG, Biven RE, et al. The presence of airway reactivity before the development of asthma. Am Rev Respir Dis. 1990;141:2–8. doi: 10.1164/ajrccm/141.1.2. [DOI] [PubMed] [Google Scholar]
- 74.Brutsche MH, Downs SH, Schindler C, et al. Bronchial hyperresponsiveness and the development of asthma and COPD in asymptomatic individuals: SAPALDIA cohort study. Thorax. 2006;61:671–77. doi: 10.1136/thx.2005.052241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992–96. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puddicombe SM, Polosa R, Richter A, et al. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–74. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 77.Wang X, Saito J, Ishida T, et al. Polymorphism of egfr Intron1 is associated with susceptibility and severity of asthma. J Asthma. 2006;43:711–15. doi: 10.1080/02770900600925247. [DOI] [PubMed] [Google Scholar]
- 78.Blanc-Brude OP, Archer F, Leoni P, et al. Factor Xa stimulates fibroblast procollagen production, proliferation, and calcium signaling via PAR1 activation. Exp Cell Res. 2005;304:16–27. doi: 10.1016/j.yexcr.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 79.Molloy CJ, Pawlowski JE, Taylor DS, et al. Thrombin receptor activation elicits rapid protein tyrosine phosphorylation and stimulation of the raf-1/MAP kinase pathway preceding delayed mitogenesis in cultured rat aortic smooth muscle cells: evidence for an obligate autocrine mechanism promoting cell proliferation induced by G-protein-coupled receptor agonist. J Clin Invest. 1996;97:1173–83. doi: 10.1172/JCI118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herbert JM, Dupuy E, Laplace MC, et al. Thrombin induces endothelial cell growth via both a proteolytic and a non-proteolytic pathway. Biochem J. 1994;303:227–31. doi: 10.1042/bj3030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanazawa H, Yoshikawa T. Upregulation of thrombin activity induced by vascular endothelial growth factor in asthmatic airways. Chest. 2007;132:1169–74. doi: 10.1378/chest.07-0945. [DOI] [PubMed] [Google Scholar]
- 82.Dery O, Corvera CU, Steinhoff M, et al. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–52. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 83.Yoshikawa T, Kanazawa H. Integrated effect of two genotypes with signal crosstalk on airway hyperresponsiveness (AHR) Int J Mol Med. 2012;30:41–48. doi: 10.3892/ijmm.2012.981. [DOI] [PubMed] [Google Scholar]
- 84.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Acevedo N, Sääf A, Söderhäll C, et al. Interaction between Retinoid Acid Receptor-Related Orphan Receptor Alpha (RORA) and Neuropeptide S Receptor 1 (NPSR1) in Asthma. PLoS One. 2013;8:e60111. doi: 10.1371/journal.pone.0060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orsmark-Pietras C, Melen E, Vendelin J, et al. Biological and genetic interaction between tenascin C and neuropeptide S receptor 1 in allergic diseases. Hum Mol Genet. 2008;17:1673–82. doi: 10.1093/hmg/ddn058. [DOI] [PubMed] [Google Scholar]
- 87.De Lobel L, Geurts P, Baele G, et al. A screening methodology based on Random Forests to improve the detection of gene-gene interactions. Eur J Hum Genet. 2010;18:1127–32. doi: 10.1038/ejhg.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ungvari I, Hullam G, Antal P, et al. Evaluation of a partial genome screening of two asthma susceptibility regions using bayesian network based bayesian multilevel analysis of relevance. PLoS One. 2012;7:e33573. doi: 10.1371/journal.pone.0033573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nomura N, Yoshikawa T, Kamoi H, et al. Induced sputum analysis in asymptomatic young adults with bronchial hyperresponsiveness to methacholine. Respirology. 2007;12:516–22. doi: 10.1111/j.1440-1843.2007.01103.x. [DOI] [PubMed] [Google Scholar]