Abstract
Adenylyl cyclase activity can be reconstituted by simple mixture of the two cytosolic domains of the enzyme after their independent synthesis in Escherichia coli. We have synthesized and purified the C1a domain of type I adenylyl cyclase and the C2 domain of the type II enzyme to assess their interactions with each other and with the activators Gsalpha and forskolin. In the absence of an activator, the fragments associate with low affinity and display low catalytic activity. This basal activity can be stimulated more than 100-fold by either forskolin or activated Gsalpha. Further, the addition of these activators increases the apparent affinity of the fragments for each other. Stimulation of catalysis by Gsalpha and forskolin is synergistic. These data suggest a model wherein either Gsalpha or forskolin enhances association of the other activator with adenylyl cyclase, as well as facilitating the interaction between the C1 and C2 domains of the enzyme.
Full text
PDF
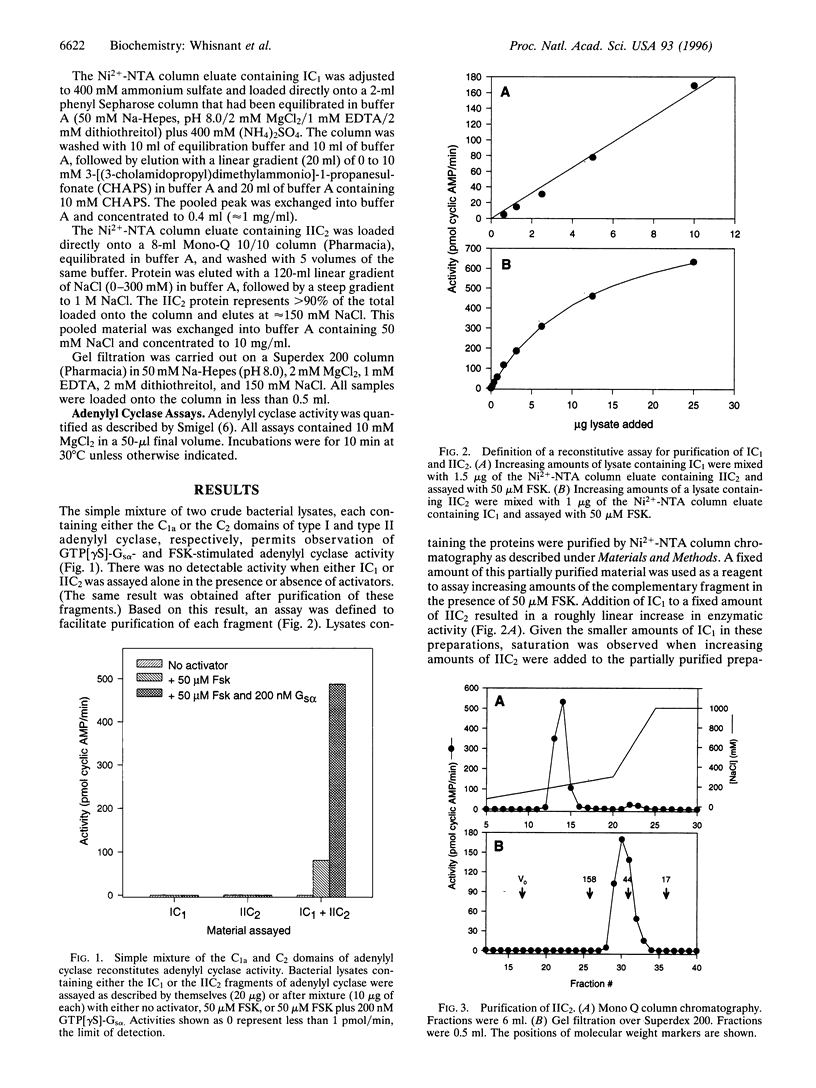
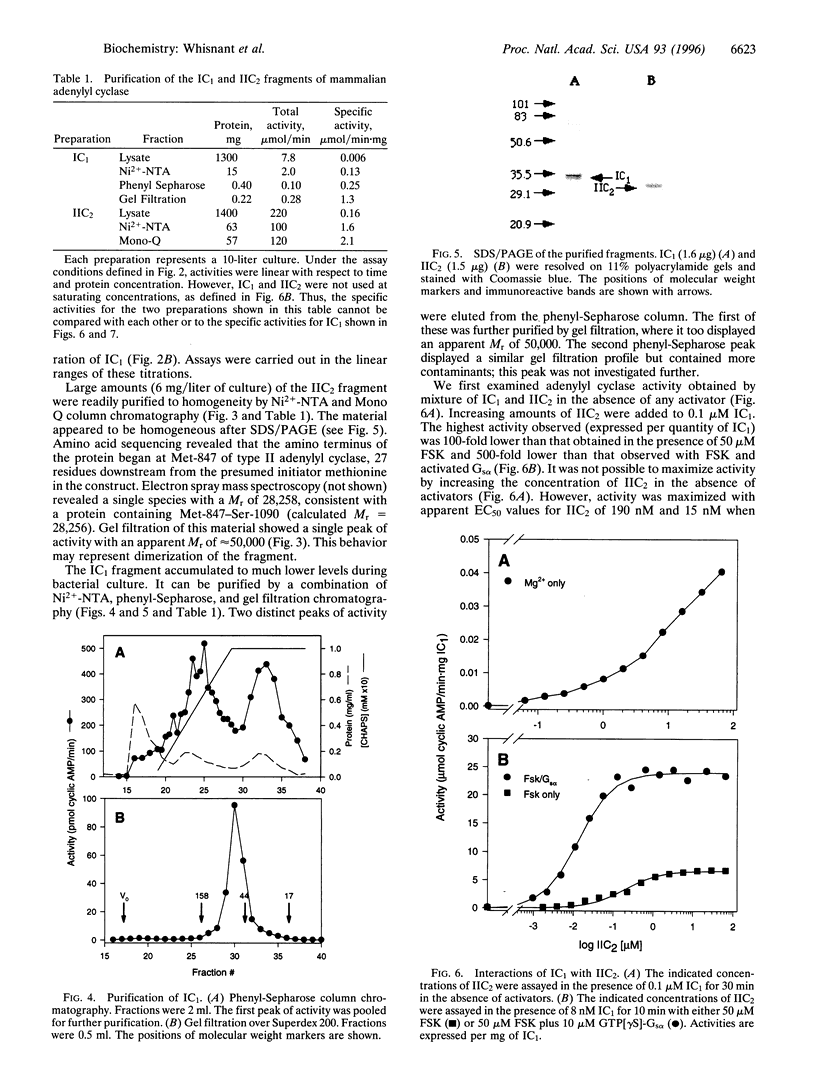
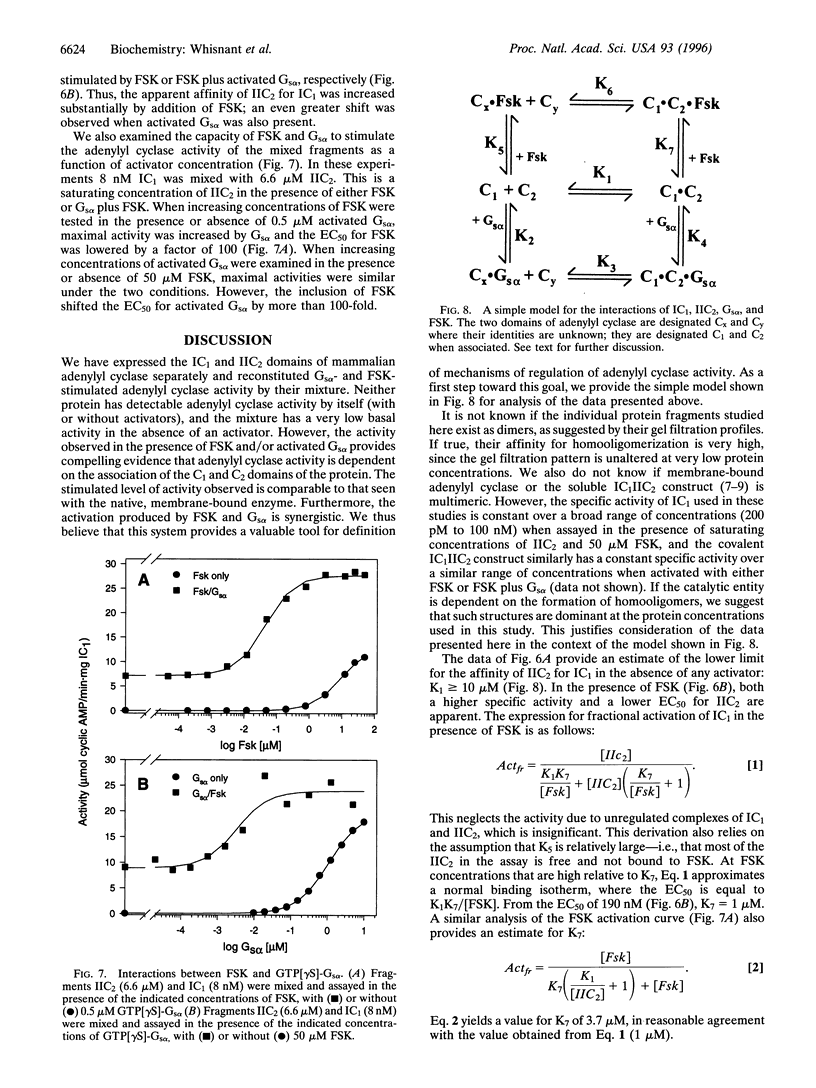

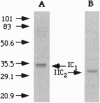
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feinstein P. G., Schrader K. A., Bakalyar H. A., Tang W. J., Krupinski J., Gilman A. G., Reed R. R. Molecular cloning and characterization of a Ca2+/calmodulin-insensitive adenylyl cyclase from rat brain. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10173–10177. doi: 10.1073/pnas.88.22.10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Echeverria D., Knox S. Increase in the size of soluble brain adenylate cyclase with activation by guanosine 5'-(beta, gamma-imino)triphosphate. J Biol Chem. 1980 Oct 25;255(20):9782–9789. [PubMed] [Google Scholar]
- Pfeuffer E., Dreher R. M., Metzger H., Pfeuffer T. Catalytic unit of adenlyate cyclase: purification and identification by affinity crosslinking. Proc Natl Acad Sci U S A. 1985 May;82(10):3086–3090. doi: 10.1073/pnas.82.10.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigel M. D. Purification of the catalyst of adenylate cyclase. J Biol Chem. 1986 Feb 5;261(4):1976–1982. [PubMed] [Google Scholar]
- Sunahara R. K., Dessauer C. W., Gilman A. G. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Construction of a soluble adenylyl cyclase activated by Gs alpha and forskolin. Science. 1995 Jun 23;268(5218):1769–1772. doi: 10.1126/science.7792604. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Krupinski J., Gilman A. G. Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J Biol Chem. 1991 May 5;266(13):8595–8603. [PubMed] [Google Scholar]
- Tang W. J., Stanzel M., Gilman A. G. Truncation and alanine-scanning mutants of type I adenylyl cyclase. Biochemistry. 1995 Nov 7;34(44):14563–14572. doi: 10.1021/bi00044a035. [DOI] [PubMed] [Google Scholar]
- Taussig R., Gilman A. G. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995 Jan 6;270(1):1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]