Abstract
The ability to inhibit expression of a mutant allele while retaining expression of wild-type protein might provide a useful approach to treating Huntington’s Disease (HD) and other inherited pathologies. The mutant form of huntingtin (HTT), the protein responsible for HD, is encoded by an mRNA containing an expanded CAG repeat. We demonstrate that peptide nucleic acid (PNA) conjugates and locked nucleic acids (LNAs) complementary to the CAG repeat selectively block expression of mutant HTT. The selectivity of inhibition is at least as good as that shown by an siRNA targeted to a deletion polymorphism. Our data suggest that antisense oligomers are promising subject for further development as an anti-HD therapeutic strategy.
Keywords: Huntington’s disease, huntingtin, allele selectivity, peptide nucleic acid, PNA, locked nucleic acid, LNA, siRNA, trinucleotide repeat, CAG repeat
THE NUCLEIC ACID BASIS FOR HUNTINGTON’S DISEASE
Huntington’s disease (HD) is an autosomal dominant inherited disorder with an incidence of 5–10 per 100,000 individuals in Europe and North America.1–3 The disease is characterized by adult onset and progressive neurodegeneration. There are no curative treatments, creating an urgent need for potential therapeutic strategies. HD is caused by the expansion of CAG trinucleotide repeats within the first exon of the huntingtin (HTT) gene, leading to disruption of protein function and neurodegeneration. Unaffected individuals have up to 35 repeats, while HD patients can have from 36 to >100 repeats.4–6 While the exact cause of HD pathology has not been conclusively determined, one hypothesis is that mutant HTT aggregates and causes neuronal abnormalities and cell death.3
One therapeutic strategy is to target the mRNA encoding HTT with siRNAs or antisense oligonucleotides. In theory, this approach might lead to decreased levels of HTT protein, decreased aggregation or other abnormalities, and slower disease progression. While unorthodox when compared to traditional strategies that employ small molecule drugs, this approach is plausible because nucleic acids can be administered by direct infusion into the central nervous system.8
TARGETING HTT mRNA AS A THERAPEUTIC STRATEGY
There have been prior reports of antisense oligonucleotides and duplex RNAs inhibiting HTT expression.9–14, One obstacle has been that most oligomers tested to date inhibit the mutant and wild-type protein expression indiscriminately. HTT is known to play an essential role in embryogenesis,15,16 neurogenesis,15 and normal adult function.15 It is possible, therefore, that agents inhibiting both mutant and wild-type HTT may induce significant side-effects in HD patients.
The ability to inhibit expression of mutant HTT while leaving expression of wild-type HTT mostly intact would be desirable. Research has focused on targeting duplex RNAs to single nucleotide or deletion polymorphisms.18–20 While this is a promising strategy, polymorphisms will often differ from patient to patient, necessitating development of a family of related compounds. It is also unclear whether duplex RNAs will be able to achieve sufficient allele-selectivity and potency.
All HD patients express mutant huntingtin encoded by mRNA containing expanded trinucleotide repeats. Agents capable of distinguishing expanded repeats from shorter wild-type repeats might provide an alternate strategy for achieving allele selectivity. Computational prediction and experimental assays indicate that trinucleotide repeat sequences within RNA form hairpin structures.21,22 The structures formed by wild-type and mutant mRNAs differ in length and will therefore possess different stabilities. While the magnitude and functional significance of such structural differences was unclear, we hypothesized that these hybridization of complementary oligomers to CAG repeats or adjacent mRNA sequences might be sensitive to these differences and that might preferentially bind the mutant mRNA and block expression of the mutant protein.
We have shown that peptide nucleic acid (PNA) peptide conjugates and locked nucleic acid (LNA) oligomers can be potent and allele-selective inhibitors of mutant HTT expression.23 Here we analyze our results with PNA-peptide conjugates and LNAs and directly compare our strategy with a method for allele-selective silencing that employs a duplex RNA targeting a deletion polymorphism.
ALLELE-SELECTIVITY OF PNA-PEPTIDE CONJUGATES
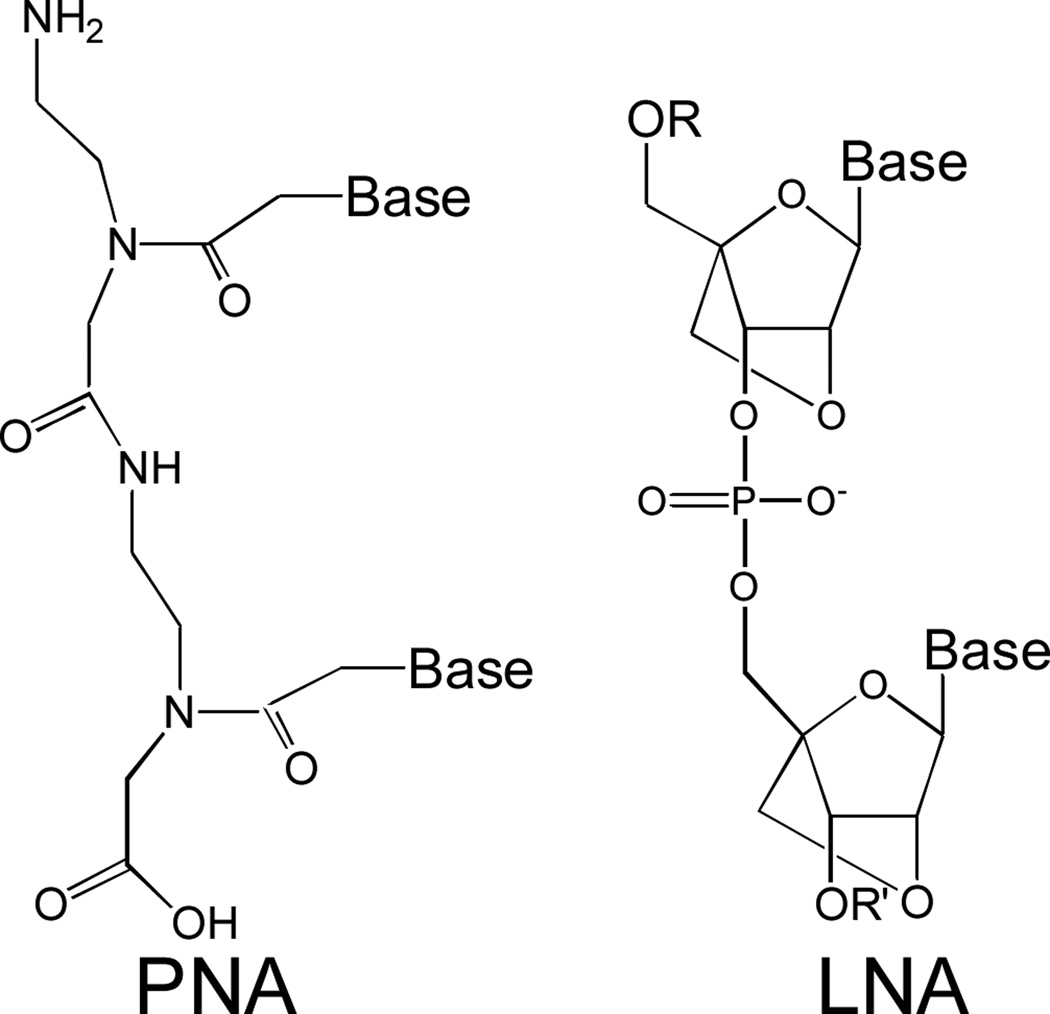
PNAs are a class of DNA/RNA mimic with an uncharged amide backbone (Fig. 1a).24 One advantage of using PNAs is that the charge-charge repulsion that characterizes the formation of RNA/DNA or RNA/RNA duplexes does not occur when PNAs bind complementary RNA sequences. The absence of repulsion increases the affinity of PNA hybridization and facilitates recognition of target sequences within RNA structure.25 Another advantage is that PNAs are synthesized using standard peptide chemistry, allowing easy addition of peptide sequences designed to produce PNA-peptide conjugates with optimal properties.26
Figure 1.
Chemical Structures of PNA and LNA.
We designed PNA-peptide conjugate REP to target the CAG repeat.23 PNAs do not efficiently enter cells, so to facilitate delivery a cationic peptide, D-Lys8 was attached to the C-termini of PNAs. This peptide was chosen because it combines efficient delivery with good physical properties and simple synthesis requirements.26
Many different patient-derived cell lines are available and these constitute an important resource for research into treatments for HD. We chose to examine cell lines that possess HTT alleles with different CAG repeat lengths. Most HD patients have CAG repeats in the range of 40–50, but repeats can be more than 100.4–6 By assaying inhibition in different cell lines we can gain insights into the potential effectiveness of our strategy for a broad range of HD patients. The HD patient-derived fibroblast cell lines used in our study were GM09197 (wild-type allele 21 repeats/mutant allele 151 repeats), GM04281 (wild-type allele 17 repeats/mutant allele 69 repeats), GM04869 (wild-type allele 15 repeats/mutant allele 47 repeats), GM04719 (wild-type allele 15 repeats/mutant allele 44 repeats), and GM04717 (wild-type allele 20 repeats/mutant allele 41 repeats).
We first characterized inhibition in GM04281 cells. For REP19 we observed that i) allele-selective inhibition persisted for up to 22 days, ii) expression of other genes containing triplet repeats was not observed when REP19 was added at 1 µM, 3-fold higher than the IC50 value for selective inhibition, iii) REP19 was not toxic to cells when used at 1 µM, and iv) REP19 protected primary neuronal cells derived from YAC128 transgenic mice (in this model mice express HTT mRNA containing 128 CAG repeats) from apoptosis after addition of glutamate.23
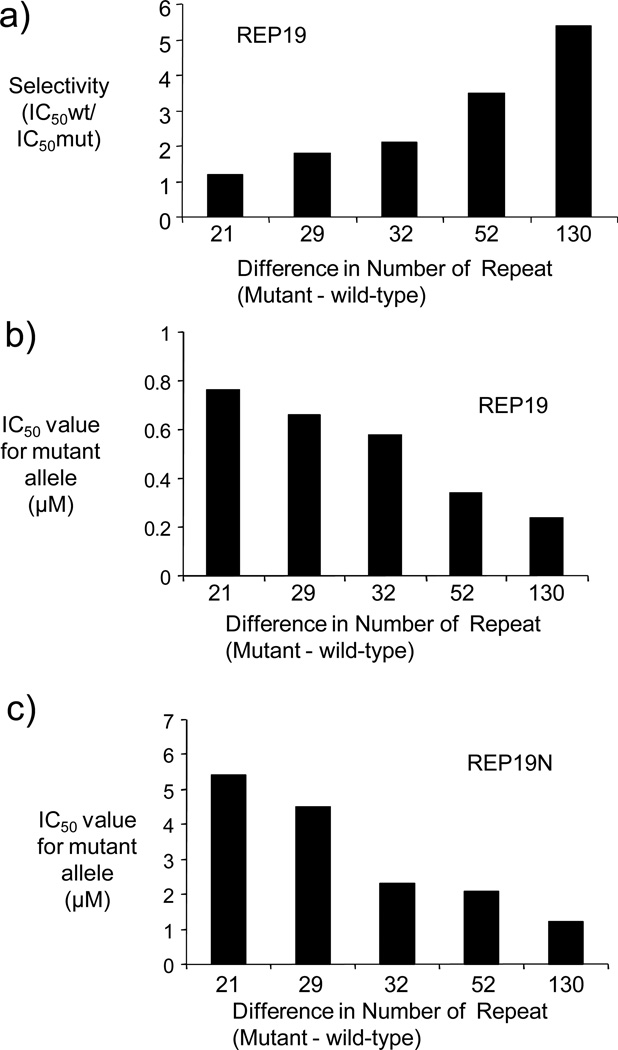
We then tested REP19 in patient-derived HD cell lines with varied numbers of repeats (Table 2).23 We observed that selectivity increased when the differential between the number of wild-type and mutant repeats increased (Fig. 2a). IC50 values for inhibiting expression of mutant HTT variants decreased (i.e. indicated enhanced potency) for variants with larger numbers of mutant repeats (Fig. 2b). These correlations are consistent with the hypothesis that larger triplet repeat structures may have more potential for allele-selective antisense inhibition.
Table 2.
C50 values (µM) for inhibition of mutant and wild-type alleles of HTT in varied cell lines.
| Cell line | GM09197 | GM04281 | GM04869 | GM04719 | GM04717 |
|---|---|---|---|---|---|
| # Mutant Repeats | 151 | 69 | 47 | 44 | 41 |
| # wt Repeats | 21 | 17 | 15 | 15 | 20 |
| Δ#mut/wt | 130 | 52 | 32 | 29 | 21 |
| REP19/wt | 1.3 ± 0.13 | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.90 ± 0.07 |
| REP19/mut | 0.24 ± 0.02 | 0.34 ± 0.03 | 0.58 ± 0.07 | 0.66 ± 0.22 | 0.76 ± 0.04 |
| Selectivity | 5.4 | 3.5 | 2.1 | 1.8 | 1.2 |
| REP19N/wt | >8* | > 16* | > 8* | > 8* | > 8* |
| REP19N/mut | 1.2 ± 0.08 | 2.1 ± 0.5 | 2.3 ± 0.3 | 4.5 ± 0.4 | 5.4 ± 1.5 |
| Selectivity | >6 | >8 | >3 | >2 | >1.5 |
| LNA/REP/wt | >0.1* | >0.1* | |||
| LNA/REP/mut | 0.004 | 0.017 | |||
| Selectivity | >25 | >6 | |||
| siRNA/S4/wt | >0.1* | ||||
| siRNA/S4/mut | 0.05 | ||||
| Selectivity | >2 | ||||
wt: IC50 value (µM) for inhibition of wild-type protein. Mut: IC50 value (µM) for inhibition of mutant protein.
Highest concentration tested.
Figure 2.
(a) Graph of selectivity versus difference repeat number (number of mutant repeats minus number of wild-type repeats) for REP19. (b) Graph of IC50 value versus repeat number for REP19. (c) Graph of IC50 value versus repeat number for REP19N.
Improving selectivity by peptide modification
The peptides that are necessary for the import of PNA are composed of amino acids in the D-configuration. This was done to reduce the likelihood of proteolytic degradation but their stability also suggests that they will remain present during and after recognition of mRNA and may affect both potency and selectivity. To begin to investigate the influence of peptide attachment on allele-selective inhibition of HTT we made a simple change. Instead of attaching peptide D-Lys8 to the C-terminus of REP19, as had been done for previous conjugates, D-Lys8 was attached to the N-terminus to create REP19N.23 This N-terminal attachment yielded improved selectivity in every cell line tested (Table 1). As with the C-terminal conjugate, The IC50 value for inhibiting expression improved as the number of CAG repeats increased (Figure 2 c). These data suggest that straightforward changes in oligomer chemistry can yield substantial improvements in allele-selectivity and that our approach has the potential to be a general one for allele-selective silencing of mutant HTT in a broad range of HD patients.
Table 1.
PNA, duplex RNAs, and LNA oligomers used in these studies.
| Name | Sequence (length) |
|---|---|
| PNA-peptide conjugates | |
| REP19 | K-GCTGCTGCTGCTGCTGCTG-K8 (19) |
| REP19N | K8-GCTGCTGCTGCTGCTGCTG-K (19) |
| -CTL | K-GCTATACCAGCGTCGTCAT-K8 (19) |
| siRNAs | |
| siRNA/S2 | GAAGAGGAGGAGGCCGACGCCTT (23) |
| siRNA/S4 | GAGGAAGAGGAGGAGGCCGACTT (23) |
| siRNA/-CTL | GCUAUACCAGCGUCGUCAUTT (21) |
| LNAs | |
| LNA/REP | gcTgcTgcTgcTgcTgcTg (19) |
| LNA/-CTL | gcTatAccAgcGtcGtcAt (19) |
PNAs are listed N to C terminal. D-amino acids are used in all peptide conjugates. K=lysine. siRNAs (antiense strands only) and LNAs are listed 5’ to 3’. Mismatched bases are underlined. For LNAs, modified bases are represented as capital letters and DNA bases are lower case.
Allele-selective inhibition by LNAs
Single stranded oligonucleotides containing locked nucleic acid (LNA) bases provide a promising strategy for development of nucleic acid-based therapeutics. LNA is an RNA analog that contains a methylene bridge between the 2'-oxygen and 4'-carbon of the ribose (Fig. 1b).27 This bridge reduces the conformational flexibility of the ribose and confers outstanding affinity to complementary hybridization. LNA bases can be placed at any position and allow the thermal stability of oligonucleotides to be precisely tailored for any application. LNA oligomers are being tested in clinical trials28,29 and this experience may have the practical benefit of facilitating clinical development of anti-HTT oligomers.
We tested LNA oligomers that contain LNA bases interspersed among DNA bases. In contrast to the neutral amide backbone of PNAs, LNAs have a negatively charged phosphodiester backbone, allowing us to use cationic lipid to introduce LNAs into cells. LNA/REP, which targets the CAG repeat, yielded allele-specific inhibition of HTT with an IC50 value of 17 nM in GM04281 patient-derived fibroblast cells.23 Concentrations of LNA that selectively blocked expression of mutant HTT did not affect other genes that contain CAG repeats and caused only a modest a modest decrease in levels of wild-type HTT. These results suggest that LNA is a good lead compound for further development.
COMPARISON: TARGETING REPEATS VERSUS POLYMORPHISMS
Other approaches towards achieving allele-selectivity have used duplex RNAs that target single-nucleotide polymorphisms (SNPs) or deletion polymorphisms associated with the mutant HTT allele.18–20 Recent work by Friedlander and colleagues is among the most promising because it i) suggests relatively potent and selective inhibition of mutant HTT, ii) targets a deletion polymorphism (D2642) that is relatively common (38% of mutant HTT alleles, 7% of wild-type alleles), and iii) the three base deletion creates the potential for better discrimination than a one base single nucleotide polymorphism.20
We chose to directly compare PNA REP19 and LNA/REP19 with the best RNA identified by Friedlander (RNA S4) and co-workers in the GM09197 cell line used by Friedlander. This cell line has 151 CAG repeats within the mutant allele, and 21 repeats in the wild-type allele. We did not test RNA S4 in the other cell lines used for these studies because they either do not contain the polymorphism (and would therefore not be susceptible to the action of S4) or have not been characterized. Both RNA S4 and LNA/REP19 were introduced into cells using cationic lipid, permitting a direct comparison of potency.
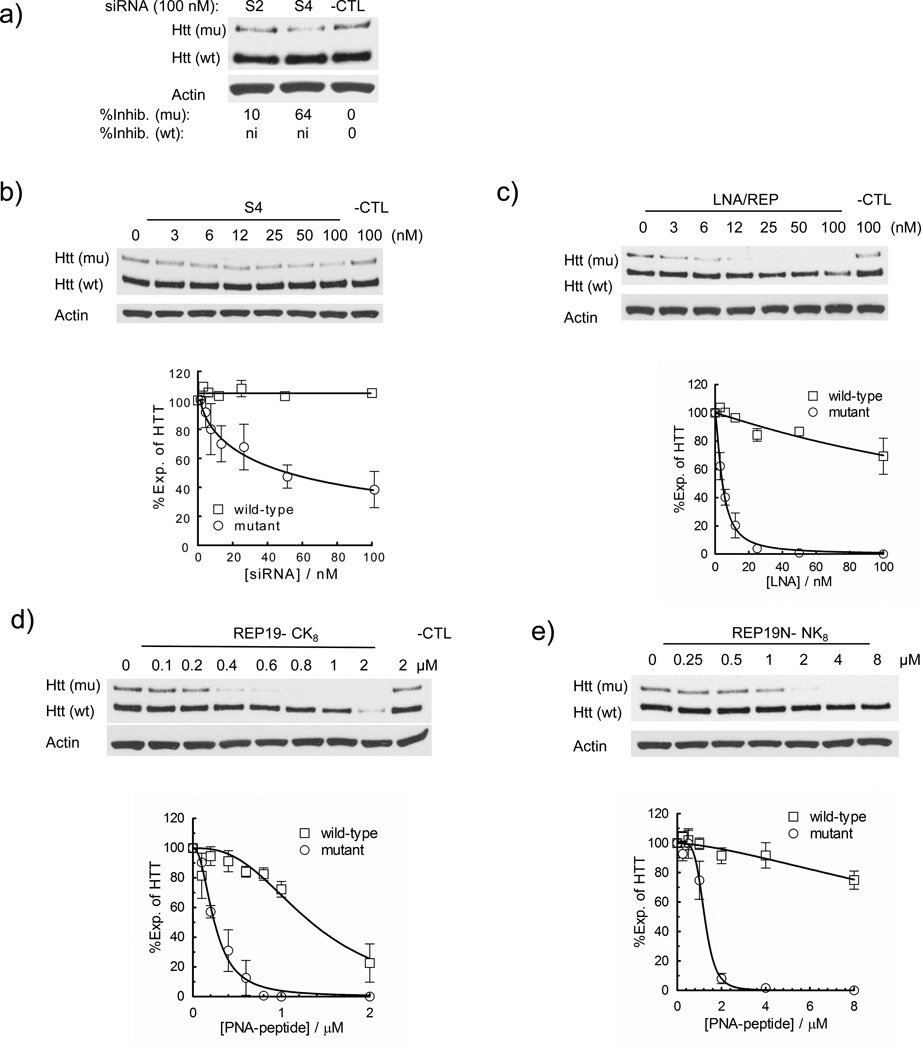
We confirmed that RNA S4 as an allele-selective inhibition of mutant HTT, with an IC50 value of 50 nM and a maximum efficacy of 60 % for inhibition of mutant HTT. No inhibition of wild-type HTT was observed (Figure 3a,b, Table 2). LNA/REP19 was more potent with an IC50 value of 4 nM for inhibition of mutant HTT and a maximum efficacy of 100 % (Figure 3c, Table 1). LNA/REP19 yielded 30 % inhibition of wild-type HTT expression when 100 nM LNA was added. At concentrations of over 100 nM the combination of lipid and RNA S4 or LNA/REP19 begins to be toxic to cells.
Figure 3.
Comparison of allele-specific silencing strategies: siRNAs that target polymorphisms versus PNAs and an LNA that target a CAG repeat. (a) Inhibition of HTT expression by two different siRNAs that target a deletion polymorphism. (b–e) Inhibition of HTT expression by siRNA S4,23 LNA/REP,23 PNA REP19, and REP19N respectively.
We also tested PNAs REP19 and REP19N in GM09197 cells. Direct comparison of IC50 values of PNA REP19 with LNA/REP19 or siRNA S4 is impossible because the PNA is delivered into cells using an attached peptide rather than cationic lipid. However quantifying inhibition allows general trends to be observed. REP19 inhibits mutant HTT with an IC50 value of 240 nM and a selectivity of 5.4-fold relative to inhibition of wild-type HTT (Figure 4d, Table 2). REP19N inhibits mutant HTT with an IC50 value of 1.2 µM and little inhibition of wild-type HTT. The potencies and selectivities towards inhibition of mutant HTT in GM09197 cells are slightly better than in the other cell lines, consistent with GM09197 cells expressing HTT mRNA with a greater number of CAG repeats.
SUMMARY
Effective therapy for HD is a major unmet medical need. Our data suggest that antisense oligonucleotides can be potent inhibitors of HTT and can selectively reduce expression of the mutant allele. Work by others has shown that antisense oligomers can be delivered to the brain in vivo, suggesting that our strategy is plausible. Priorities for future research will be identifying compounds that combine good biodistribution with improved allele-selectivities and inhibitory potencies. While our experiments suggest that inhibition by PNAs and LNAs that target CAG repeats is more effective in one model cell line than inhibition by an siRNA that targets a deletion polymorphism, the approaches are fundamentally different and both should be pursued.
ACKNOWLEDGEMENTS
This work was supported by the High-Q foundation, the National Institutes of Health (NIGMS 73042), the Robert A. Welch Foundation (I-1244), and a McKnight Foundation Neuroscience of Brain Disorders Award.
REFERENCES
- 1.Borrell-Pages, Zala D, Humbert S, Saudou F. Huntington’s disease: from huntingtin function and dysfunction to therapeutic strategies. Cell. Mol. Life Sci. 2006;63:2642–2660. doi: 10.1007/s00018-006-6242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker FO. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 3.Gusella JF, MacDonald ME. Huntington’s disease: seeing the pathogenic process through a genetic lens. Trends Biochem. Sci. 2006;31:533–540. doi: 10.1016/j.tibs.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Duyao M, et al. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat. Genet. 1993;4:387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- 5.Kremer B, et al. A worldwide study of the Huntington’s disease mutation: The sensitivity and specificity of measuring CAG repeats. New Engl. J. Med. 1994;330:1401–1406. doi: 10.1056/NEJM199405193302001. [DOI] [PubMed] [Google Scholar]
- 6.The U.S.-Venezuela collaborative research project. Wexler N. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl. Acad. Sci. USA. 2004;101:3498–3503. doi: 10.1073/pnas.0308679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Souza EB, Cload ST, Pendergrast PS, Sah DW. Novel therapeutic modalities to address nondrugable protein interaction targets. Neuropsychopharmacology Reviews. 2008;34:142–158. doi: 10.1038/npp.2008.115. [DOI] [PubMed] [Google Scholar]
- 8.Smith RA, et al. Antisense oligonucleotide therapy for neurodegenerative disease. J. Clin. Invest. 2006;116:2290–2296. doi: 10.1172/JCI25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasholt L, et al. Antisense downregulation of mutant huntingtin in a cell model. J. Gene Med. 2003;5:528–538. doi: 10.1002/jgm.378. [DOI] [PubMed] [Google Scholar]
- 10.Boado RJ, Kazantsev A, Apostol BL, Thompson LM, Pardridge WM. Antisense-mediated down-regulation of the mutant human huntingtin gene. J. Pharmacol. Exp. Ther. 2000;295:239–243. [PubMed] [Google Scholar]
- 11.Harper SQ, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc. Natl. Acad. Sci. USA. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denovan-Wright EM, Davidson BL. RNAi: a potential therapy for the dominantly inherited nucleotide repeat diseases. Gene Ther. 2006;13:525–531. doi: 10.1038/sj.gt.3302664. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y-L, et al. Clinico-pathological rescue of a model mouse of Huntington’s disease by siRNA. Neurosci. Res. 2005;53:241–249. doi: 10.1016/j.neures.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 14.DiFiglia M, et al. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc. Natl. Acad. Sci. USA. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasir J, et al. Targeted disruption of the Huntington’s disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell. 1995;81:811–823. doi: 10.1016/0092-8674(95)90542-1. [DOI] [PubMed] [Google Scholar]
- 16.Zeitlin S, Liu J-P, Chapman DL, Papaioannou VE, Efstratiadis A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nat. Genet. 1995;11:155–163. doi: 10.1038/ng1095-155. [DOI] [PubMed] [Google Scholar]
- 17.White JK, et al. Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nat. Genet. 1997;17:404–410. doi: 10.1038/ng1297-404. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz DS, et al. Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2006;2:1307–1318. doi: 10.1371/journal.pgen.0020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Bilsen PHJ, et al. Identification and allele-specific silencing of the mutant huntingtin allele in Huntington’s disease patient-derived fibroblasts. Hum. Gene Ther. 2008;19:710–718. doi: 10.1089/hum.2007.116. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Engelman J, Friedlander RMJ. Allele-specific silencing of mutant Hunington’s disease gene. J. Neurochem. 2009;108:82–90. doi: 10.1111/j.1471-4159.2008.05734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobczak K, de Mezer M, Michlewski G, Krol J, Krzyzosiak WJ. RNA structure of trinucleotide repeats associated with human neurological diseases. Nucl. Acids Res. 2003;31:5469–5482. doi: 10.1093/nar/gkg766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gacy AM, Goellner G, Juranic N, Macura S, McMurray CT. Trinucleotide repeats that expand in human disease form hairpin structures in vitro. Cell. 1995;81:533–540. doi: 10.1016/0092-8674(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 23.Hu J, Matsui M, Gagnon KT, Schwartz JC, Gabillet S, Arar K, Wu J, Bezprozvanny I, Corey DR. Allele-specific silencing of mutant huntingtin and ataxin-3 genes by targeting expanded CAG repeats in mRNAs. Nat. Biotech. 2009 doi: 10.1038/nbt.1539. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen PG, Egholm M, Berg RH, Buchardt O. Sequence–selective recognition of DNA by strand displacement with a thymine–substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 25.Marin VL, Armitage BA. RNA guanine quadruplex invasion by complementary and homologous PNA probes. J. Am. Chem. Soc. 2005;127:8032–8033. doi: 10.1021/ja051102y. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Corey DR. Inhibiting gene expression with peptide nucleic acid (PNA)-peptide conjugates that target chromosomal DNA. Biochemistry. 2007;46:7581–7589. doi: 10.1021/bi700230a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vester B, Wengel J. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry. 2004;43:13233–13241. doi: 10.1021/bi0485732. [DOI] [PubMed] [Google Scholar]
- 28.Frieden M, Orum H. The application of locked nucleic acids in the treatment of cancer. IDrugs. 2006;9:706–711. [PubMed] [Google Scholar]
- 29.Koch T, et al. Locked nucleic acid: Therapeutic properties and therapeutic aspects. In: Kurreck J, editor. Published in Therapeutic Oligonucleotides. RSC Biomolecular Sciences, Royal Society of Chemistry; 2008. pp. 103–141. [Google Scholar]