Abstract
Hypertension is a disorder affecting millions worldwide, and is a leading cause of death and debilitation in the United States. It is widely accepted that during hypertension and other cardiovascular diseases the vasculature exhibits endothelial dysfunction; a deficit in the relaxatory ability of the vessel, attributed to a lack of nitric oxide (NO) bioavailability. Recently, the one electron redox variant of NO, nitroxyl anion (NO−) has emerged as an endothelium-derived relaxing factor (EDRF) and a candidate for endothelium-derived hyperpolarizing factor (EDRF). NO− is thought to exist protonated (HNO) in vivo, which would make this species more resistant to scavenging. However, no studies have investigated the role of this redox species during hypertension, and whether the vasculature loses the ability to relax to HNO. Thus, we hypothesize that aorta from angiotensin II (AngII)-hypertensive mice will exhibit a preserved relaxation response to Angeli’s Salt, an HNO donor. Male C57Bl6 mice, aged 12–14 weeks were implanted with mini-osmotic pumps containing AngII (90ng/min, 14 days plus high salt chow) or sham surgery. Aorta were excised, cleaned and used to perform functional studies in a myograph. We found that aorta from AngII-hypertensive mice exhibited a significant endothelial dysfunction as demonstrated by a decrease in acetylcholine (ACh)-mediated relaxation. However, vessels from hypertensive mice exhibited a preserved response to Angeli’s Salt (AS), the HNO donor. To confirm that relaxation responses to HNO were maintained, concentration response curves (CRCs) to ACh were performed in the presence of scavengers to both NO and HNO (carboxy-PTIO and L-cys, resp.). We found that ACh-mediated relaxation responses were significantly decreased in aorta from sham and almost completely abolished in aorta from AngII-treated mice. Vessels incubated with L-cys exhibited a modest decrease in ACh-mediated relaxations responses. These data demonstrate that aorta from AngII-treated hypertensive mice exhibit a preserved relaxation response to AS, an HNO donor, regardless of a significant endothelial dysfunction.
Keywords: nitroxyl anion, HNO, vascular, aorta, angiotensin II hypertension
1.1 Introduction
Hypertension is a leading contributor to death and disability in the United States and globally. Although a variety of factors including diabetes, smoking and other life style choices can be correlated with hypertension, there is still a subset of the population which becomes hypertensive without the presence of these risk factors. These patients develop what is known as essential hypertension and frequently do not consistently respond to commonly used antihypertensive medications.
Among other factors, it is widely accepted that vascular tone contributes to vascular resistance, and changes in vascular resistance can alter systemic blood pressure. Many investigators have demonstrated, using different hypertensive animal models, that increased blood pressure can also alter vascular contractility and relaxation through a variety of processes. One such pathway is through a decrease in nitric oxide (NO) production and/or bioavailability, as NO is known to be a potent modulator of vascular tone. [1] NO is produced through the one electron reduction of L-arginine to citrulline and NO by nitric oxide synthase (NOS). Since the discovery of an endothelium-derived relaxing factor (EDRF) and the determination of NO to be the EDRF, the vast majority of research has focused on this redox species of NO. Recent evidence suggests that a redox variant of NO, nitroxyl anion (NO−), may mediate relaxation through potassium (K+) channel activation and VSM hyperpolarization leading investigators to suggest that NO− is an endogenously-derived EDHF. [2]
Very little research exists on the two redox variants of NO; the charged nitrosonium cation (NO+) and the one-electron product, nitroxyl anion (NO−). [1,3] It was thought that NO− would exist as an anion (pKa 4.7) at a physiological pH; however, this assumption was corrected in 2002, when investigators determined the actual pKa to be around 11.4 and that at physiological pH NO− exists as HNO. [4–6] It was also determined that this conjugated weak acid, HNO, would be able to cross cellular membranes, leading investigators to divert research to this understudied molecule. [5] It is now known that the physiology, pharmacology and biochemistry of HNO are vastly different than that of NO. [7–8]
HNO and NO can both be produced through the conversion of L-arginine to NO by nitric oxide synthase (NOS), in the presence of required substrates; arginine, NADPH and oxygen with the co-factors calmodulin and tetrahydrobiopterin (BH4) (for constitutive NOS). [9–11] However, HNO is mainly produced when NOS is uncoupled or in the absence of BH4. [10,12–15] Under similar conditions, the product of uncoupled NOS, N-hydroxyl-L-arginine, can also lead to HNO production. [16] During hypoxic conditions, NO can be generated via reduction of nitrites on metal sites and from conversion via xanthine oxidase (XO). Interestingly, it was demonstrated that through a similar, yet aerobic pathway, HNO is also produced. [9,17–18] Furthermore, HNO can be generated via reduction of NO by mitochondrial cytochrome c (mCyC) and non-enzymatically from the decomposition of S-nitrosothiols. Other reports suggest HNO production to be a product of the catalytic turnover of NOS. [19–20] [1,21–22]
Moreover, the mechanism by which HNO elicits its effects varies from NO. Given the chemistry of HNO, the production and target site may be limited to the membrane, suggesting channels and other membrane-bound enzymes as possible downstream targets. [9,23] This corroborates reports of HNO S-nitrosylating the ryanodine receptor and myofilaments, causing both lusitropy and inotropy in cardiac tissue. [24–25] It has also been shown that HNO can activate adenosine-triphosphate (ATP)-sensitive potassium channels (KATP) in the coronary vasculature and voltage-gated potassium channels (K+V) in mesenteric arteries. [1,26–27] There is also evidence suggesting that both NO and HNO can activate soluble guanylate cyclase (sGC), while accumulation of cyclic guanosine monophosphate (cGMP) has only been shown in large conduit vessels. [3,28–29] In contrast to NO donors, infusion of HNO donors such as Angeli’s Salt does not lead to an increase in systemic cGMP, but to an increase in calcitonin gene-related peptide (CGRP). CGRP is a potent vasodilator released from neurons and has been described as a biomarker of HNO activity. [3,30] Although there is an increasing body of knowledge regarding HNO-mediated relaxation, no current studies have investigated the relative role that HNO plays during hypertension. Given the stability of HNO as compared to NO, the ability of HNO to maintain its ability to mediate vasorelaxation during hypertension may preclude HNO to be a possible therapeutic agent to exploit. We hypothesize that HNO-mediated relaxation will be preserved in aorta from angiotensin II (AngII) hypertensive mice.
2.0 MATERIALS AND METHODS
Male C57bl/6 mice, weighing between 25–30 grams were obtained from Jackson Laborotories (Bar Harbor, ME). Mice were maintained on a 12-hour light dark cycle, housed five per cage and allowed access to chow and water ad libitum. Isoflurane (10%) in oxygen was used for surgeries with carbon dioxide (CO2) for euthanasia. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Georgia Health Sciences University Committee on the Use of Animals in Research and Education.
2.1 Blood Pressure Telemetry Studies
Mice were anesthetized using isofluorane and a DSI Data Transmitter (Data Sciences International, St. Paul, MN) was implanted in the left carotid artery, routed and secured sub-scapularly. Animals were allowed to recover; systolic/diastolic pressures, heart rate and activity were collected for 18 hours per day/night. Data were analyzed using Power Lab (AD Instruments, Colorado Springs, CO). The blood pressure data from these mice are shared, as the mesenteric arteries and aorta were used in two separate papers.
2.2 Functional Studies
After euthanasia with CO2, the mesentery was rapidly excised and bathed in ice-cold physiological salt solution (PSS) (NaCl 120 mM, KCl 4.7 mM, KH2PO4 1.18 mM, NaHCO3 14.9 mM, dextrose 5.6 mM, CaCl2·H2O, 0.06 mM EDTA). Increased concentrations of EDTA were used in PSS buffer to aid in preventing the extracellular conversion of HNO to NO. Aorta were carefully isolated and mounted as ring preparations on two stainless steel pins in a myograph (Danish MyoTech, Aarhus, Denmark). Vessels were maintained at 37° C and continuously aerated with 95% O2, 5% CO2 and allowed to stabilize for at least 45 mins, at an optimal passive force of 5.0 mN. After stabilization, tissues were contracted with KCl (120 mM) solution to determine the reactivity of the vascular smooth muscle cells. To determine the viability of the endothelium, contraction was stimulated via phenylephrine (Phe; 1 µM) followed by acetylcholine (ACh; 10 µM). Vessels were then washed before performing concentration response curves (CRC) and after each CRC. CRCs to ACh or Angeli’s Salt (AS, Cayman Chemical, Ann Arbor, MI) in Phe-contracted vessels was performed in the presence of vehicle or the following inhibitors: carboxy-PTIO (CPTIO, nitric oxide scavenger), L-cysteine (L-cys, nitroxyl anion scavenger), 4-aminopyridine (4-AP, K+V channel blocker). CPTIO was obtained from Cayman Chemical and 4-AP obtained from Tocris Bioscience, Ellisville, MO. All other chemicals and drugs were purchased from Sigma Aldrich, St. Louis, MO. Force measurements were collected using Chart™ Software (ADI Instruments, Colorado Springs, CO) for PowerLab data acquisition systems (ADI Instruments).
2.3 Statistical Analysis
Agonist concentration-response curves were fitted using a nonlinear interactive fitting program (GraphPad Prism, Graph Pad Software Inc., San Diego CA), and values expressed as percent of maximal relaxation graphed against increasing molar concentrations of agonist. Agonist potencies and maximum response are expressed as negative logarithm of the molar concentration of agonist producing 50% of the maximum response (EC50) and maximum effect elicited by the agonist (Rmax), respectively. Non-linear regression analysis was used to determine EC50 values, where Rmax was normalized to 100 percent for calculations. Data are expressed as mean±SEM (n), where n is the number of experiments performed. Statistical analysis of the concentration-response curves was performed by using the F test for comparisons of best-fit data between groups (EC50 and Rmax). For CRCs where a best-fit analysis could not be performed, absolute Rmax values were averaged and Students’ t-test performed for significance.
2.4 Drugs
Carboxy-PTIO was suspended in DMSO and Angeli’s Salt was prepared using a 0.01M NaOH solution. All other stock solutions were prepared by using water. Stock solutions originally diluted in DMSO or ethanol was used with a final concentration of less than 0.003% v/v in the muscle bath; this concentration has been demonstrated to have no effect on vascular reactivity. Additionally, solutions containing vehicle levels of ethanol and DMSO were also used throughout the experimental protocol to control for non-specific effects.
3.0 RESULTS
3.1 Angiotensin II treated mice exhibited an increase in mean arterial pressure, as shown by telemetry
Before drug treatment, MAP, HR and activity data were obtained during a control period of no less than 4 days in all mice. Mice treated with AngII for two weeks exhibited a significant increase in MAP by day 3 (160±5mmHg, p<0.05). There were no observed changes in MAP, HR or activity for the sham surgery mice, whether given normal chow or high salt chow.
3.2 Aorta from AngII mice did not exhibit a decrease in AS-mediated relaxation responses despite a reduction in ACh-mediated relaxation reponses
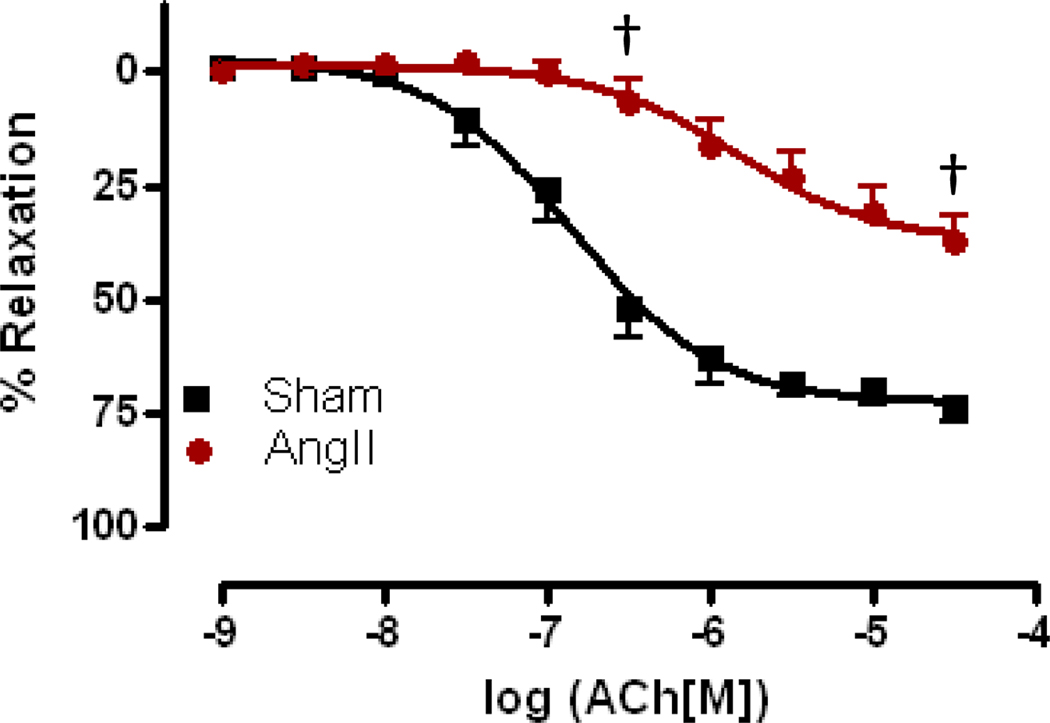
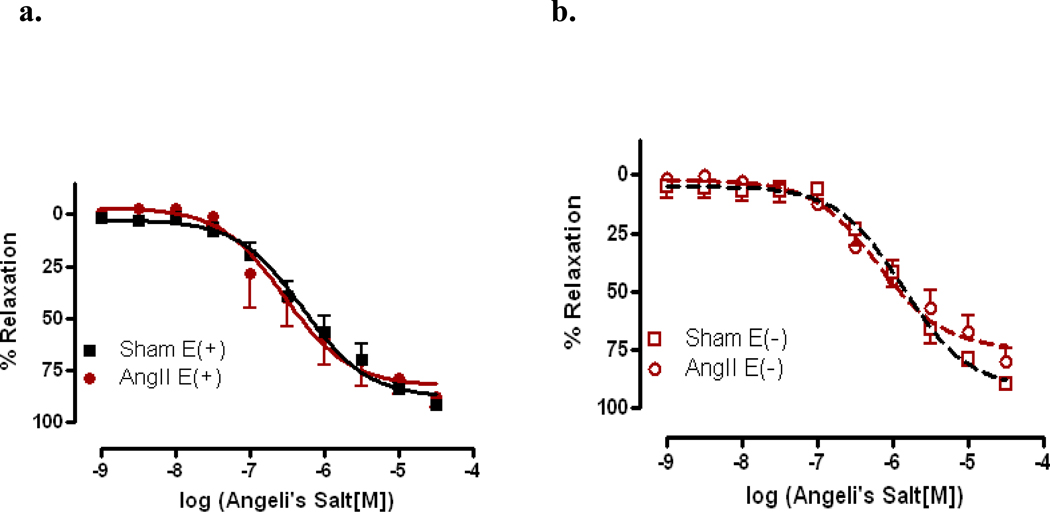
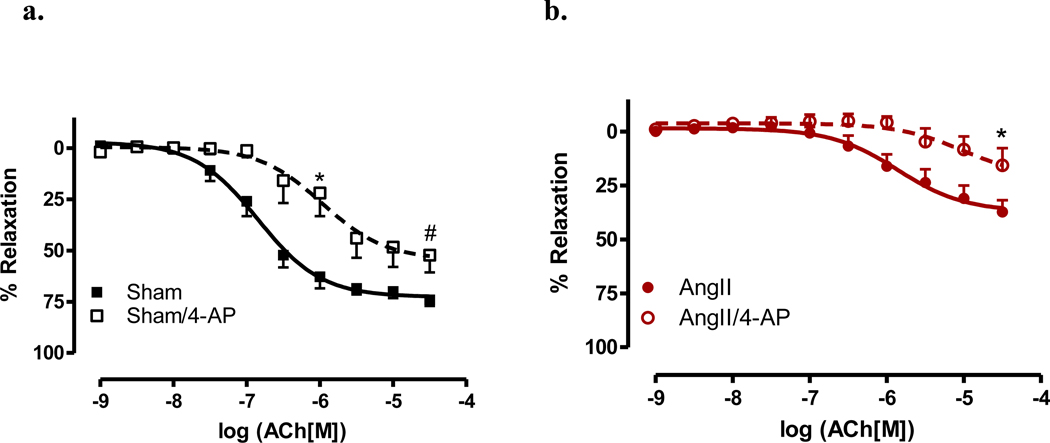
Aorta from AngII hypertensive mice exhibited a significant decrease in sensitivity to ACh as compared to sham aorta (EC50 −5.86±0.18 vs. −6.85±0.09, p<0.001). There was also a reduction in the maximal relaxation in AngII-treated aorta as compared to sham aorta (Rmax 37.00%±3.66 vs. 72.56%±2.46, p<0.001) (Figure 1). However, we observed no change in AS-mediated relaxation responses (Figures 2a and 2b) in AngII-treated mice as compared to vessels from sham mice. Similar results were obtained whether aortas were intact (E+, Figure 2a) or denuded (E−, Figure 2b).
Figure 1. Aorta from AngII-hypertensive mice exhibit endothelial dysfunction.
Concentration response curves to ACh were performed in Phe (1 µM) contracted aorta. ACh-mediated relaxation responses were assessed in aorta from AngII-treated (AngII) and sham (Sham) mice. Relaxation responses were calculated relative to the maximal contraction elicited by Phe. Data are represented as mean ± SEM; n=12–18. †p<0.001, EC50 and Rmax values of AngII vs. Sham.
Figure 2. Angeli’s Salt-mediated relaxation responses were preserved in intact and denuded aorta from AngII hypertensive mice.
Concentration response curves to the nitroxyl anion donor, Angeli’s Salt, were performed in Phe (1 µM) contracted aorta. Relaxation responses to nitroxyl anion were assessed in intact aorta from AngII-treated (AngII,) and sham (Sham) mice. Nitroxyl anion-mediated relaxation was determined in aorta from intact, E(+), (a) AngII-treated (AngII) and sham (Sham) mice and denuded, E− (b). Relaxation responses were calculated relative to the maximal contraction elicited by Phe. Data are represented as mean ± SEM; n=12–16.
3.3 Aorta from AngII hypertensive mice are dependent upon nitric oxide for relaxation
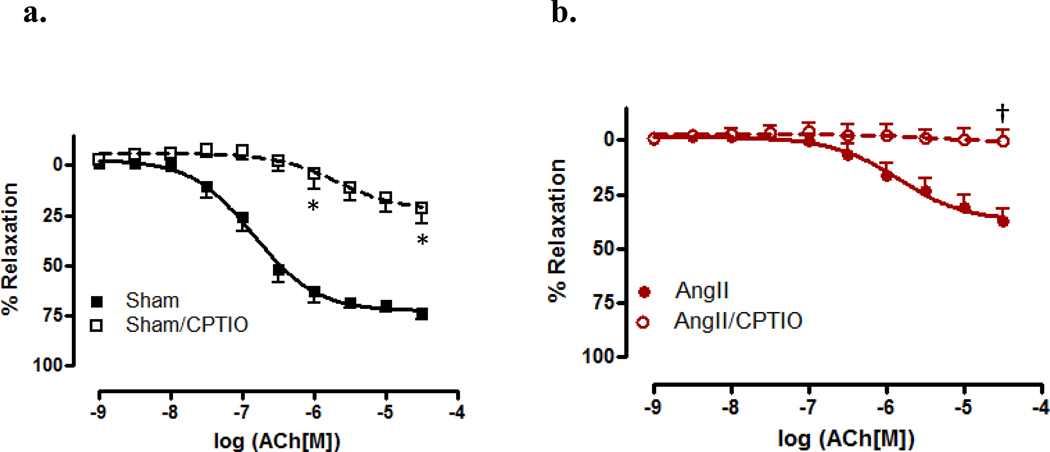
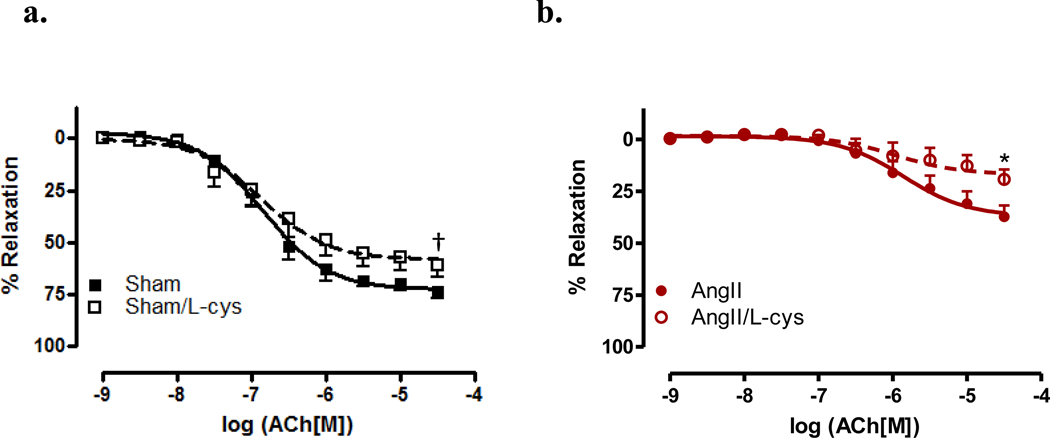
To further investigate the role of HNO in vasorelaxation, scavengers of NO and HNO (CPTIO and L-cys, resp.) were used. Aortas were incubated with CPTIO and CRCs performed to ACh. In the presence of CPTIO, vessels from sham mice exhibited a significant decrease in both sensitivity (EC50 −5.68 ± 0.34 vs. −6.85 ± 0.09, p<0.05) and maximal relaxation (Rmax 22.29% ± 5.48 vs. 72.56% ± 2.46, p<0.05) (Figure 3a) to ACh. When aortas from AngII hypertensive mice were incubated with CPTIO, there was an almost complete inhibition of relaxation to ACh (Rmax p<0.001)(Figure 3b). As shown in Figure 4, aortas were incubated with the HNO scavenger, L-cys, which has been demonstrated as a mechanism to differentiate between HNO and NO. [26,31–32] A significant decrease in maximal relaxation was observed in vessels incubated with L-cys as compared to vehicle in sham (Rmax 58.00% ± 3.43 vs. 72.56% ± 2.46, p<0.001) and AngII treated mice (Rmax 16.80% ± 3.37 vs. 36.99% ± 3.66, p<0.05)(Figure 4b). These data suggest a significant dependence upon NO for vasorelaxation, and that NO bioavailability is decreased during AngII hypertension.
Figure 3. Scavenging nitric oxide decreases ACh-mediated relaxation responses in aorta from AngII-treated and sham mice.
Concentration response curves to ACh were performed in Phe (1 µM) contracted aorta from sham (Sham,)(a) and AngII (AngII)(b) in the presence of the nitric oxide scavenger, carboxy-PTIO (200 µM). Data are represented as mean ± SEM; n=7–9. *p<0.05, EC50 and Rmax values of CPTIO vs. vehicle in sham; †p<0.001, Rmax values of CPTIO vs. vehicle in AngII.
Figure 4. Nitroxyl anion does not primarily mediate endothelium dependent vasorelaxation in aorta from both sham and AngII-treated mice.
Concentration response curves to ACh were performed in Phe (1 µM) contracted first aorta from sham (Sham)(a) and AngII (AngII)(b) in the presence of the nitroxyl anion scavenger, L-cys (3 mM). Data are represented as mean ± SEM; n=9–11. †p<0.001, Rmax values of L-cys vs. vehicle in sham, *p<0.05, Rmax values of L-cys vs. vehicle in AngII.
3.4 Aorta exhibit a decrease in ACh-mediated relaxation with voltage-gated potassium channel blockade
The K+V channel has been demonstrated to be specifically activated by HNO in rat and mouse mesenteric arteries. Given this, the role of K+V channels in this model of hypertension was investigated. Aortas were incubated with 4-AP, which has been previously demonstrated to be a specific K+V channel blocker. [2,26] In Figure 5, aortas were incubated with the K+V channel blocker or vehicle and CRCs to ACh were performed. Vessels from sham animals exhibited a rightward shift in sensitivity to ACh (EC50 −5.97 ± 0.22 vs. −6.85 ± 0.09, p<0.05), with a significant decrease in the maximal relaxation responses (Rmax 54.41% ± 6.05 vs. 72.56% ± 2.46, p<0.01) (Figure 5a). Aorta from AngII hypertensive mice also exhibited a decrease in maximal relaxation responses (Rmax 21.50% ± 10.09 vs. 37.00% ± 3.65, p<0.01 (Figure 5b). These data suggest that the K+V channel may modulate a portion of endothelium-mediated relaxation.
Figure 5. Voltage-gated potassium channel blockade decreases relaxation in aorta.
Concentration response curves to ACh were performed in Phe (1 µM) contracted aorta from sham (Sham)(a) and AngII (AngII)(b) in the presence of the voltaged-gated potassium channel blocker, 4-AP (100 mM). Data are represented as mean ± SEM; n=6–9. *p<0.05, EC50 values of 4-AP vs. vehicle in sham and Rmax values of 4-AP vs. vehicle in AngII, #p<0.01, Rmax values of 4-AP vs. vehicle in sham.
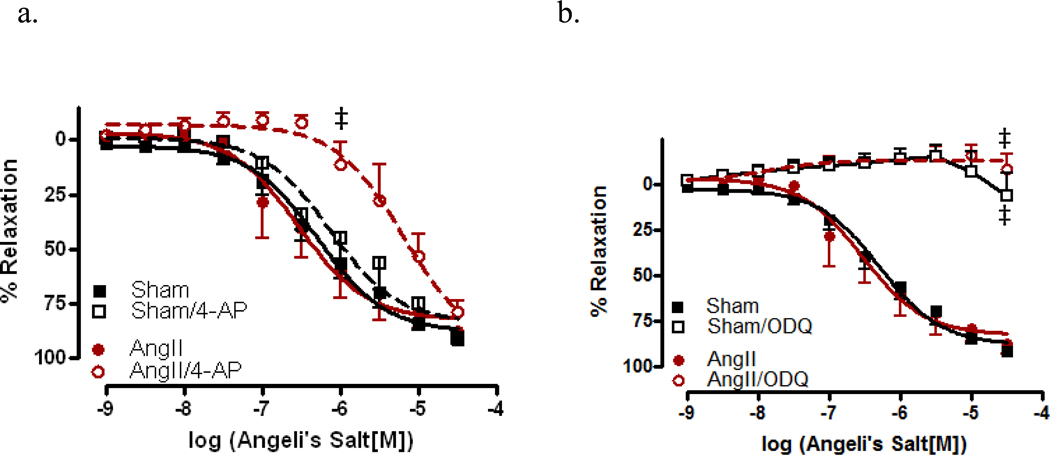
3.5 AS-mediated relaxation is soluble guanylate cyclase dependent
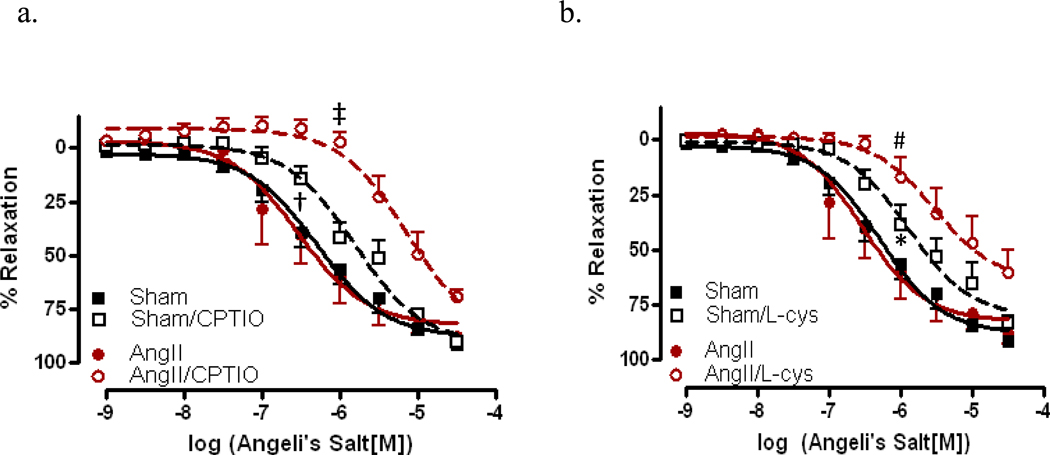
To assist in determining the mechanism involved in HNO-mediated relaxation, CRCs to AS were performed in aorta from sham and AngII-treated mice. Vessels were incubated with 4-AP, the KV+ channel blocker, as shown in Figure 6a. No significant differences in sensitivity to AS were observed aorta from sham mice; however, vessels from AngII-treated mice exhibited a significant decrease in AS-mediated relaxation (−5.19 ± 0.15 vs.−6.54 ± 0.16, p<0.0001). In vessels incubated with ODQ, the sGC inhibitor (Figure 6b), aorta from both sham (Rmax 27.0%) and AngII (Rmax 11.5%) –treated mice exhibited almost a complete inhibition of relaxation, suggesting that in the aorta, AS-mediated relaxation is largely dependent upon sGC for relaxation responses. CRCs to AS were also performed in the presence of the NO and HNO scavengers, CPTIO and L-cys, resp. We found that vessels from both sham (EC50 −5.82 ± 0.08, p<0.001) and AngII-treated (EC50 −5.18 ± 0.14, p<0.001) mice, incubated with CPTIO exhibited a significantly decreased sensitivity to AS (Figure 7a). When similar curves were performed in the presence of L-cys, aorta from both sham (EC50 −5.89 ± 0.12, p<0.05) and AngII-treated (EC50 −5.53 ± 0.21, p<0.01) mice exhibited a decrease in AS-mediated relaxation responses (Figure 7b).
Figure 6. Relaxation responses to the nitroxyl anion donor, Angeli’s Salt, are mediated through soluble guanylate cyclase.
Concentration response curves to AS were performed in Phe (1 µM) contracted aorta from AngII (AngII) and sham (Sham) in the presence of the (a) voltage-gated potassium channel blocker, 4-AP (100 mM) and the (b) soluble guanylate cyclase inhibitor, ODQ (1 µM). Data are represented as mean ± SEM; n=5–8. ‡p<0.0001, EC50 values of 4-AP vs. vehicle in AngII, ‡p<0.0001, Rmax values of ODQ vs. vehicle in sham and AngII.
Figure 7. Relaxation responses to the nitroxyl anion donor, Angeli’s Salt, are reduced with nitric oxide and nitroxyl anion scavenging.
Concentration response curves to AS were performed in Phe (1 µM) contracted first aorta from AngII (AngII) and sham (Sham) in the presence of the (a) NO scavenger, CPTIO (200 µM) and the (b) HNO scavenger, L-cys (3 mM). Data are represented as mean ± SEM; n=5–8. *p<0.05, EC50 values of L-cys vs. vehicle in sham; #p<0.01 EC50 values of L-cys vs. vehicle in AngII; †p<0.001, EC50 values of CPTIO vs. vehicle in sham, ‡p<0.0001, EC50 values ODQ vs. vehicle in AngII.
4.0 DISCUSSION
The free radical species of nitrogen oxide, NO, is the most well known and well-studied, in contrast to its redox congeners: NO− and NO+. As mentioned previously, studies involving NO− were not pursued due to the fact that NO− was thought to exist as an anion in vivo; however, recently it has been determined that NO− would actually be protonated (HNO). [4–6,33] As the protonated species, HNO, no channel or ion pore is needed for intracellular access, thus making HNO a possible mediator in cell signaling pathways. [5] Studies have been performed using the HNO/NO− donor, AS, which decomposes to yield NO− and NO2 to determine the mechanism by which this species mediates relaxation in the vasculature. [34–37] Although literature shows a definite role for HNO-mediated relaxation, no studies to date have investigated the role which HNO plays in vessels from hypertensive animals. In this study, we set out to determine if HNO-mediated relaxation responses would be preserved in the aorta from AngII hypertensive mice, given that HNO is thought to exhibit decreased reactivity in the presence of reactive oxygen species (ROS).
Previous studies have demonstrated that AS induces relaxation in vascular and nonvascular smooth muscle including: aorta, mesenteric artery, cornonary artery, anococcygeus and gastric fundus. [1,26,31,35,38–40] In the present study, we also found that the aorta from mice exhibited a similar half-maximal relaxation reponse to AS, 0.65 µM, when compared to other tissues (Figure 2a). What was interesting was our observation that relaxation responses to AS were maintained in aorta from AngII-treated mice, whether the vessels were intact or denuded (Figure 2). When CRCs to ACh were performed, aortas from hypertensive mice exhibited a significantly decreased sensitivity and maximal relaxation response, suggesting there is a profound endothelial dysfunction (Figure 1). This study is the first to demonstrate this maintenance of HNO-mediated vasorelaxation, and this phenomenon was in contrast to other findings from this laboratory showing that mesenteric arteries from salt-loaded AngII-hypertensive mice exhibit a decrease in HNO-mediated relaxation. [41–42] These data demonstrate regional differences in vascular reactivity of HNO during hypertension.
In responses mediated by ACh, we found that incubation with CPTIO (Figure 3) produced a marked decreased in relaxation, while incubation with L-cys (Figure 4) only produced a modest decrease in relaxation responses. These data indicate that endogenous production of NO, such as by ACh-mediated mechanisms, is scavenged in the presence of CPTIO. The presence of CPTIO in CRCs performed to ACh reduced the response in aorta from sham, confirming the role that NO plays in aortic vascular tone. Conversely, the almost complete abrogation of ACh-mediated relaxation responses in aorta from AngII-treated mice, when incubated with CPTIO, also signifies the loss of NO bioavailability for relaxation during AngII hypertension. L-cys incubation produced a modest, though significant decrease in ACh-mediated relaxation in vessels from sham and AngII-treated, mice revealing little reliance upon HNO for relaxation as compared to NO. When compared to our data using the HNO donor, AS, we believe that these data may also suggest that there may be different mechanisms for relaxation when HNO is produced endogenously versus using a pharmacological donor.
In order to investigate mechanisms for relaxation, we also performed CRCs to AS and ACh in the presence of the sGC inhibitor, ODQ, and the KV+ channel blocker, 4-AP. When CRCs to ACh were performed in the presence of 4-AP, aorta from sham and AngII-treated mice exhibited a decrease in ACh-mediated relaxation responses (Figure 5). In preliminary results using mesenteric arteries, we found that KV+ channel blockade has a profound effect on endothelium-mediated relaxation, which, given that resistance vessels rely heavily upon EDHF for relaxation, is expected. Our current data demonstrates a role for KV+ channels in modulating aortic vascular tone and possibly KV+ channels in hypertension. To further investigate this role, CRCs were performed to AS in the presence of the KV+ channel antagonist, with surprising results. Only vessels from AngII hypertensive mice exhibited a decrease in AS-mediated relaxation (Figure 6a). When similar CRCs were performed in the presence of ODQ, which inhibits sGC, we observed a complete inhibition of AS-mediated relaxation (Figure 6b). We believe these data reveal that AS-mediated relaxation is completely dependent upon sGC for relaxation, and again suggesting that endogenously produced HNO may differ in mechanism from exogenously applied HNO.
All CRCs were performed in the presence of the copper (Cu(II)+) chelator, EDTA, in order to prevent or decrease the extracellular conversion of HNO to NO, as shown by other investigators. [27,31,35,43] Using NO-sensing probes, Favaloro and colleagues demonstrated that Cu+ present in the PSS used during experiments will oxidize NO− to NO. [27] Although the use of EDTA will limit the extracellular conversion of NO− to NO, there is limited evidence regarding the intracellular conversion between these two molecules. In our experiments, we found that both L-cys and CPTIO attenuated AS-mediated relaxation responses in aorta from sham and AngII-treated mice. Although this is the first study to investigate HNO-mediated relaxation in aorta from AngII hypertensive mice, there are other studies to demonstrate that both scavengers can reduce AS-mediated relaxation responses. In a study performed by Ellis and coworkers, they found that AS mediated relaxations differently in the aorta than in the non-vascular tissue, anococcygeus muscle. They also found that CPTIO itself, oxidizes NO− to NO, albeit less than 2% of the total amount of AS added. [35] In our present study, we also observed a decrease in AS-mediated relaxation responses in the presence of CPTIO. Interestingly, the scavenging effect of CPTIO was greater in aorta from the AngII-treated mice. Given that a possible intracellular oxidative effect may be inter-converting these two molecules, the fact that this effect was increased in aorta from hypertensive mice gives us insight into different possible oxidative environments of these vessels. Other laboratories have shown that there are increases in superoxide dismutase (SOD) activity during AngII hypertension, which may also explain our findings. [44] If vascular disease and hypertension lead to increases in expression and/or activity of other metal containing enzymes such as xanthine oxidase (XO), which is highly likely given the oxidative environment during these conditions, this would also contribute to changes in HNO/NO interconversion. Additionally, with increasing concentrations of AS (> 10 µM), there have been shown to be increases in NO, along with nitrite production. [27] Other investigators have ruled out a possibility of nitrites to be mediating the vast majority of effects seen by AS, given that nitrites mediate vascular relaxation at concentrations much higher than what is present with AS decomposition. [27,31] We also observed a further attenuation of AS-mediated relaxation responses in aorta from AngII-treated mice when incubated with L-cys as compared to aorta from sham mice incubated with L-cys. Investigators have used bolus doses of AS and found that incubation with millimolar levels of L-cys significantly decreases the maximal relaxation responses. L-cys, in lower concentrations, is suggested to block NO-mediated vasorelaxation. With low concentrations (< 300 µM) of L-cys, Furchgott and Feelisch et al. found that these concentrations blocked the response to NO and to EDRF, which they surmised to be through the formation of superoxide during the auto-oxidation of L-cys. [31–32,45–48] Moreover, data suggest that, via S-nitrosothiol production, L-cys may potentiate NO-mediated responses in the vasculature when used at high concentrations (> 1mM). [31–32,47] In our preparation, we did not find a potentiation of ACh-mediated relaxation responses in the presence of L-cys [3 mM]. This again calls into question the oxidative environment of the aorta, as there were observed differences in AS-mediated relaxation responses in the presence of L-cys in aorta from sham and AngII-treated mice.
4.1 Conclusions
Overall, our data are the first to demonstrate that aorta from AngII-treated hypertensive mice, while displaying endothelial dysfunction also exhibit a preservation of HNO-mediated relaxation responses. We also demonstrate that there may be a difference in the oxidative environment of the aorta, as compared to other vascular and non-vascular smooth muscle tissues.
Acknowledgements
The authors of this manuscript are grateful to Mrs. Zidonia Carneiro for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Favaloro JL, Kemp-Harper BK. The nitroxyl anion (hno) is a potent dilator of rat coronary vasculature. Cardiovasc Res. 2007;73:587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. A role for nitroxyl (hno) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irvine JC, Ritchie RH, Favaloro JL, Andrews KL, Widdop RE, Kemp-Harper BK. Nitroxyl (hno): The cinderella of the nitric oxide story. Trends Pharmacol Sci. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Gratzel M, Taniguchi S, Henglein A. A pulse radiolytic study of short-lived byproducts on nitric oxide reduction in aqueous solution. Ber Bunsenges Phys Chem. 1970;74:1003–1010. [Google Scholar]
- 5.Bartberger MD, Fukuto JM, Houk KN. On the acidity and reactivity of hno in aqueous solution and biological systems. Proc Natl Acad Sci U S A. 2001;98:2194–2198. doi: 10.1073/pnas.041481598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafirovich V, Lymar SV. Nitroxyl and its anion in aqueous solutions: Spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc Natl Acad Sci U S A. 2002;99:7340–7345. doi: 10.1073/pnas.112202099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The physiological chemistry and biological activity of nitroxyl (hno): The neglected, misunderstood, and enigmatic nitrogen oxide. Chem Res Toxicol. 2005;18:790–801. doi: 10.1021/tx0496800. [DOI] [PubMed] [Google Scholar]
- 8.Fukuto JM, Switzer CH, Miranda KM, Wink DA. Nitroxyl (hno): Chemistry, biochemistry, and pharmacology. Annu Rev Pharmacol Toxicol. 2005;45:335–355. doi: 10.1146/annurev.pharmtox.45.120403.095959. [DOI] [PubMed] [Google Scholar]
- 9.Flores-Santana W, Switzer C, Ridnour LA, Basudhar D, Mancardi D, Donzelli S, Thomas DD, Miranda KM, Fukuto JM, Wink DA. Comparing the chemical biology of no and hno. Arch Pharm Res. 2009;32:1139–1153. doi: 10.1007/s12272-009-1805-x. [DOI] [PubMed] [Google Scholar]
- 10.Adak S, Wang Q, Stuehr DJ. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. Implications for mechanism. J Biol Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- 11.Sessa WC. Enos at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs AJ, Fukuto JM, Ignarro LJ. Formation of free nitric oxide from l-arginine by nitric oxide synthase: Direct enhancement of generation by superoxide dismutase. Proc Natl Acad Sci U S A. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt HH, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. No .No from no synthase. Proc Natl Acad Sci U S A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil'yano NY, Stepanov SI, Konevega LV, Noskin LA. Intracellular no concentration and its changes in carcinoma cells and cultured human endotheliocytes under the influence of inhibitors and inductors of nitric oxide synthases. Bull Exp Biol Med. 2007;143:248–250. doi: 10.1007/s10517-007-0062-6. [DOI] [PubMed] [Google Scholar]
- 16.Pufahl RA, Wishnok JS, Marletta MA. Hydrogen peroxide-supported oxidation of ng-hydroxy-l-arginine by nitric oxide synthase. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- 17.Saleem M, Ohshima H. Xanthine oxidase converts nitric oxide to nitroxyl that inactivates the enzyme. Biochem Biophys Res Commun. 2004;315:455–462. doi: 10.1016/j.bbrc.2004.01.081. [DOI] [PubMed] [Google Scholar]
- 18.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: Role in blood flow regulation. Free Radic Biol Med. 2004;36:707–717. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Donzelli S, Espey MG, Thomas DD, Mancardi D, Tocchetti CG, Ridnour LA, Paolocci N, King SB, Miranda KM, Lazzarino G, Fukuto JM, Wink DA. Discriminating formation of hno from other reactive nitrogen oxide species. Free Radic Biol Med. 2006;40:1056–1066. doi: 10.1016/j.freeradbiomed.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 20.Donzelli S, Switzer CH, Thomas DD, Ridnour LA, Espey MG, Isenberg JS, Tocchetti CG, King SB, Lazzarino G, Miranda KM, Roberts DD, Feelisch M, Wink DA. The activation of metabolites of nitric oxide synthase by metals is both redox and oxygen dependent: A new feature of nitrogen oxide signaling. Antioxid Redox Signal. 2006;8:1363–1371. doi: 10.1089/ars.2006.8.1363. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe M, C C. Reactions of nitric oxide with mitochondrial cytochrome c: A novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem J. 1998;332:9–19. doi: 10.1042/bj3320009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnelle DR, S SJ. No+, no−, and no− donation by s-nitrosothiols: Implications for regulation of physiological functions by s-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 23.Lopez BE, Wink DA, Fukuto JM. The inhibition of glyceraldehyde-3-phosphate dehydrogenase by nitroxyl (hno) Arch Biochem Biophys. 2007;465:430–436. doi: 10.1016/j.abb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Cheong E, Tumbev V, Abramson J, Salama G, Stoyanovsky DA. Nitroxyl triggers ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium. 2005;37:87–96. doi: 10.1016/j.ceca.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favaloro JL, Kemp-Harper BK. Redox variants of no (no{middle dot} and hno) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am J Physiol Heart Circ Physiol. 2009;296:H1274–H1280. doi: 10.1152/ajpheart.00008.2009. [DOI] [PubMed] [Google Scholar]
- 27.Irvine JC, Favaloro JL, Kemp-Harper BK. No− activates soluble guanylate cyclase and kv channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 28.Paolocci N, Wink DA. The shy angeli and his elusive creature: The hno route to vasodilation. Am J Physiol Heart Circ Physiol. 2009;296:H1217–H1220. doi: 10.1152/ajpheart.00243.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H SA, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis A, Li CG, Rand MJ. Differential actions of l-cysteine on responses to nitric oxide, nitroxyl anions and edrf in the rat aorta. Br J Pharmacol. 2000;129:315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pino RZ, Feelisch M. Bioassay discrimination between nitric oxide (no.) and nitroxyl (no−) using l-cysteine. Biochem Biophys Res Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- 33.Bartberger MD, Liu W, Ford E, Miranda KM, Switzer C, Fukuto JM, Farmer PJ, Wink DA, Houk KN. The reduction potential of nitric oxide (no) and its importance to no biochemistry. Proc Natl Acad Sci U S A. 2002;99:10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amatore C, Arbault S, Ducrocq C, Hu S, Tapsoba I. Angeli's salt (na2n2o3) is a precursor of hno and no: A voltammetric study of the reactive intermediates released by angeli's salt decomposition. ChemMedChem. 2007;2:898–903. doi: 10.1002/cmdc.200700016. [DOI] [PubMed] [Google Scholar]
- 35.Ellis A, Lu H, Li CG, Rand MJ. Effects of agents that inactivate free radical no (no*) on nitroxyl anion-mediated relaxations, and on the detection of no* released from the nitroxyl anion donor angeli's salt. Br J Pharmacol. 2001;134:521–528. doi: 10.1038/sj.bjp.0704287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liochev SI, Fridovich I. The mode of decomposition of angeli's salt (na2n2o3) and the effects thereon of oxygen, nitrite, superoxide dismutase, and glutathione. Free Radic Biol Med. 2003;34:1399–1404. doi: 10.1016/s0891-5849(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 37.Miranda KM, Dutton AS, Ridnour LA, Foreman CA, Ford E, Paolocci N, Katori T, Tocchetti CG, Mancardi D, Thomas DD, Espey MG, Houk KN, Fukuto JM, Wink DA. Mechanism of aerobic decomposition of angeli's salt (sodium trioxodinitrate) at physiological ph. J Am Chem Soc. 2005;127:722–731. doi: 10.1021/ja045480z. [DOI] [PubMed] [Google Scholar]
- 38.Li CG, Karagiannis J, Rand MJ. Comparison of the redox forms of nitrogen monoxide with the nitrergic transmitter in the rat anococcygeus muscle. Br J Pharmacol. 1999;127:826–834. doi: 10.1038/sj.bjp.0702540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. Nitroxyl anion donor, angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49:885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 40.Guilmard C, Auguet M, Chabrier PE. Comparison between endothelial and neuronal nitric oxide pathways in rat aorta and gastric fundus. Nitric Oxide. 1998;2:147–154. doi: 10.1006/niox.1998.0170. [DOI] [PubMed] [Google Scholar]
- 41.Wynne BM, Labazi H, Tostes RC, Webb RC. Experimental Biology. Anaheim, California: 2010. Nitroxyl anion mediates vasorelaxation in salt-loaded angii hypertensive mesenteric arteries. [Google Scholar]
- 42.Wynne BM, Labazi H, Tostes RC, Webb RC. Mesenteric arteries from angiotensin ii hypertensive mice exhibit a decreased relaxation response to nitroxyl anion when given a high salt diet. American Heart Association-Council for High Blood Pressure Research. Chicago, Illinois. Hypertension. 2009 [Google Scholar]
- 43.Wanstall JC, Jeffery TK, Gambino A, Lovren F, Triggle CR. Vascular smooth muscle relaxation mediated by nitric oxide donors: A comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol. 2001;134:463–472. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang HY, Kao PF, Chen TH, Tomlinson B, Ko WC, Chan P. Effects of the angiotensin ii type 1 receptor antagonist valsartan on the expression of superoxide dismutase in hypertensive patients. J Clin Pharmacol. 2007;47:397–403. doi: 10.1177/0091270006296762. [DOI] [PubMed] [Google Scholar]
- 45.Misra HP. Generation of superoxide free radical during the autooxidation of thiols. J Biol Chem. 1974;219:2151–2155. [PubMed] [Google Scholar]
- 46.Saez G, Thornalley PJ, Hill HAO, Hems R, Bannister JV. The production of free radicals during the autoxidation of cysteine and their effect on isolated rat hepatocytes. Biochem Biophys Acta. 1982;719:24–31. doi: 10.1016/0304-4165(82)90302-6. [DOI] [PubMed] [Google Scholar]
- 47.Feelisch M, Te Poel M, Zamora R, Deussen A, Moncada S. Understanding the controversy over the identity of edrf. Nature. 1994;368:62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- 48.Jia L, Furchgott RF. Inhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1993;267:371–378. [PubMed] [Google Scholar]