Abstract
Solid-liquid extraction of phytic acid (PA) from rice bran was optimized by the maximization of the yield using response surface methodology. A Box-Behnken design was used to monitor the effects of three processing parameters of extraction on the PA yield, including ratio of acid solution to raw material (mL/g), hydrochloric acid concentration (mol/L), and extraction time (h). The results showed that the optimal conditions were acid solution/raw material of 8.5:1 (mL/g), HCl concentration 0.62 mol/L and extraction time 5.5 h. Validation tests indicated that the actual yield of PA was (2.15 ± 0.02)% with RSD = 1.92% (n = 5) under the optimized conditions, which was in good agreement with predicted yield. Antioxidant assays suggested that the extracted PA had weaker DPPH, hydroxyl and superoxide free-radical-scavenging capabilities than vitamin C at the same concentration of 0.5 mg/mL.
Keywords: Phytic acid, Rice bran, Extraction, Antioxidant activity
Introduction
Sustainable food production and waste valorization have become important issues in modern life. Rice bran is a byproduct from milling and has many nutraceuticals, including vegetable oils, phytic acid (PA), and phytosterol. PA, inositol hexakisphosphate (IP6), is an organic compound containing phosphorus. Many studies have shown that PA has a broad spectrum of biological activities, such as antioxidant (Ahn et al. 2003), anticancer (Norazalina et al. 2010; Schröterová et al. 2010), neuroprotection (Xu et al. 2008), prevention of atherosclerosis (Grases et al. 2007), and formation of renal stone (Grases et al. 2000). Therefore, more and more nutritionists pay attention on the amount of phytate in food (Amaro et al. 2009). China has become the world’s largest producer of paddy, with annual yield of over 200 million tons (Itagi and Singh 2011). However, nearly 90% of rice bran was directly made into animal feeds because of low processing cost. In order to promote utilization of rice bran and develop high-valued products, investigation of PA extraction from rice bran was carried out in our lab.
Solid-liquid extraction using acid solutions, such as HCl and H2SO4, is the most common method used for the extraction of PA from rice bran (Wu et al. 2009). Preliminary trials in our laboratory showed that three extraction conditions, ratio of acid solution to raw material (mL/g), hydrochloric acid concentration (mol/L) and extraction time (h), might have significant influence on the yield. Response surface methodology (RSM) is a collection of statistical and mathematical techniques and is effective for responses that are influenced by many factors and their interactions (Myers and Montgomery 2002). To maximize levels of PA yield, these extraction conditions were optimized in this study. Up to now, it is the first study to describe the optimization of solid-liquid extraction process of PA from rice bran using RSM.
Materials and methods
Materials
Rice bran was supplied by Jiashan Industry and Trade of Grain and Oil Food Co.,Ltd (Zhejiang Province, China), with 9.5% water content. After finely milled with a muller (HX-200, Zhejiang Yongkang Xi’an Hardware Officinal Instrument Factory, Yongkang, China) and sieved through a 20 mesh sieve, the fine material was packed in plastic bag and stored at −20 °C. PA (sodium salt), 95%, w/w; 2,2-diphenyl-1-picrylhydrazyl (DPPH); deoxyribose, 97%, w/w; nicotinamide adenine dinucleotide, reduced dipotassium salt (NADH), 96.5%, w/w; phenazine methosulfate (PMS), 96%, w/w; and nitro blue tetrazolium (NBT) chloride, 98%, w/w, were purchased from Sigma-Aldrich Chemical Co. (St. Louis, USA). Ion-exchange resin (AG1-X8 of Clˉ, 200–400 mesh) was obtained from Bio-Rad Laboratories (Richmond, USA). Qualitative filter paper was produced by Hangzhou Xinhua Paper Industry Co.,Ltd (Hangzhou, China). All other chemicals used in this study were of analytical grade. The WADE reagent consisted of 0.15 g FeCl3·6H2O and 1.5 g sulphosalicylic acid in 500 mL water.
Extraction of PA
About 5.0 g of pretreated rice bran was put into a 200 mL beaker and extracted under different conditions at room temperature. The ranges of three variables were listed in Table 1. After extraction, the extract was filtered through filter paper, and deionized water was added to bring the final volume of the filtrate to 100 mL.
Table 1.
Code and level of independent variable chosen for Box-Behnken design
| Variables | Symbol | Levels | |||
|---|---|---|---|---|---|
| Coded | Uncoded | −1 | 0 | −1 | |
| Acid solvent/raw material (mL/g) | x1 | X 1 | 6:1 | 8:1 | 10:1 |
| HCl concentration (mol/L) | x2 | X 2 | 0.4 | 0.6 | 0.8 |
| Extraction time (h) | x3 | X 3 | 4 | 5 | 6 |
Determination of PA yield
PA content of the sample extract was determined by the spectrophotometric method of Latta and Eskin (1980) with some modifications. A chromatographic column (1.5 × 30 cm) was filtered with 2 g of AG1-X8 anion-exchange resin and washed with 60 mL of 1.62 mol/L HCl followed by 80 mL deionized water. About 40 mL of the extract were poured through the column followed by 60 mL of 0.1 mol/L NaCl and the eluant was discarded. The 60 mL of 0.7 mol/L NaCl was added to the column. The eluting solution was collected, which the final volume was made up to 100 mL. 1 mL of the WADE reagent was added to 3 mL of the purified extract and the absorbance was read at 500 nm (Hitachi UV-3000, Japan). PA (sodium salt) was used to prepare the standard curve which was used to calculate the actual PA content in the extract.
The extraction yield of PA was calculated by the following equation:
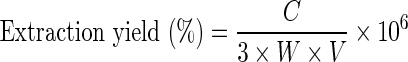 |
1 |
where C is the concentration of PA in the purified extract, g/mL; W is the amount of rice bran, g; V is the volume of the unpurified extract, mL.
Experimental design
Box-Behnken design (BBD) was employed to statistically optimize the formulation parameters and evaluate main effects, interaction effects and quadratic effects of the formulation ingredients on the extraction yields of PA (Ajay et al. 2010; Mahajan et al. 2010). According to the principle of BBD, acid solution/raw material, HCl concentration and extraction time were taken as the variables tested in a 17-run experiment. As shown in Table 1, three factors chosen for this study were designated as X1, X2, and X3, and were prescribed into three levels, coded +1, 0, –1 for high, intermediate, low value, respectively. Test variables were coded according to the following equation:
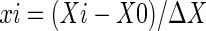 |
2 |
where xi is the coded value of an independent variable; Xi is the actual value of an independent variable; X0 is the actual value of an independent variable at central point; and ΔX is the step change value of an independent variable. All experiments were performed in triplicate and the averages of PA yield were taken as response.
For predicting the optimal point, a second-order polynomial model was fitted to correlate relationship between independent variables and response (PA yield). For the three factors, the equation was
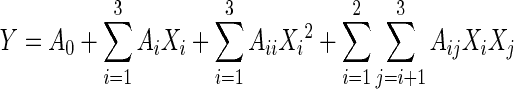 |
3 |
where Y is the response variables (PA yields in real values). A0, Ai, Aj and Aij are the regression coefficients of variables for intercept, linear, quadratic and interaction terms, respectively. Xi and Xj are independent variables (i ≠ j).
Analysis of the experimental design and data were carried out using Design-Expert (Version 7.1.3). The models were predicted through statistical analysis and regression analysis (ANOVA), and the fitness of the polynomial model equation was expressed by the coefficient of determination R2. Models and regression coefficients were considered significant when p-values were lower than 0.05. The optimal extraction conditions of acid solution/raw material, HCl concentration and extraction time were calculated by the software MATLAB (Version 7.0.1) (Shampine and Thompson 2001).
DPPH Free Radical scavenging activity
The free radical scavenging activity of the extracted solution was measured by DPPH test according to the method of Shimada et al. (1992) with some modifications. The 0.2 mM solution of DPPH in ethanol was prepared daily before UV measurements. 1 mL of the purified extract with a concentration of 0.5 mg/mL PA was thoroughly mixed with 2 mL of freshly prepared DPPH and 2 mL of ethanol. The mixture was shaken well, allowed to stand for 30 min in the dark, and the absorbance was then measured at 517 nm against a blank. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity, which was analyzed from the graph plotted of inhibition percentage against compound concentration. Vitamin C (Vc) was used as positive control. The experiments were carried out in triplicate and averaged. The capability to scavenge the DPPH radical was calculated using the following equation:
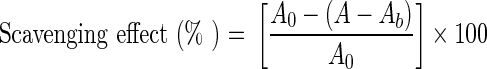 |
4 |
where A0 was the absorbance of DPPH solution without sample solution; A was the absorbance of the test sample mixed with DPPH solution and Ab was the absorbance of the sample without DPPH solution.
Hydroxyl free radical scavenging activity
The hydroxyl radical assay was carried out using the method of Ghiselli et al. (1998) with some modifications. 0.1 mL of the purified extract (0.5 mg/mL PA) was mixed with 0.6 mL of reaction buffer [20 mM phosphate buffer (pH 7.4), 2.67 mM deoxyribose, and 100 μM EDTA], 0.2 mL of 0.4 mM ferrous ammonium sulfate, 0.05 mL of 2.0 mM Vc, and 0.05 mL of 10 mM H2O2 was then added to the reaction solution. The reaction solution was incubated for 15 min at 37 °C, and then 1 mL of 1% thiobarbituric acid (TBA) and 1 mL of 2% trichloroacetic acid (TCA) were added to terminate the reaction. The mixture was boiled for 15 min and cooled to room temperature. The absorbance of the mixture was measured at 532 nm against blank. The capability of scavenge hydroxyl radical was calculated according to the following equation:
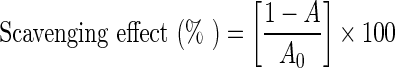 |
5 |
where A0 was the absorbance of mixture solution without sample; A was the absorbance of the test sample mixed with reaction solution.
Superoxide free radical scavenging activity
The superoxide radical scavenging assay was assessed according to the method of Qi et al. (2005). Superoxide radicals were generated in a PMS/NADH system for being assayed in the reduction of NBT. The reaction mixture, containing the purified extract with 0.5 mg/mL PA, Tris-HCl (16 mM, pH 8.0), NADH (338 μM), NBT (72 μM) and PMS (30 μM) was incubated at room temperature for 5 min and the absorbance was read at 560 nm. The capability of scavenging superoxide radical was also calculated using the Eq. 5.
Results and discussion
Extraction model and statistical analysis
There were a total of 17 runs for optimizing the three individual parameters in the current BBD which was applied to the production of PA from rice bran. The response values at different experimental combination for coded variables were listed in Table 2. The PA yields ranged from 1.86% to 2.14%. By applying multiple regression analysis on the experimental data, the response variable and the test variables were related by the following second-order polynomial equation:
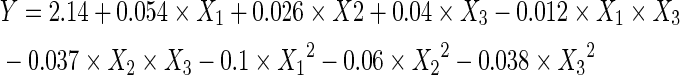 |
6 |
Table 2.
BBD and the response values for the PA yields
| Run | X1 (Acid solution/raw material, mL/g) | X2 (HCl concentration, mol/L) | X3 (Extraction time, h) | Response (PA yield, %) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 1.86 |
| 2 | 0 | −1 | 1 | 2.11 |
| 3 | −1 | 1 | 0 | 1.94 |
| 4 | 0 | 0 | 0 | 2.14 |
| 5 | −1 | 0 | −1 | 1.92 |
| 6 | 1 | 0 | −1 | 2.01 |
| 7 | 0 | 0 | 0 | 2.13 |
| 8 | 1 | −1 | 0 | 2.01 |
| 9 | 0 | 1 | −1 | 2.04 |
| 10 | 0 | 0 | 0 | 2.13 |
| 11 | 1 | 1 | 0 | 2.09 |
| 12 | 0 | 1 | 1 | 2.06 |
| 13 | 0 | 0 | 0 | 2.14 |
| 14 | 0 | −1 | −1 | 1.94 |
| 15 | 0 | 0 | 0 | 2.14 |
| 16 | 1 | 0 | 1 | 2.05 |
| 17 | −1 | 0 | 1 | 2.01 |
The ANOVA of the quadratic regression model showed that the values of the determination coefficient (R2) and the adjusted determination coefficient (Adj. R2) were 0.9539 and 0.8946, respectively, which indicated that a high degree of correlation between the observed and predicted values. Moreover, a low value of coefficient of the variation (CV = 1.40%) indicated a high degree of precision and a good deal of reliability of the experimental values (Garg and Singh 2010).
The P values are used to check the significance of each coefficient, which in turn may indicate the pattern of the interaction between the variables. The coefficient estimate for the parameter optimization suggested that three independent variables (X1, X2, and X3), one interaction term (X2X3), and all quadratic terms (X1X1, X2X2, X3X3) had significant effect on the PA yield (P < 0.05). The results of the study showed that acid solution/raw material was the most significant single factor which influenced the PA yield followed by extraction time and HCl concentration.
Optimization of the procedure by RSM
Eq. 6 allowed the prediction of the effects of the three factors on the PA yield. Three independent response surface plots and their respective contour plots were shown in Figs. 1 and 2. Two variables within the experimental range were depicted in 3D surface plots while the other variable was kept constant at zero level. The shapes of the contour plots, circular or elliptical indicated whether the mutual interactions between the variables were significant or not (Liang et al. 2010; Muralidhar et al. 2001).
Fig. 1.
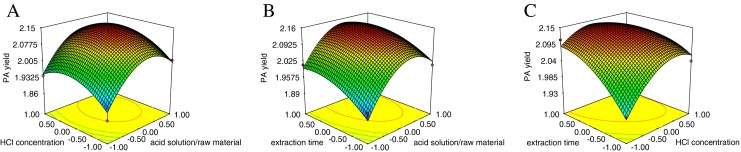
Response surface plots for the effects of (a) acid solution/raw material and HCl concentration; (b) acid solution/raw material and extraction time; (c) HCl concentration and extraction time on the PA yield
Fig. 2.
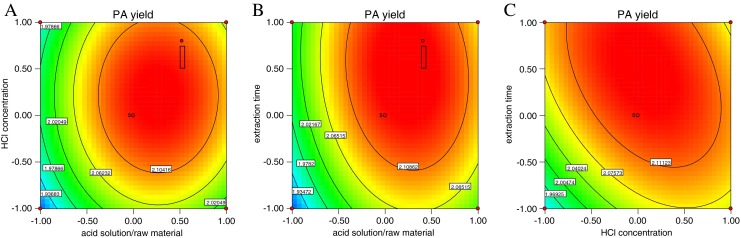
Contour plots for the effects of (a) acid solution/raw material and HCl concentration; (b) acid solution/raw material and extraction time; (c) HCl concentration and extraction time on the PA yield
As shown in Figs. 1 and 2, the increased ratio of acid solution/raw material (X1) and HCl concentration (X2) up to a threshold level led to increased PA yield (see Figs. 1a, b, 2a and b). Beyond this level, the PA yield slightly decreased, which indicated that a greater yield could be achieved if the moderate acid solution/raw material and HCl concentration were selected. Meanwhile, extraction time (X3) had a positive impact on the PA production. There is an increase in the PA yield with an increase in extraction time (see Figs. 1c and 2c).
Therefore, it could be concluded that the optimal conditions for solid-liquid extraction of PA from rice bran were acid solution/raw material 8.5:1, HCl concentration 0.62 mol/L, and extraction time 5.5 h.
Validation of the model
In order to validate the adequacy of the model Eq. 6, a verification experiment was carried out under the optimized conditions mentioned above. Under the optimal conditions, the model predicted a maximum response of 2.16%. A mean value of (2.15 ± 0.02)% with RSD = 1.92% (n = 5), obtained from actual experiments, demonstrated the validation of the optimized extraction model. The good correlation between these results undoubtedly confirmed that the model was adequate for reflecting the predicted optimization.
Antioxidant activity analysis
Table 3 showed the antioxidant capabilities of the extracted PA from rice bran compared with those of Vc at the same concentration of 0.5 mg/mL. The DPPH, hydroxyl and superoxide free-radical-scavenging effects of PA were 25.81%, 15.93%, 5.90%, respectively, which indicated PA extracted from rice bran had weaker antioxidant activities than Vc.
Table 3.
Antioxidant effect of PA from rice bran and Vc at 0.5 mg/mL
| Sample | PA | Vc |
|---|---|---|
| DPPH·(%) | 25.8 ± 2.32 | 46.6 ± 2.05 |
| ·OH–(%) | 15.9 ± 1.85 | 37.1 ± 1.46 |
| ·O–2(%) | 5.9 ± 1.39 | 100 |
Conclusions
An efficient process of solid-liquid extraction had been developed for the PA extraction from rice bran. BBD was successfully employed to optimize extraction parameters in this work. Results indicated that acid solution/raw material 8.5:1, HCl concentration 0.62 mol/L and extraction time 5.5 h were the best conditions to extract PA from rice bran. The maximum PA yield was (2.15 ± 0.02)% (n = 5) under these optimal conditions. Antioxidant assays showed that PA had weaker DPPH, hydroxyl and superoxide free-radical-scavenging activities than Vc at the same concentration of 0.5 mg/mL. This study can be useful to the development of industrial extraction of PA from rice bran, including further studies concerning the optimal number of sequential steps to enhance the efficacy of a large-scale extraction system.
Acknowledgements
Financial supports for this work from the Education Bureau Project for Scientific Research of Zhejiang Province (No. Y200803745) and the Key Brainstorm Project for Science and Technology of Zhejiang Province (No. 2007 C12019) were gratefully acknowledged.
References
- Ahn HJ, Kim JH, Yook HS, Byun MW. Irradiation effects on free radical scavenging and antioxidant activity of phytic acid. J Food Sci. 2003;68:2221–2224. doi: 10.1111/j.1365-2621.2003.tb05750.x. [DOI] [Google Scholar]
- Ajay P, Ramana KV, Bawa AS. Simplification and optimization of deMan Rogosa Sharpe (MRS) medium for enhanced production of bacteriocin by Weissella paramesenteroides DFR-8. J Food Sci Technol. 2010;47(3):258–265. doi: 10.1007/s13197-010-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro R, Murillo M, González Z, Escalona A, Hernández L. Optimization of the treatment of wheat samples for the determination of phytic acid by HPLC with refractive index detection. J AOAC Int. 2009;92:873–878. [PubMed] [Google Scholar]
- Garg SK, Singh DS. Optimization of extrusion conditions for defatted soy-rice blend extrudates. J Food Sci Technol. 2010;47(6):606–612. doi: 10.1007/s13197-010-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli A, Nardini M, Baldi A, Scaccini C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J Agri Food Chem. 1998;46:361–367. doi: 10.1021/jf970486b. [DOI] [PubMed] [Google Scholar]
- Grases F, Simonet BM, March JG, Prieto RM. Inositol hexakisphophate in urine: the relationship between oral intake and urinary excretion. BJU Int. 2000;85:138–142. doi: 10.1046/j.1464-410x.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- Grases F, Sanchis P, Perello J, Isern B, Prieto RM. Effect of crystallization inhibitors on vascular calcifications induced by vitamin D–A pilot study in Sprague-Dawley rats. Cir J. 2007;71:1152–1156. doi: 10.1253/circj.71.1152. [DOI] [PubMed] [Google Scholar]
- Itagi HBN, Singh V (2011) Preparation, nutritional composition, functional properties and antioxidant activities of multigrain composite mixes. J Food Sci Technol. doi:10.1007/s13197-011-0267-6 [DOI] [PMC free article] [PubMed]
- Latta M, Eskin M. A simple and rapid colorimetric method for phytate determination. J Agri Food Chem. 1980;28:1313–1315. doi: 10.1021/jf60232a049. [DOI] [Google Scholar]
- Liang J, Li F, Fang Y, Yang W, An X, Zhao L, Xin Z, Hu Q. Response surface methodology in the optimization of tea polyphenold-loaded chitosan nanoclusters formulations. Euro Food Res Technol. 2010;231:917–924. doi: 10.1007/s00217-010-1341-4. [DOI] [Google Scholar]
- Mahajan BVC, Kaur T, Gill MIS, Dhaliwal HS, Ghuman BS, Chahil BS. Studies on optimization of ripening techniques for banana. J Food Sci Technol. 2010;47(3):315–319. doi: 10.1007/s13197-010-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhar RV, Chirumamila RR, Marchant R, Nigam P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem Eng J. 2001;9:17–23. doi: 10.1016/S1369-703X(01)00117-6. [DOI] [Google Scholar]
- Myers RH, Montgomery DC. Response Surface Methodology: Process and Product in Optimization using Designed Experiments. New York: John Wiley & Sons Inc; 2002. pp. 521–690. [Google Scholar]
- Norazalina S, Norhaizan ME, Hairuszah I, Norashareena MS. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp Toxicol Pathol. 2010;62:259–268. doi: 10.1016/j.etp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Qi HM, Zhang QB, Zhao TT, Chen R, Zhang H, Niu XZ, Li Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol. 2005;37:195–199. doi: 10.1016/j.ijbiomac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Schröterová L, Hasková P, Rudolf E, Cervinka M. Effect of phytic acid and inositol on the proliferation and apotosis of cells derived from colorectal carcinoma. Oncol Rep. 2010;23:787–793. [PubMed] [Google Scholar]
- Shampine LF, Thompson S. Solving DDEs in MATLAB. Appl Nume Math. 2001;37:441–458. doi: 10.1016/S0168-9274(00)00055-6. [DOI] [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agri Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Wu P, Tian JC, Walker CE, Wang FC. Determination of phytic acid in cereals–A brief review. Int J Food Sci Technol. 2009;44:1671–1676. doi: 10.1111/j.1365-2621.2009.01991.x. [DOI] [Google Scholar]
- Xu Q, Kanthasamy AG, Reddy MB. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology. 2008;245:101–108. doi: 10.1016/j.tox.2007.12.017. [DOI] [PubMed] [Google Scholar]


