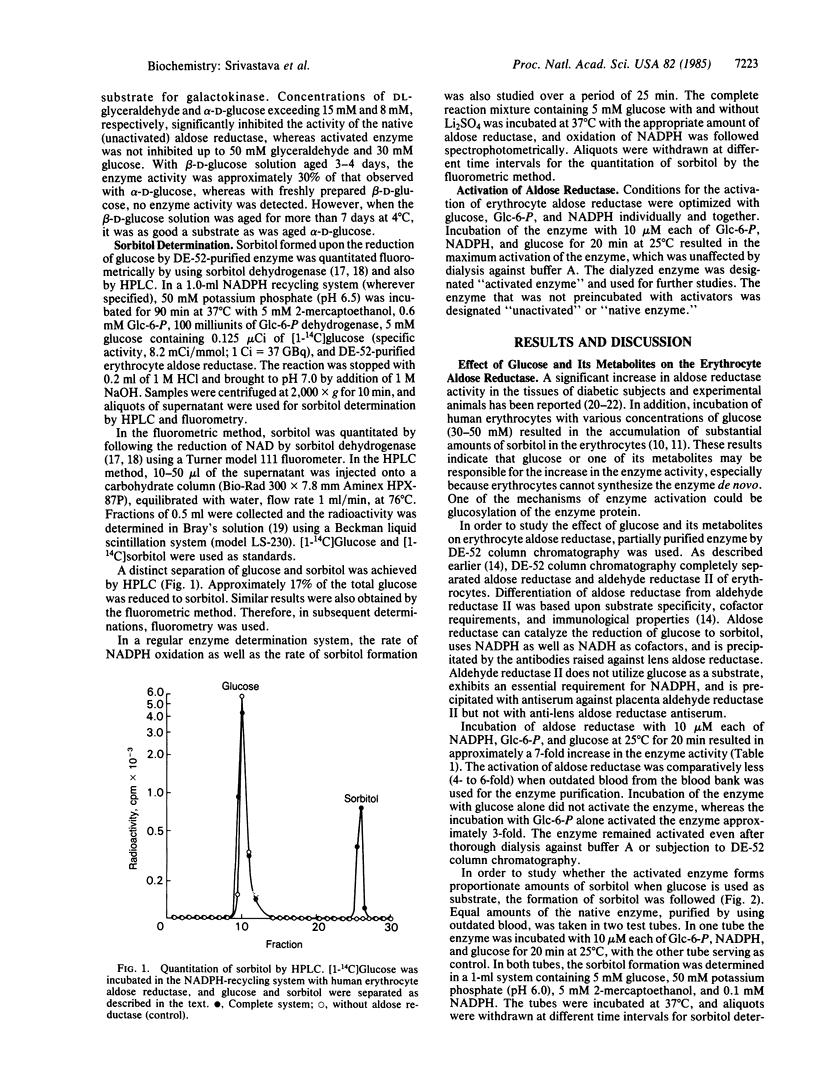
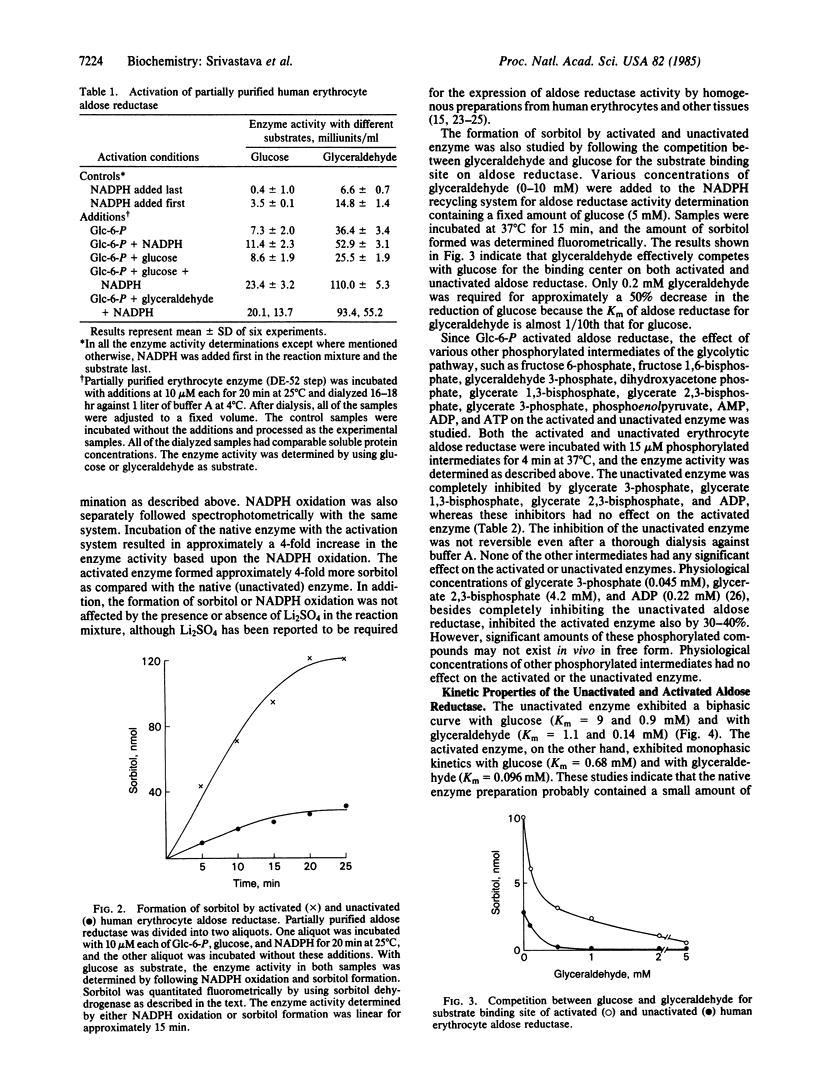
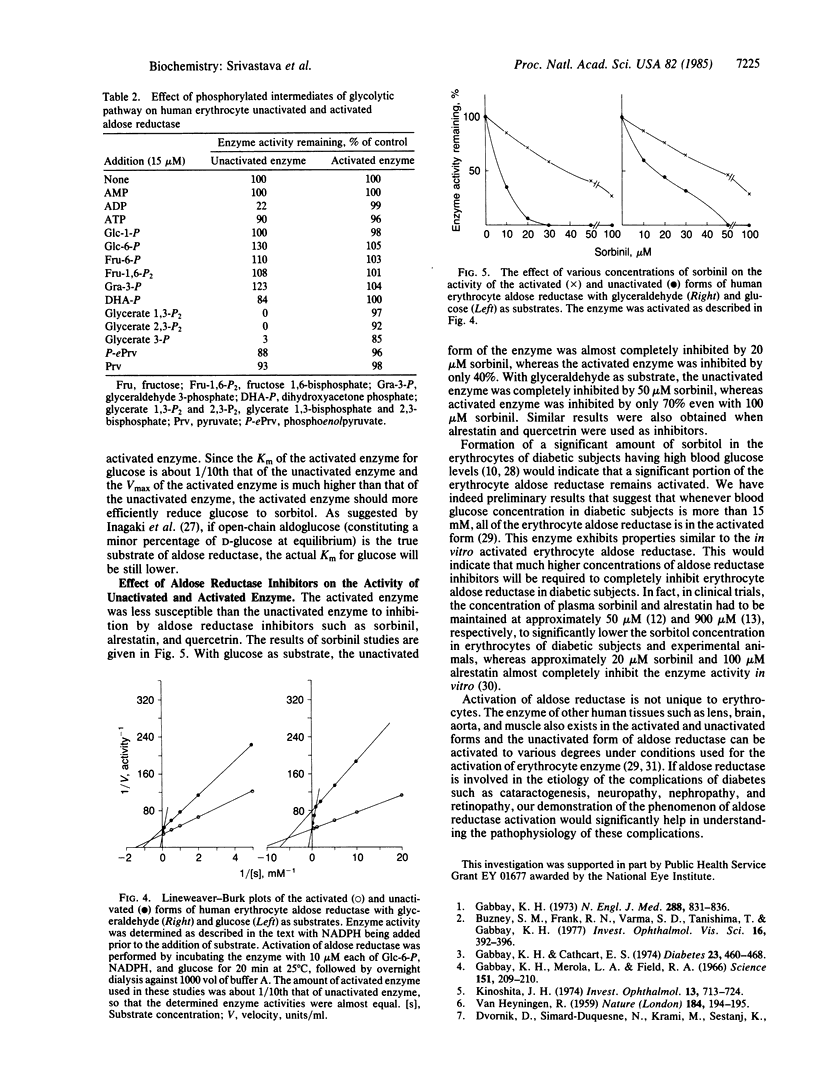
Abstract
Aldose reductase (alditol:NADP+ 1-oxidoreductase, EC 1.1.1.21) has been partially purified from human erythrocytes by DEAE-cellulose (DE-52) column chromatography. This enzyme is activated severalfold upon incubation with 10 microM each glucose 6-phosphate, NADPH, and glucose. The activation of the enzyme was confirmed by following the oxidation of NADPH as well as the formation of sorbitol with glucose as substrate. The activated form of aldose reductase exhibited monophasic kinetics with both glyceraldehyde and glucose (Km of glucose = 0.68 mM and Km of glyceraldehyde = 0.096 mM), whereas the native (unactivated) enzyme exhibited biphasic kinetics (Km of glucose = 9.0 and 0.9 mM and Km of glyceraldehyde = 1.1 and 0.14 mM). The unactivated enzyme was strongly inhibited by aldose reductase inhibitors such as sorbinil, alrestatin, and quercetrin, and by phosphorylated intermediates such as ADP, glycerate 3-phosphate, glycerate 1,3-bisphosphate, and glycerate 2,3-trisphosphate. The activated form of the enzyme was less susceptible to inhibition by aldose reductase inhibitors and phosphorylated intermediates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS L., OLKEN H. G. AN AUTOMATED FLUOROMETRIC METHOD FOR DETERMINATION OF LACTIC DEHYDROGENASE IN SERUM. Clin Chem. 1965 Aug;11:748–762. [PubMed] [Google Scholar]
- Beutler E., Matsumoto F. A rapid simplified assay for galactokinase activity in whole blood. J Lab Clin Med. 1973 Nov;82(5):818–821. [PubMed] [Google Scholar]
- Buzney S. M., Frank R. N., Varma S. D., Tanishima T., Gabbay K. H. Aldose reductase in retinal mural cells. Invest Ophthalmol Vis Sci. 1977 May;16(5):392–396. [PubMed] [Google Scholar]
- Crabbe M. J., Bron A. J., Peckar C. O., Petchey M., Ting H. H., Howard-Williams J. NADPH-oxidising activity in lens and erythrocytes in diabetic and nondiabetic patients with cataract. Br J Ophthalmol. 1983 Oct;67(10):696–699. doi: 10.1136/bjo.67.10.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromlish J. A., Flynn T. G. Purification and characterization of two aldose reductase isoenzymes from rabbit muscle. J Biol Chem. 1983 Mar 10;258(5):3416–3424. [PubMed] [Google Scholar]
- Das B., Srivastava S. K. Purification and properties of aldose reductase and aldehyde reductase II from human erythrocyte. Arch Biochem Biophys. 1985 May 1;238(2):670–679. doi: 10.1016/0003-9861(85)90213-9. [DOI] [PubMed] [Google Scholar]
- Dvornik E., Simard-Duquesne N., Krami M., Sestanj K., Gabbay K. H., Kinoshita J. H., Varma S. D., Merola L. O. Polyol accumulation in galactosemic and diabetic rats: control by an aldose reductase inhibitor. Science. 1973 Dec 14;182(4117):1146–1148. doi: 10.1126/science.182.4117.1146. [DOI] [PubMed] [Google Scholar]
- Foulds G., O'Brien M. M., Bianchine J. R., Gabbay K. H. Kinetics of an orally absorbed aldose reductase inhibitor, sorbinil. Clin Pharmacol Ther. 1981 Nov;30(5):693–700. doi: 10.1038/clpt.1981.222. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Cathcart E. S. Purification and immunologic identification of aldose reductases. Diabetes. 1974 May;23(5):460–468. doi: 10.2337/diab.23.5.460. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Merola L. O., Field R. A. Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science. 1966 Jan 14;151(3707):209–210. doi: 10.1126/science.151.3707.209. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Spack N., Loo S., Hirsch H. J., Ackil A. A. Aldose reductase inhibition: studies with alrestatin. Metabolism. 1979 Apr;28(4 Suppl 1):471–476. doi: 10.1016/0026-0495(79)90059-3. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973 Apr 19;288(16):831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- HAYMAN S., KINOSHITA J. H. ISOLATION AND PROPERTIES OF LENS ALDOSE REDUCTASE. J Biol Chem. 1965 Feb;240:877–882. [PubMed] [Google Scholar]
- Heaf D. J., Galton D. J. Sorbitol and other polyols in lens, adipose tissue and urine in diabetes mellitus. Clin Chim Acta. 1975 Aug 18;63(1):41–47. doi: 10.1016/0009-8981(75)90376-9. [DOI] [PubMed] [Google Scholar]
- Illegal fishing. Lancet. 1981 Dec 5;2(8258):1268–1269. [PubMed] [Google Scholar]
- Inagaki K., Miwa I., Okuda J. Affinity purification and glucose specificity of aldose reductase from bovine lens. Arch Biochem Biophys. 1982 Jun;216(1):337–344. doi: 10.1016/0003-9861(82)90219-3. [DOI] [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Leissing N., McGuinness E. T. Sorbitol dehydrogenase from rat liver. Methods Enzymol. 1982;89(Pt 500):135–140. [PubMed] [Google Scholar]
- Malone J. I., Leavengood H., Peterson M. J., O'Brien M. M., Page M. G., Aldinger C. E. Red blood cell sorbitol as an indicator of polyol pathway activity. Inhibition by sorbinil in insulin-dependent diabetic subjects. Diabetes. 1984 Jan;33(1):45–49. doi: 10.2337/diab.33.1.45. [DOI] [PubMed] [Google Scholar]
- Morrison A. D., Clements R. S., Jr, Travis S. B., Oski F., Winegrad A. I. Glucose utilization by the polyol pathway in human erythrocytes. Biochem Biophys Res Commun. 1970 Jul 13;40(1):199–205. doi: 10.1016/0006-291x(70)91066-1. [DOI] [PubMed] [Google Scholar]
- PIRIE A., VANHEYNINGEN R. THE EFFECT OF DIABETES ON THE CONTENT OF SORBITOL, GLUCOSE, FRUCTOSE AND INOSITOL IN THE HUMAN LENS. Exp Eye Res. 1964 Jun;3:124–131. doi: 10.1016/s0014-4835(64)80027-0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H., Brown J. H., Petrash J. M. Formation of sorbitol 6-phosphate by bovine and human lens aldose reductase, sorbitol dehydrogenase and sorbitol kinase. Biochim Biophys Acta. 1982 Aug 6;717(2):210–214. doi: 10.1016/0304-4165(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H., Hair G. A., Das B. Aldose and aldehyde reductases in human tissues. Biochim Biophys Acta. 1984 Aug 21;800(3):220–227. doi: 10.1016/0304-4165(84)90399-4. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Petrash J. M., Sadana I. J., Ansari N. H., Partridge C. A. Susceptibility of aldehyde and aldose reductases of human tissues to aldose reductase inhibitors. Curr Eye Res. 1982;2(6):407–410. doi: 10.3109/02713688209000786. [DOI] [PubMed] [Google Scholar]
- Travis S. F., Morrison A. D., Clements R. S., Jr, Winegrad A. I., Oski F. A. Metabolic alterations in the human erythrocyte produced by increases in glucose concentration. The role of the polyol pathway. J Clin Invest. 1971 Oct;50(10):2104–2112. doi: 10.1172/JCI106704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S. D., Schocket S. S., Richards R. D. Implications of aldose reductase in cataracts in human diabetes. Invest Ophthalmol Vis Sci. 1979 Mar;18(3):237–241. [PubMed] [Google Scholar]


