Abstract
Bleach oxidizes trimethylsilyl cyanide to generate an electrophilic cyanating reagent that readily reacts with an amine nucleophile. This oxidative N-cyanation reaction allows for the preparation of disubstituted cyanamides from amines without using highly toxic cyanogen halides.
Cyanamide is a valuable functional group in medicinal and coordination chemistry.1 It is frequently used for urea, thiourea, and guanidine synthesis.2 It can also be transformed easily into heterocycles such as 2-aminoxazole, 2-hetero-imidazole, 5-aminotetrazole, and 2-aminopyridine.3 The most straightforward method for cyanamide synthesis is the electrophilic cyanation of amines using cyanogen halides.4 Cyanogen chloride, produced by reacting sodium cyanide with chlorine gas,5 is a poisonous gas (bp 13 °C and mp −7 °C).6 Cyanogen bromide is a solid and therefore a safer N-cyanating reagent.7 However, it has high vapor pressure (126 torr) and low melting and boiling points (mp 52 °C and bp 62 °C). This reagent should therefore be handled very carefully.
We have been interested in developing new oxidation reactions8 and synthesizing highly nitrogenated natural products.9 During the development of a vanadium catalyst system for the oxidative Strecker reactions,8b we found that secondary amines can be cyanated at either the a-C- or N-position depending on the oxidant used. We studied the origin of this selectivity and found a convenient way to generate an electrophilic cyanating reagent in situ. This new oxidative method allows for the preparation of cyanamides from amines without using highly toxic cyanogen halides.
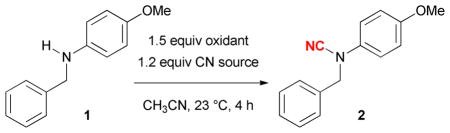
We examined the ability of a variety of oxidants in promoting the N-cyanation of N-(4-methoxyphenyl)-benzylamine (1) (Table 1). We used trimethylsilyl cyanide (TMSCN) as the cyanide source and acetonitrile as the solvent. While most of the oxidants we examined gave little or no cyanamide 2 (Entries 1–8), NaClO (household bleach, 10–15% NaClO in water) promoted a smooth N-cyanation (entry 9). However, no reaction occurred when we used sodium cyanide (NaCN) as the cyanide source (entry 10). Using water as a co-solvent did not improve the N-cyanation of 1 for entries 7, 8, and 10.
Table 1.
Development of the oxidative N-cyanation reactiona
| |||
|---|---|---|---|
| entry | oxidant | CN source | yield |
| 1 | TBHP | TMSCN | 0% |
| 2 | H2O2 | TMSCN | 0% |
| 3 | Oxone | TMSCN | 0% |
| 4 | mCPBA | TMSCN | <5% |
| 5 | O2 | TMSCN | 0% |
| 6 | PhIO | TMSCN | 0% |
| 7 | NaBrO3 | TMSCN | 0% |
| 8 | NaClO2 | TMSCN | 0% |
| 9 | NaClO(aq) | TMSCN | 70% |
| 10 | NaClO(aq) | NaCN | 0% |
Reaction conditions: 1 (0.1 mmol), oxidant (0.15 mmol), TMSCN (0.12 mmol), 1 mL solvent.
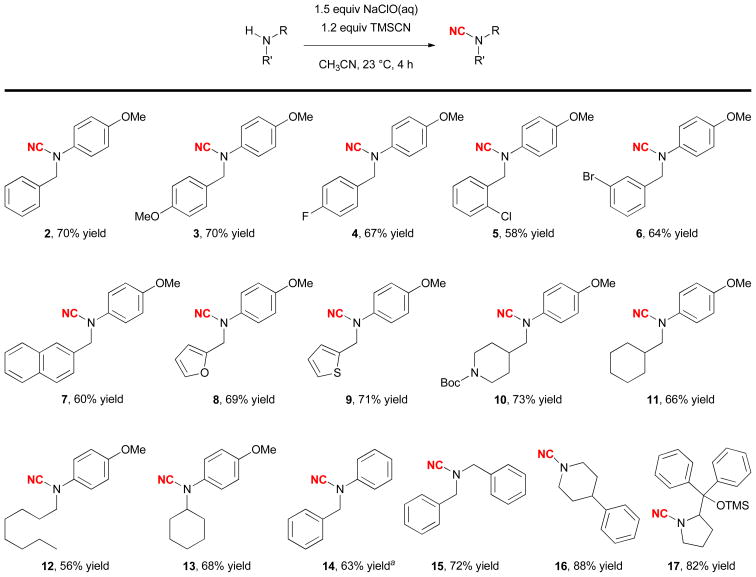
The generality of this N-cyanation reaction is shown in Figure 1. This method is useful for preparing both arylalkylcyanamides (2–14) and dialkylcyanamides (15–17). A range of functional groups can be tolerated, including the methoxyl (3), halogen (F, Cl, Br) (4–6), tert-butyloxycarbonyl (Boc) (10), and trimethylsilyloxyl (TMSO) (17) groups. The reactive naphthyl, furyl, and thiophenyl groups were also compatible (7–9).
Figure 1.
Scope of the N-cyanation reaction. aReaction conditions: 3.0 equiv NaClO (aq), 2.0 equiv TMSCN, 24 h.
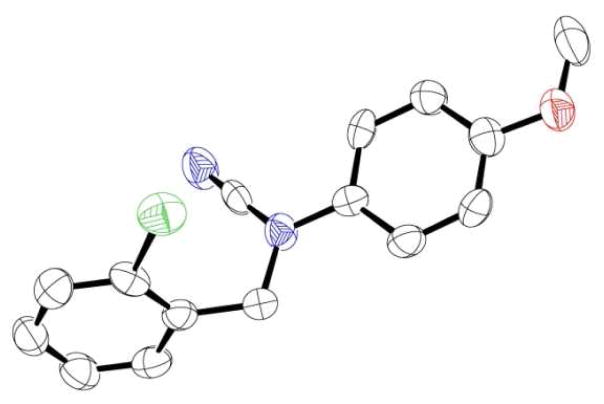
While our initial studies focused on the cyanation of the more nucleophilic PMP-alkylamines (2–13), the 4-methoxyl group was not needed for the reactivity. Cyanation of N-phenylbenzylamine gave 14 smoothly. However, the reaction was slower and an increased amount of the reagents and extended reaction time were required. This reaction could also be used to functionalize dialkylamines. Cyanation of dialkylamines proceeded smoothly, giving cyanamides 15–17 in high yields. We have also obtained a single crystal of 5 and used X-ray analysis to confirm its structure (Figure 2).
Figure 2.
Crystal structure of 5.
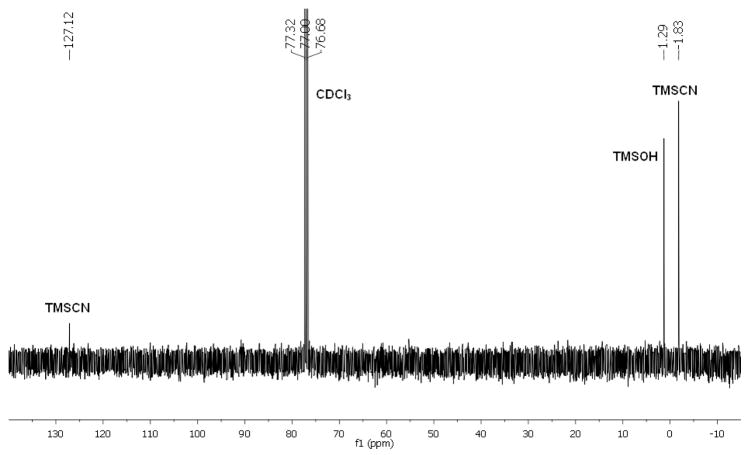
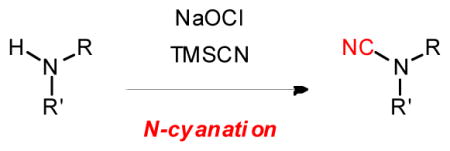
We believe that NaClO oxidized TMSCN instead of the amines4c in this N-cyanation reaction. We found that NaClO reacted with TMSCN but not 1 according to 13C NMR analyses (Figure 3).10 The reaction between NaClO and TMSCN was rapid and exothermic. It was accompanied by gas evolution and a change of solution pH to 11. The silyl group of TMSCN may activate NaClO for the oxidation of CN because replacing TMSCN with NaCN resulted in no reaction. We suspect that mixing NaClO with TMSCN gave cyanogen chloride (ClCN), which reacted with amines to give cyanamides (Figure 4).
Figure 3.
13C NMR spectrum of the reaction of TMSCN and NaClO in CDCl3 after 5 min.
Figure 4.
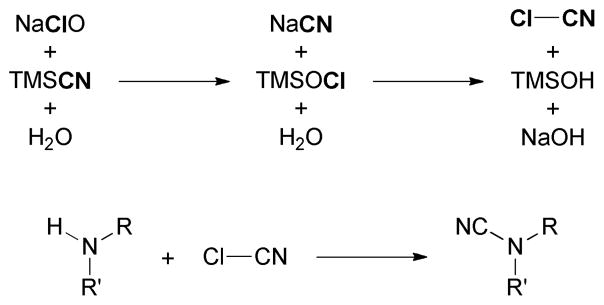
Proposed mechanism for the N-cyanation reaction.
In summary, we have developed an operationally simple method for generating an electrophilic cyanating reagent in situ from TMSCN and NaClO. It is useful for synthesizing a wide range of cyanamides from amines. We are exploring further synthetic utilities of this CN-umpolung reaction.
Supplementary Material
Acknowledgments
Financial support was provided by NIH/NIGMS (R01-GM079554) and the Welch Foundation (I-1596). We thank Dr. Vincent Lynch (University of Texas at Austin) for performing the X-ray analysis of 5.
Footnotes
Supporting Information Available. Experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Nekrasov DD. Russ J Org Chem. 2004;40:1387–1402. [Google Scholar]; (b) Larraufie MH, Maestri G, Malacria M, Ollivier C, Fensterbank L, Lacôte E. Synthesis. 2012;44:1279–1292. doi: 10.1021/ol3026439. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kim SH, Park BR, Kim JN. Bull Korean Chem Soc. 2011;32:716–718. [Google Scholar]; (b) Hua G, Zhang Q, Li Y, Slawin AMZ, Woollins JD. Tetrahedron. 2009;65:6074–6082. [Google Scholar]; (c) Katritzky AR, Rogovoy BV. Arkivoc. 2005:49–87. [Google Scholar]; (d) Simig G, Lempert K, Tamás J, Czira G. Tetrahedron. 1975;31:1195–1200. [Google Scholar]; (e) Reddy NL, Hu LY, Cotter RE, Fischer JB, Wong WJ, McBurney RN, Weber E, Holmes DL, Wong ST, Prasad R, Keanal JFW. J Med Chem. 1994;37:260–267. doi: 10.1021/jm00028a009. [DOI] [PubMed] [Google Scholar]; (f) Zahariev S, Guarnaccia C, Lamba D, Čemažar M, Pongor S. Tetrahedron Lett. 2004;45:9423–9426. [Google Scholar]; (g) Larraufie MH, Ollivier C, Fensterbank L, Malacria M, Lacôte E. Angew Chem Int Ed. 2010;49:2178–2181. doi: 10.1002/anie.200907237. [DOI] [PubMed] [Google Scholar]; (h) Looper RE, Haussener TJ, Mack JBC. J Org Chem. 2011;76:6967–6971. doi: 10.1021/jo201264j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zhou L, Chen J, Zhou J, Yeung YY. Org Lett. 2011;13:5804–5807. doi: 10.1021/ol202402y. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ross WJ, Harrison RG, Jolley MRJ, Neville MC, Todd A, Verge JP, Dawson W, Sweatman WJF. J Med Chem. 1979;22:412–417. doi: 10.1021/jm00190a011. [DOI] [PubMed] [Google Scholar]; (b) Knapp S, Kukkola PJ. J Org Chem. 1990;55:1632–1636. [Google Scholar]; (c) Devan B, Rajagopalan K. Tetrahedron Lett. 1994;35:1585–1586. [Google Scholar]; (d) Giles RL, Sullivan JD, Steiner AM, Looper RE. Angew Chem Int Ed. 2009;48:3116–3120. doi: 10.1002/anie.200900160. [DOI] [PubMed] [Google Scholar]; (e) Giles RL, Nkansah RA, Looper RE. J Org Chem. 2010;75:261–264. doi: 10.1021/jo902326d. [DOI] [PubMed] [Google Scholar]; (f) Demko ZP, Sharpless KB. Org Lett. 2001;3:4091–4094. doi: 10.1021/ol010220x. [DOI] [PubMed] [Google Scholar]; (g) Duan M, Aquino C, Ferris R, Kazmierski WM, Kenakin T, Koble C, Wheelan P, Watson C, Youngman M. Bioorg Med Chem Lett. 2009;19:1610–1613. doi: 10.1016/j.bmcl.2009.02.014. [DOI] [PubMed] [Google Scholar]; (h) Stolley RM, Maczka MT, Louie J. Eur J Org Chem. 2011:3815–3824. doi: 10.1002/ejoc.201100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) van Leusen AM, Jagt JC. Tetrahedron Lett. 1970;11:967–970. [Google Scholar]; (b) Cockerill AF, Deacon A, Osborne RGHDJ, Prime DM, Ross WJ, Todd A, Verge JP. Synthesis. 1976:591–593. [Google Scholar]; (c) Troyanskii ÉI, Ioffe VA, Mizintsev VV, Nikishin GI. Bull Acad Sci USSR Div Chem Sci. 1984;33:1661–1667. [Google Scholar]; (d) Kaupp G, Schmeyers J, Boy J. Chem Eur J. 1998;4:2467–2474. [Google Scholar]; (e) Kamijo S, Jin T, Yamamoto Y. Angew Chem Int Ed. 2002;41:1780–1782. doi: 10.1002/1521-3773(20020517)41:10<1780::aid-anie1780>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; (f) Wong FF, Chen CY, Yeh MY. Synlett. 2006:559–562. [Google Scholar]; (g) Ghosh H, Yella R, Ali AR, Sahoo SK, Patel BK. Tetrahedron Lett. 2009;50:2407–2410. [Google Scholar]; (h) Ramana T, Saha P, Das M, Punniyamurthy T. Org Lett. 2010;12:84–87. doi: 10.1021/ol9024088. [DOI] [PubMed] [Google Scholar]
- 5.Coleman GH, Leeper RW, Schulze CC. Inorganic Syntheses. 1946;2:90–94. [Google Scholar]
- 6.For safety information, see the Emergency Response Safety and Health Database published by the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention: http://www.cdc.gov/niosh/ershdb/EmergencyResponseCard_29750039.html.
- 7.For safety information, see the Hazardous Substances Data Bank on the Toxicology Data Network of the U.S. National Library of Medicine, National Institutes of Health: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@DOCNO+708.
- 8.(a) Xia JB, Cormier KW, Chen C. Chem Sci. 2012;3:2240–2245. doi: 10.1039/C2SC20178J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu C, Xia J-B, Chen C. Tetrahedron Lett. doi: 10.1016/j.tetlet.2013.11.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xia JB, Zhu C, Chen C. J Am Chem Soc. 2013;135:17494–17500. doi: 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Lu J, Tan X, Chen C. J Am Chem Soc. 2007;129:7768–7769. doi: 10.1021/ja072844p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ma Z, Lu J, Wang X, Chen C. Chem Commun. 2011;47:427–429. doi: 10.1039/c0cc02214d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang X, Lu ZMJ, Tan X, Chen C. J Am Chem Soc. 2011;133:15350–15353. doi: 10.1021/ja207386q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang X, Wang X, Tan X, Lu J, Cormier KW, Ma Z, Chen C. J Am Chem Soc. 2012;134:18834–18842. doi: 10.1021/ja309172t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Hamada T, Manabe K, Kobayashi S. Chem Eur J. 2006;12:1205–1215. doi: 10.1002/chem.200500673. [DOI] [PubMed] [Google Scholar]; (b) Liu X, Qin B, Zhou X, He B, Feng X. J Am Chem Soc. 2005;127:12224–12225. doi: 10.1021/ja052629d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.