Abstract
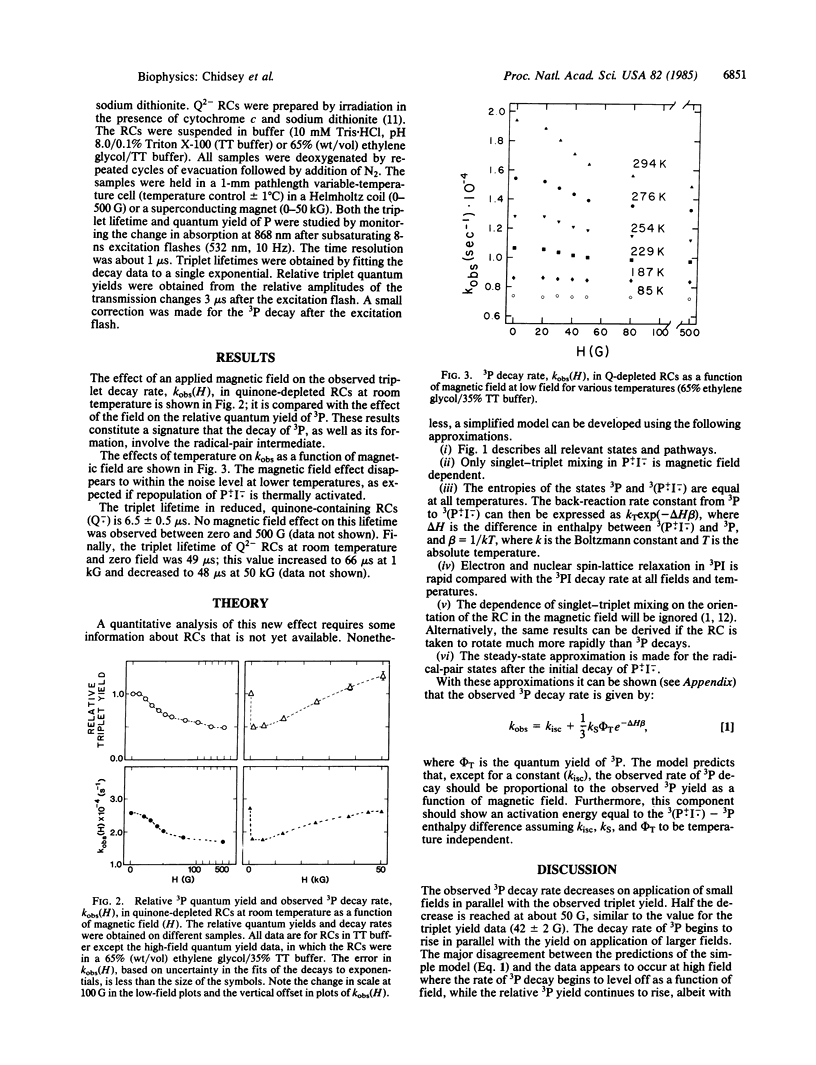
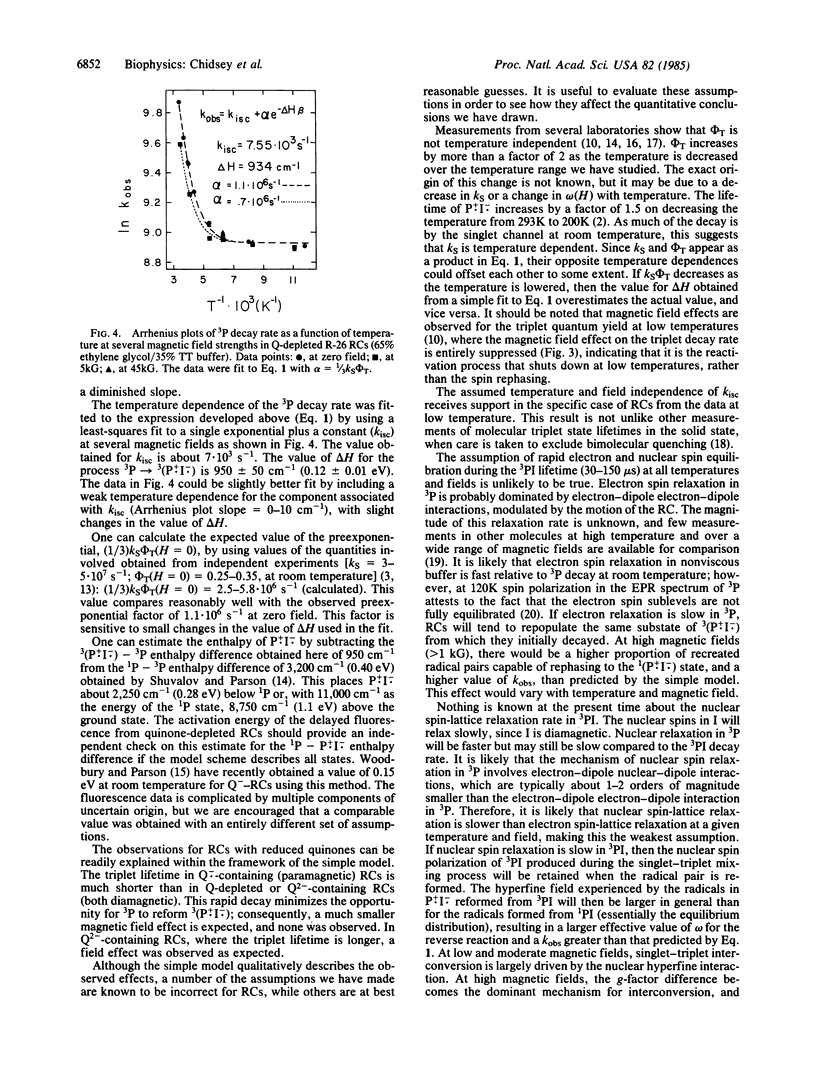
The lifetime of the molecular triplet state formed by recombination of the radical ion pair in quinonedepleted bacterial photosynthetic reaction centers is found to depend on applied magnetic field strength. It is suggested that this magnetic field effect results from thermally activated repopulation of the same radical ion pair that generates the triplet. Consistent with this hypothesis, the magnetic field effect on the triplet lifetime disappears at low temperature where the triplet state decays exclusively by ordinary intersystem crossing. This activated pathway for the decay of the triplet state can explain the strong temperature dependence of the triplet decay rate. A detailed theoretical treatment of the problem within a set of physically reasonable assumptions relates the observed temperature dependence of the triplet decay rate to the energy gap between the radical ion pair intermediate and the triplet state. This energy gap is estimated to be about 950 cm-1 (0.12 eV). Combined with an estimate of the energy of the donor excited state, we obtain an energy gap between the excited singlet state of the donor and the radical ion pair of 2,250 cm-1 (0.28 eV).
Keywords: photosynthesis, electron transfer, magnetic field effects
Full text
PDF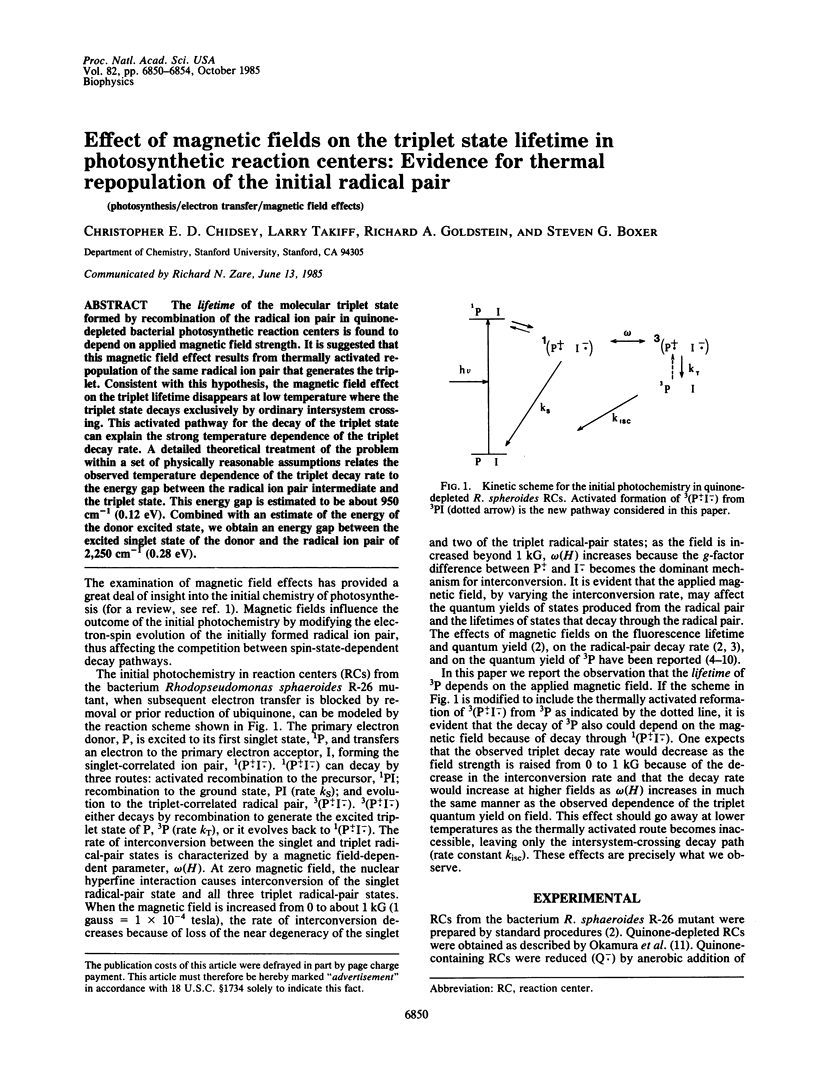


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Schaafsma T. J., Parson W. W. Magnetic field effects on radical pair intermediates in bacterial photosynthesis. Biochim Biophys Acta. 1977 Aug 10;461(2):297–305. doi: 10.1016/0005-2728(77)90179-7. [DOI] [PubMed] [Google Scholar]
- Boxer S. G., Chidsey C. E., Roelofs M. G. Anisotropic magnetic interactions in the primary radical ion-pair of photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4632–4636. doi: 10.1073/pnas.79.15.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn R., Michel-Beyerle M. E. On the mechanism of magnetic field effects in bacterial photosynthesis. Biophys J. 1979 Jun;26(3):489–498. doi: 10.1016/S0006-3495(79)85266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff A. J., Rademaker H., van Grondelle R., Duysens L. N. On the magnetic field dependence of the yield of the triplet state in reaction centers of photosynthetic bacteria. Biochim Biophys Acta. 1977 Jun 9;460(3):547–554. doi: 10.1016/0005-2728(77)90094-9. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Bowman M. K., Budil D. E., Tang J., Wraight C. A., Closs G. L. Magnetic characterization of the primary state of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5532–5536. doi: 10.1073/pnas.79.18.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik A., Krüger H. W., Orthuber H., Haberkorn R., Michel-Beyerle M. E., Scheer H. Recombination dynamics in bacterial photosynthetic reaction centers. Biophys J. 1982 Jul;39(1):91–99. doi: 10.1016/S0006-3495(82)84494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Parson W. W. Energies and kinetics of radical pairs involving bacteriochlorophyll and bacteriopheophytin in bacterial reaction centers. Proc Natl Acad Sci U S A. 1981 Feb;78(2):957–961. doi: 10.1073/pnas.78.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H. J., Schulten K., Weller A. Electron transfer and spin exchange contributing to the magnetic field dependence of the primary photochemical reaction of bacterial photosynthesis. Biochim Biophys Acta. 1978 May 10;502(2):255–268. doi: 10.1016/0005-2728(78)90047-6. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Parson W. W. Nanosecond fluorescence from isolated photosynthetic reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Nov 26;767(2):345–361. doi: 10.1016/0005-2728(84)90205-6. [DOI] [PubMed] [Google Scholar]
- Wraight C. A., Leigh J. S., Dutton P. L., Clayton R. K. The triplet state of reaction center bacteriochlorophyll: determination of a relative quantum yeild. Biochim Biophys Acta. 1974 Mar 26;333(3):401–408. doi: 10.1016/0005-2728(74)90123-6. [DOI] [PubMed] [Google Scholar]


