Abstract
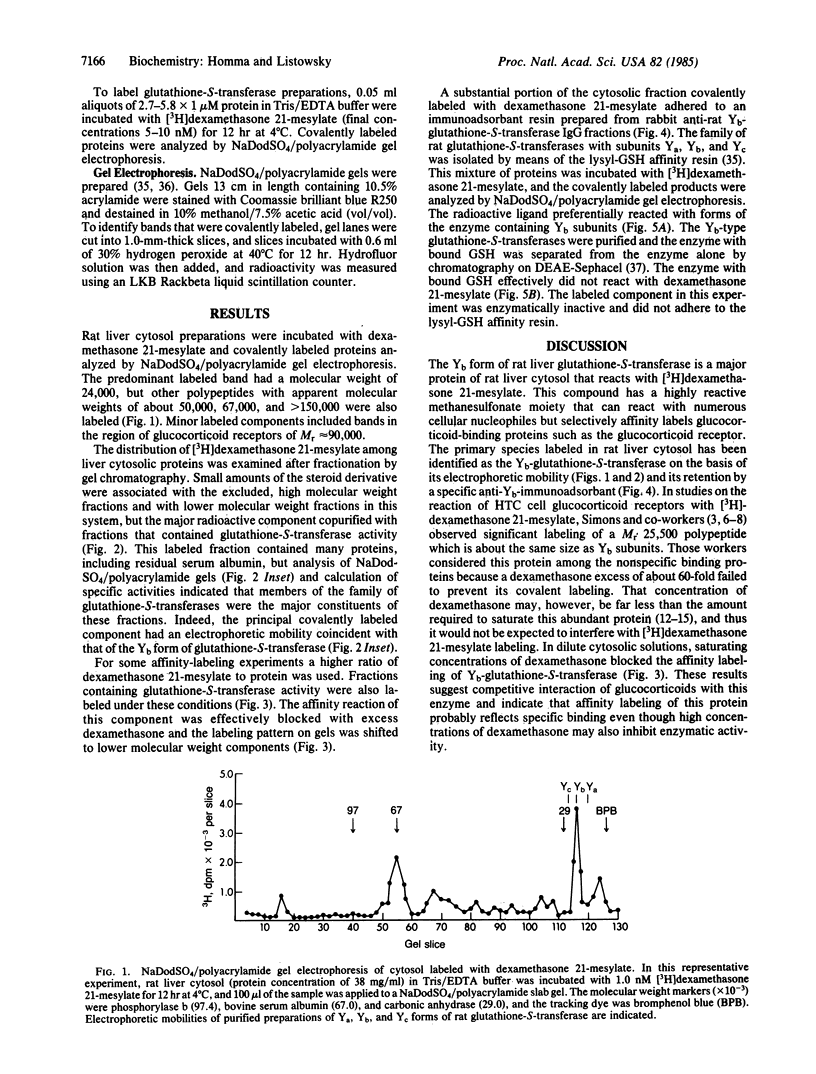
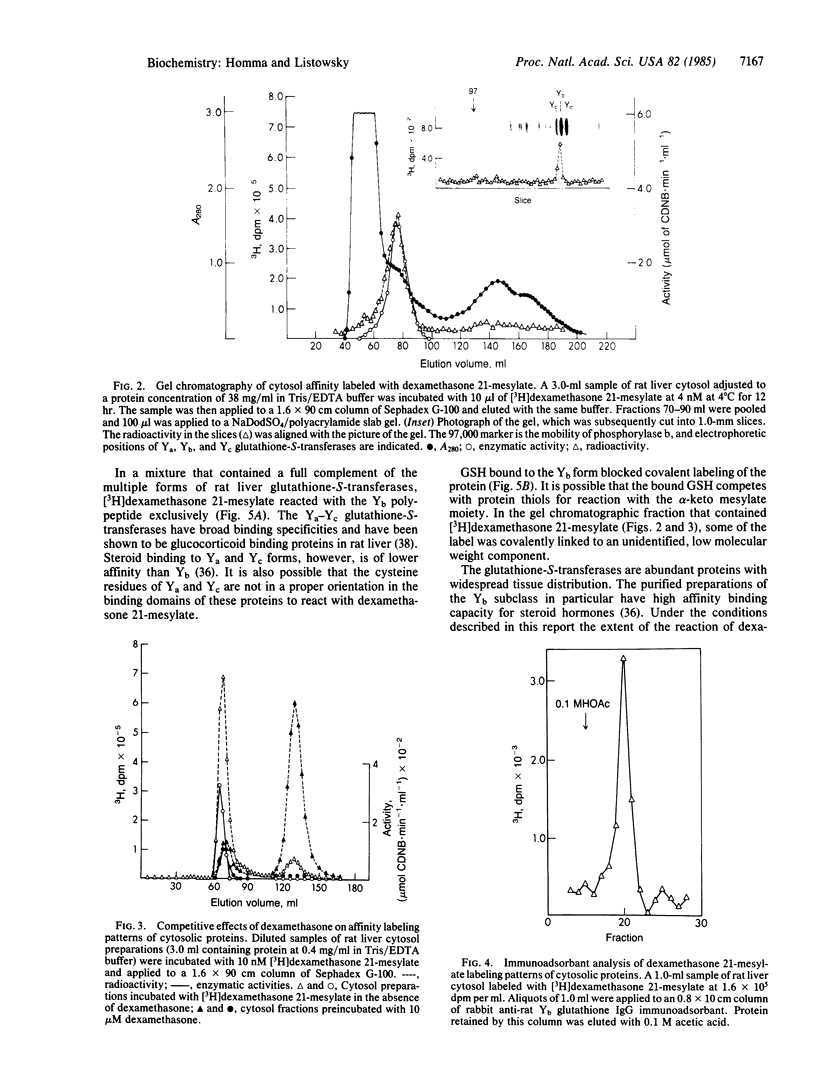
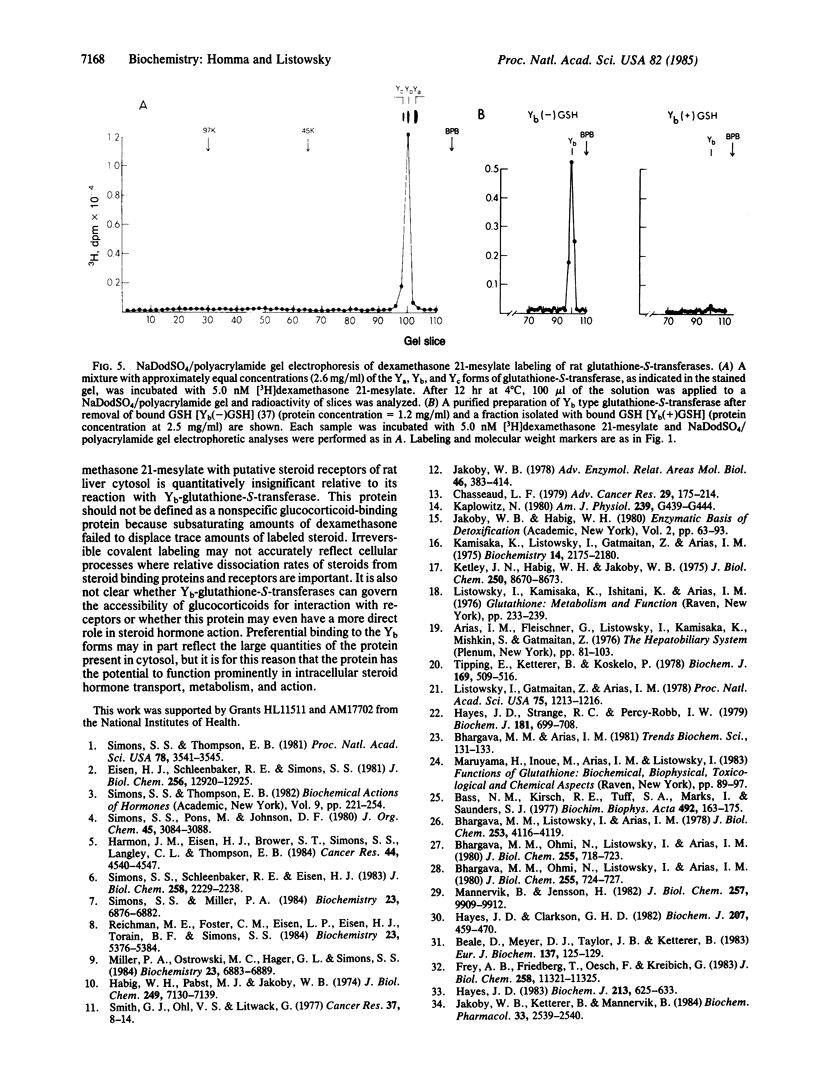
Dexamethasone 21-methanesulfonate, an affinity label for glucocorticoid-binding proteins, was incubated with rat liver cytosol preparations. The predominant covalently labeled component was identified as Yb-glutathione-S-transferase on the basis of chromatographic properties, electrophoretic mobility, and specific retention by an anti-Yb-immunoadsorbent. Affinity labeling of this protein was blocked by excess dexamethasone. Preferential reactivity of dexamethasone 21-methanesulfonate with the Yb subclass of glutathione-S-transferase (glutathione transferase, EC 2.5.1.18) was also evident with mixtures containing the multiple forms of the enzyme. Yb-glutathione-S-transferase, the nonsaturable glucocorticoid-binding component of rat liver cytosol should, therefore, be reclassified; because of its high concentration and selective interaction with steroids, this enzyme may be an intracellular glucocorticoid-binding protein and, thereby, influence transport, metabolism, and action of the steroids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M., Kirsch R. E., Tuff S. A., Marks I., Saunders S. J. Ligandin heterogeneity : evidence that the two non-identical subunits are the monomers of two distinct proteins. Biochim Biophys Acta. 1977 May 27;492(1):163–175. doi: 10.1016/0005-2795(77)90223-9. [DOI] [PubMed] [Google Scholar]
- Beale D., Meyer D. J., Taylor J. B., Ketterer B. Evidence that the Yb subunits of hepatic glutathione transferases represent two different but related families of polypeptides. Eur J Biochem. 1983 Dec 1;137(1-2):125–129. doi: 10.1111/j.1432-1033.1983.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Bhargava M. M., Listowsky I., Arias I. M. Studies on subunit structure and evidence that ligandin is a heterodimer. J Biol Chem. 1978 Jun 25;253(12):4116–4119. [PubMed] [Google Scholar]
- Bhargava M. M., Ohmi N., Listowsky I., Arias I. M. Structural, catalytic, binding, and immunological properties associated with each of the two subunits of rat liver ligandin. J Biol Chem. 1980 Jan 25;255(2):718–723. [PubMed] [Google Scholar]
- Bhargava M. M., Ohmi N., Listowsky I., Arias I. M. Subunit composition, organic anion binding, catalytic and immunological properties of ligandin from rat testis. J Biol Chem. 1980 Jan 25;255(2):724–727. [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Eisen H. J., Schleenbaker R. E., Simons S. S., Jr Affinity labeling of the rat liver glucocorticoid receptor with dexamethasone 21-mesylate. Identification of covalently labeled receptor by immunochemical methods. J Biol Chem. 1981 Dec 25;256(24):12920–12925. [PubMed] [Google Scholar]
- Frey A. B., Friedberg T., Oesch F., Kreibich G. Studies on the subunit composition of rat liver glutathione S-transferases. J Biol Chem. 1983 Sep 25;258(18):11321–11325. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Harmon J. M., Eisen H. J., Brower S. T., Simons S. S., Jr, Langley C. L., Thompson E. B. Identification of human leukemic glucocorticoid receptors using affinity labeling and anti-human glucocorticoid receptor antibodies. Cancer Res. 1984 Oct;44(10):4540–4547. [PubMed] [Google Scholar]
- Hayes J. D., Clarkson G. H. Purification and characterization of three forms of glutathione S-transferase A. A comparative study of the major YaYa-, YbYb- and YcYc-containing glutathione S-transferases. Biochem J. 1982 Dec 1;207(3):459–470. doi: 10.1042/bj2070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Rat liver glutathione S-transferases. A study of the structure of the basic YbYb-containing enzymes. Biochem J. 1983 Sep 1;213(3):625–633. doi: 10.1042/bj2130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C., Percy-Robb I. W. Identification of two lithocholic acid-binding proteins. Separation of ligandin from glutathione S-transferase B. Biochem J. 1979 Sep 1;181(3):699–708. doi: 10.1042/bj1810699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H., Listowsky I. Temperature dependent redistribution among the multiple forms of rat Yb-glutathione-S-transferase. Biochem Biophys Res Commun. 1985 Mar 15;127(2):425–432. doi: 10.1016/s0006-291x(85)80178-9. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Gatmaitan Z., Arias I. M. Interactions of bilirubin and other ligands with ligandin. Biochemistry. 1975 May 20;14(10):2175–2180. doi: 10.1021/bi00681a021. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N. Physiological significance of glutathione S-transferases. Am J Physiol. 1980 Dec;239(6):G439–G444. doi: 10.1152/ajpgi.1980.239.6.G439. [DOI] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Listowsky I., Gatmaitan Z., Arias I. M. Ligandin retains and albumin loses bilirubin binding capacity in liver cytosol. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1213–1216. doi: 10.1073/pnas.75.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Maruyama H., Arias I. M., Listowsky I. Distinctions between the multiple cationic forms of rat liver glutathione S-transferase. J Biol Chem. 1984 Oct 25;259(20):12444–12448. [PubMed] [Google Scholar]
- Maruyama H., Listowsky I. Preferential binding of steroids by anionic forms of rat glutathione S-transferase. J Biol Chem. 1984 Oct 25;259(20):12449–12455. [PubMed] [Google Scholar]
- Miller P. A., Ostrowski M. C., Hager G. L., Simons S. S., Jr Covalent and noncovalent receptor-glucocorticoid complexes preferentially bind to the same regions of the long terminal repeat of murine mammary tumor virus proviral DNA. Biochemistry. 1984 Dec 18;23(26):6883–6889. doi: 10.1021/bi00321a093. [DOI] [PubMed] [Google Scholar]
- Reichman M. E., Foster C. M., Eisen L. P., Eisen H. J., Torain B. F., Simons S. S., Jr Limited proteolysis of covalently labeled glucocorticoid receptors as a probe of receptor structure. Biochemistry. 1984 Oct 23;23(22):5376–5384. doi: 10.1021/bi00317a042. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Miller P. A. Comparison of DNA binding properties of activated, covalent and noncovalent glucocorticoid receptor-steroid complexes from HTC cells. Biochemistry. 1984 Dec 18;23(26):6876–6882. doi: 10.1021/bi00321a092. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Schleenbaker R. E., Eisen H. J. Activation of covalent affinity labeled glucocorticoid receptor-steroid complexes. J Biol Chem. 1983 Feb 25;258(4):2229–2238. [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B. Dexamethasone 21-mesylate: an affinity label of glucocorticoid receptors from rat hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3541–3545. doi: 10.1073/pnas.78.6.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. J., Ohl V. S., Litwack G. Ligandin, the glutathione S-transferases, and chemically induced hepatocarcinogenesis: a review. Cancer Res. 1977 Jan;37(1):8–14. [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Koskelo P. The binding of porphyrins by ligandin. Biochem J. 1978 Mar 1;169(3):509–516. doi: 10.1042/bj1690509. [DOI] [PMC free article] [PubMed] [Google Scholar]