Abstract
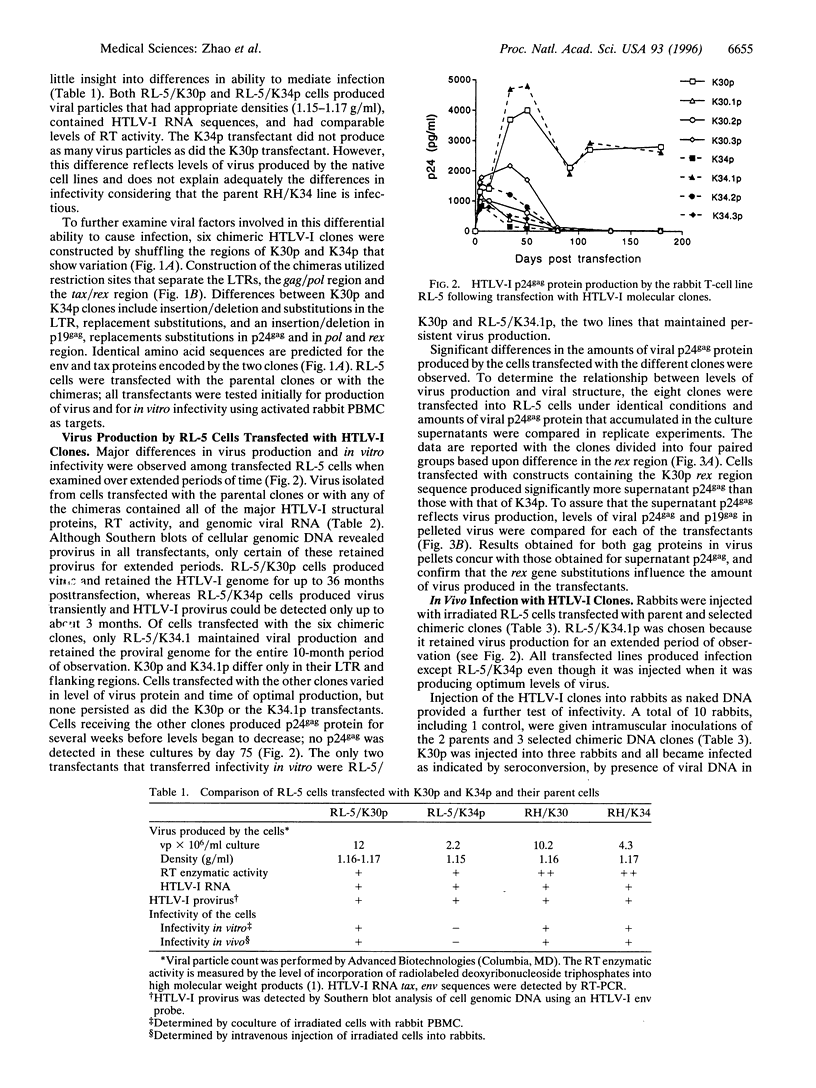
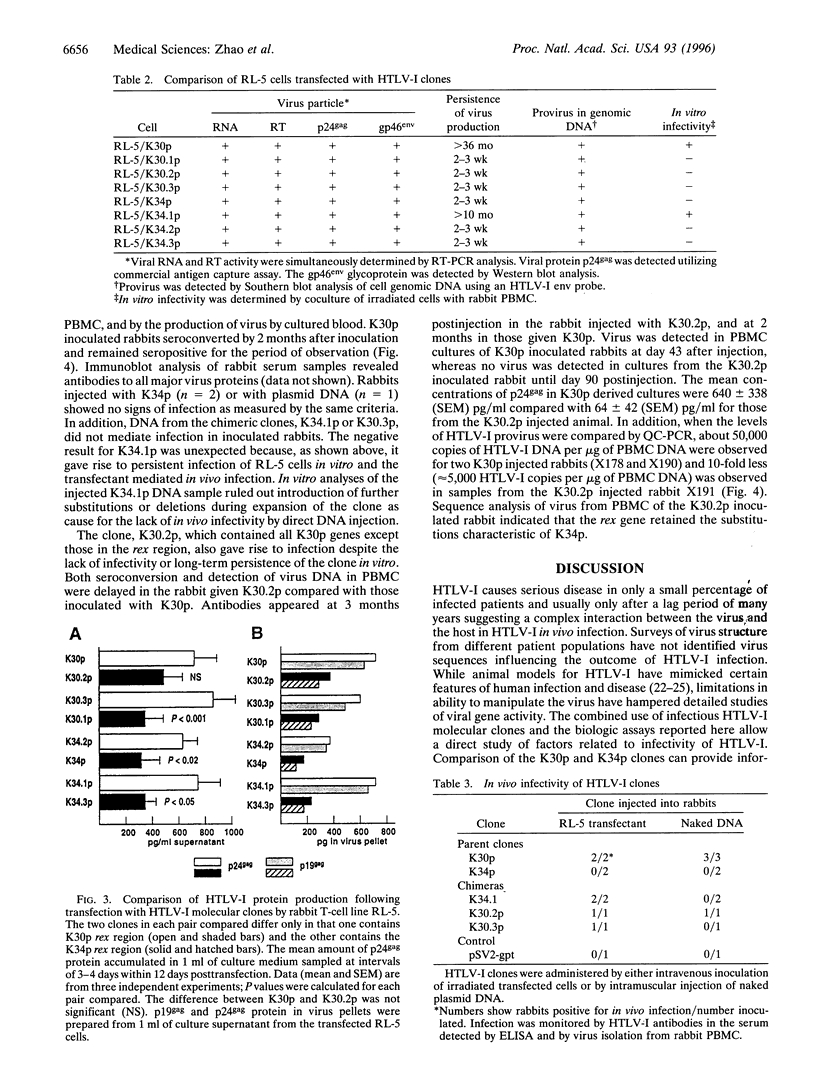
Two human T-cell leukemia virus type I (HTLV-I) molecular clones, K30p and K34p were derived from HTLV-I-infected rabbit cell lines. K30p and K34p differ by 18 bp with changes in the long terminal repeats (LTRs) as well as in the gag, pol, and rex but not tax or env gene products. Cells transfected with clone K30p were infectious in vitro and injection of the K30p transfectants or naked K30p DNA into rabbits leads to chronic infection. In contrast, K34p did not mediate infection in vitro or in vivo, although the cell line from which it was derived is fully infectious and K34p transfectants produce intact virus particles. To localize differences involved in the ability of the clones to cause infection, six chimeric HTLV-I clones were constructed by shuffling corresponding fragments containing the substitutions in the LTRs, the gag/pol region and the rex region between K30p and K34p. Cells transfected with any of the six chimeras produced virus, but higher levels of virus were produced by cells transfected with those constructs containing the K30p rex region. Virus production was transient except in cells transfected with K30p or with a chimera consisting of the entire protein coding region of K30p flanked by K34p LTRs; only the transfectants showing persistent virus production mediated in vitro infection. In vivo infection in rabbits following intramuscular DNA injection was mediated by K30p as well as by a chimera of K30p containing the K34p rex gene. Comparisons revealed that virus production was greater and appeared earlier in rabbits injected with K30p. These data suggest that several defects in the K34p clone preclude infectivity and furthermore, provide systems to explore functions of HTLV-I genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Y. F., Gilmartin G. M., Hanly S. M., Nevins J. R., Greene W. C. The HTLV-I Rex response element mediates a novel form of mRNA polyadenylation. Cell. 1991 Feb 22;64(4):727–737. doi: 10.1016/0092-8674(91)90502-p. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Mechanism of action of regulatory proteins encoded by complex retroviruses. Microbiol Rev. 1992 Sep;56(3):375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Meléndez L. V., Hunt R. D., King N. W., Anver M., Fraser C. E., Barahona H., Baggs R. B. Herpesvirus saimiri: VII. Induction of malignant lymphoma in New Zealand white rabbits. J Natl Cancer Inst. 1974 Dec;53(6):1803–1807. [PubMed] [Google Scholar]
- Derse D., Mikovits J., Polianova M., Felber B. K., Ruscetti F. Virions released from cells transfected with a molecular clone of human T-cell leukemia virus type I give rise to primary and secondary infections of T cells. J Virol. 1995 Mar;69(3):1907–1912. doi: 10.1128/jvi.69.3.1907-1912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Hall W. W. Human T cell lymphotropic virus type I and cutaneous T cell leukemia/lymphoma. J Exp Med. 1994 Nov 1;180(5):1581–1585. doi: 10.1084/jem.180.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Raine C. S., Mingioli E. S., McFarlin D. E. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988 Feb 11;331(6156):540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- Kimata J. T., Wong F. H., Wang J. J., Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994 Nov 1;204(2):656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- Koralnik I. J., Gessain A., Klotman M. E., Lo Monico A., Berneman Z. N., Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., Koprowski H. Neurological disorders associated with HTLV-1. Curr Top Microbiol Immunol. 1990;160:100–119. [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Román G. C., Osame M. Identity of HTLV-I-associated tropical spastic paraparesis and HTLV-I-associated myelopathy. Lancet. 1988 Mar 19;1(8586):651–651. doi: 10.1016/s0140-6736(88)91452-3. [DOI] [PubMed] [Google Scholar]
- Sawasdikosol S., Hague B. F., Zhao T. M., Bowers F. S., Simpson R. M., Robinson M., Kindt T. J. Selection of rabbit CD4- CD8- T cell receptor-gamma/delta cells by in vitro transformation with human T lymphotropic virus-I. J Exp Med. 1993 Oct 1;178(4):1337–1345. doi: 10.1084/jem.178.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto A., Kawanishi M., Matsuda S., Ogawa K., Eguchi T., Miyoshi I. Induction of preleukemic stage of adult T cell leukemia-like disease in rabbits. Jpn J Cancer Res. 1987 Nov;78(11):1150–1155. [PubMed] [Google Scholar]
- Seto A., Kawanishi M., Matsuda S., Ogawa K., Miyoshi I. Adult T cell leukemia-like disease experimentally induced in rabbits. Jpn J Cancer Res. 1988 Mar;79(3):335–341. doi: 10.1111/j.1349-7006.1988.tb01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto A., Kawanishi M., Matsuda S., Ogawa K. Seronegative virus carriers in the infection of rabbits with human T lymphotropic virus type I. J Exp Med. 1988 Dec 1;168(6):2409–2414. doi: 10.1084/jem.168.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. M., Leno M., Hubbard B. S., Kindt T. J. Cutaneous manifestations of human T cell leukemia virus type I infection in an experimental model. J Infect Dis. 1996 Mar;173(3):722–726. doi: 10.1093/infdis/173.3.722. [DOI] [PubMed] [Google Scholar]
- Simpson R. M., Zhao T. M., Hubbard B. S., Sawasdikosol S., Kindt T. J. Experimental acute adult T cell leukemia-lymphoma is associated with thymic atrophy in human T cell leukemia virus type I infection. Lab Invest. 1996 Mar;74(3):696–710. [PubMed] [Google Scholar]
- Wang B., Ugen K. E., Srikantan V., Agadjanyan M. G., Dang K., Refaeli Y., Sato A. I., Boyer J., Williams W. V., Weiner D. B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T. M., Robinson M. A., Bowers F. S., Kindt T. J. Characterization of an infectious molecular clone of human T-cell leukemia virus type I. J Virol. 1995 Apr;69(4):2024–2030. doi: 10.1128/jvi.69.4.2024-2030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
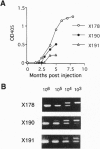
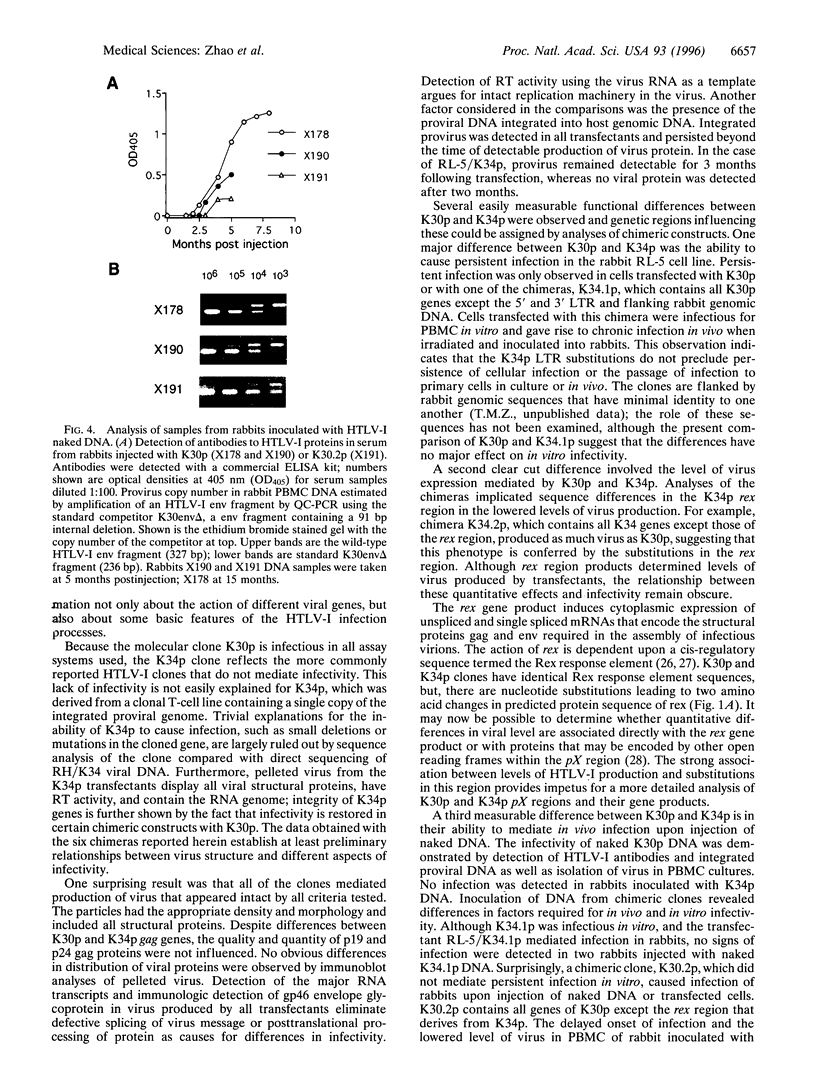
- Zhao T. M., Robinson M. A., Sawasdikosol S., Simpson R. M., Kindt T. J. Variation in HTLV-I sequences from rabbit cell lines with diverse in vivo effects. Virology. 1993 Jul;195(1):271–274. doi: 10.1006/viro.1993.1373. [DOI] [PubMed] [Google Scholar]