SUMMARY
Mononuclear phagocytes, including monocytes, macrophages and dendritic cells, contribute to tissue integrity, as well as innate and adaptive immune defense. Emerging evidence for labour division indicates that manipulation of these cells could bear therapeutic potential. However, specific ontogenies of individual populations and the overall functional organisation of the cellular network are not well-defined. Here we report a fate mapping study of the murine monocyte and macrophage compartment taking advantage of constitutive and conditional CX3CR1 promoter-driven Cre recombinase expression. We have demonstrated that major tissue resident macrophage populations, including liver Kupffer cells, lung alveolar, splenic and peritoneal macrophages, are established prior to birth and maintain themselves subsequently during adulthood independent of replenishment by blood monocytes. Furthermore, we have established that the short-lived Ly6C+ monocytes constitute obligatory steady state precursors of blood-resident Ly6C− cells and that the abundance of Ly6C+ blood monocytes dynamically controls the circulation life span of their progeny.
INTRODUCTION
The mononuclear phagocyte system (van Furth et al., 1972) today comprises monocytes, macrophages and dendritic cells (DCs), as well as their respective committed bone marrow (BM) - resident progenitors (Geissmann et al., 2010). Collectively, these cells play central roles in the maintenance of tissue integrity during development and its restoration after injury, as well as the initiation and resolution of innate and adaptive immunity.
Historically, mononuclear phagocyte subpopulations have been defined according to anatomic location and surface marker profiles. More recently, this definition has been extended to comprise dependence of subpopulations on specific growth and transcription factors (Hashimoto et al., 2011; Lawrence and Natoli, 2011), subset-specific gene expression signatures (Robbins et al., 2008) and distinct ontogenies (Geissmann et al., 2010).
A major breakthrough in defining mononuclear phagocyte development in the adult organism was the identification of a clonotypic BM-resident founder cell, termed Macrophage-DC precursor (MDP), that gives rise to peripheral mononuclear phagocytes while having lost granulocyte potential (Fogg et al., 2006). MDPs differentiate within the BM into monocytes (Varol et al., 2007) and dedicated DC precursors, the preDCs (Liu et al., 2009). Both these cell types are subsequently released into the circulation to allow repopulation of peripheral tissue macrophages and classical Fms-like tyrosine kinase 3 ligand (Flt3L) -dependent DCs, respectively. The MDP-preDC route is critical to ensure the constant replenishment of the ephemeral DC compartment. The relative importance of local proliferation versus recruitment from monocytes in the maintenance of tissue macrophage homeostasis has been debated since the original definition of the mononuclear phagocyte system ((Hume, 2006; Hume et al., 2002). The original studies of Kupffer cell turnover in the liver, for example, concluded that very few resident MΦ's are actively in cycle (Crofton et al., 1978). This proliferation is ablated by glucocorticoids, which also removes blood monocytes. The authors concluded, that the proliferating cells were monocytederived. That conclusion was however undermined by later evidence that glucocorticoids oppose growth factor activity, including the macrophage growth factor, CSF-1 (Hume and Gordon, 1984).
In certain situations, such as helminth-associated T helper-2 cell milieus, expansion of local macrophages can rely solely on proliferation of tissue-resident macrophages exclusive of monocyte influx (Jenkins et al., 2011). Under inflammatory conditions resident macrophages are complemented by recruited monocytes that differentiate in situ into macrophages. However, when analyzed the contribution of these infiltrating cells was found to be transient (Ajami et al., 2011; Leuschner et al., 2012), while resident macrophages bring about the resolution of the inflammatory response and restore macrophage homeostasis (Davies et al., 2011).
Myeloid cells, including mononuclear phagocytes, are known to arise from two successive hematopoetic waves, referred to as 'primitive' and 'definitive', respectively (Orkin and Zon, 2008). As best exemplified for the brain microglia (Alliot et al., 1999; Ginhoux et al., 2010), mononuclear phagocytes can be generated during development from primitive macrophages generated in the yolk sac, an extra-embryonic tissue. Once established, microglia cells maintain themselves throughout adult life by virtue of longevity and limited self-renewal without input from definitive hematopoesis (Ajami et al., 2007; Ginhoux et al., 2010; Mildner et al., 2007). Geissmann and coworkers recently provided evidence of the existence of a macrophage lineage lineage that is independent of the transcription factor myb (Schulz et al., 2012). In addition to microglia, autonomy from definitive hematopoesis was shown for F4/80bright CD11bint tissue macrophage populations, including Kupffer and Langerhans’ cells.
The chemokine receptor CX3CR1 is widely expressed in the mononuclear phagocyte system. Indeed, mice harbouring a GFP reporter in the CX3CR1 locus (Jung et al., 2000) have been instrumental in identifying and defining MDPs (Fogg et al., 2006), pre-DCs (Liu et al., 2009), as well as tissue-resident mononuclear phagocyte populations (Bar-On et al.; Lewis et al., 2011; Niess et al., 2005; Varol et al., 2009). Moreover, discrete expression of CX3CR1/GFP in Cx3cr1gfp animals led to the identification of two monocyte subsets in mice, characterized as CX3CR1int Ly6C+ and CX3CR1hi Ly6C− cells ((Geissmann et al., 2003; Palframan et al., 2001), corroborating earlier studies on human blood (Passlick et al., 1989). CX3CR1int Ly6C+ monocytes are the correlate of human CD14+ CD16+ and CD14+ CD16− monocytes (Cros et al., 2010) and poised to traffic to sites of infection and inflammation (Geissmann et al., 2003; Serbina et al., 2008). CX3CR1hi Ly6C−’patrolling’ monocytes – the correlate of human CD14dim CD16+ cells (Cros et al., 2010) - have been shown to adhere and crawl along the luminal surface of endothelial cells (Auffray et al., 2007). In absence of inflammation CX3CR1int Ly6C+ monocytes can return to the BM and differentiate into CX3CR1hi Ly6C− cells (Varol et al., 2007). However, it is unclear whether the generation of CX3CR1hi Ly6C− monocytes requires CX3CR1int Ly6C+ monocytes as obligatory intermediate or whether CX3CR1hi Ly6C− monocytes can - like CX3CR1int Ly6C+ monocytes (Varol et al., 2007) - also originate directly from MDPs. Moreover, if alternative pathways exist their relative contribution to the steady state generation of monocytes remains unclear.
Here, report a fate mapping study of the murine mononuclear phagocyte compartment using mice that harbour genes encoding conditional or constitutive active Cre recombinase in their CX3CR1 loci. We have demonstrated that tissue resident macrophage populations, including peritoneal, splenic and lung macrophages, as well as liver Kupffer cells, are established prior to birth and in adult steady state disconnected from monocyte input. Side-by-side comparison of Cx3cr1cre and Cx3cr1creER mice crossed to reporter animals, as well as Cx3cr1gfp mice, combined with bromodeoxyuridine (BrdU) pulsing experiments, adoptive transfers and the analysis of Ccr2-deficient mixed BM chimeras, provided critical insights into monocyte dynamics. Specifically, we have established that CX3CR1int Ly6C+ monocytes form in steady state a short-lived obligatory precursor intermediate for the generation of Ly6C− monocytes and, presumably as CSF-1 sink, dynamically control the life span of their progeny.
RESULTS
Dual origins of resident macrophages
To exploit the pronounced activity of the CX3CR1 promoter in the mononuclear phagocyte system for its study, we manipulated the murine CX3CR1 loci to harbour Cre recombinase genes. Specifically, we replaced the Cx3cr1 gene with genes encoding either cre recombinase or a cre recombinase fusion to a mutant estrogen ligand-binding domain that requires the presence of the estrogen antagonist tamoxifen for activity (CreERT2) (Metzger et al., 1995), yielding Cx3cr1cre and Cx3cr1creER mice (Fig. 1A).
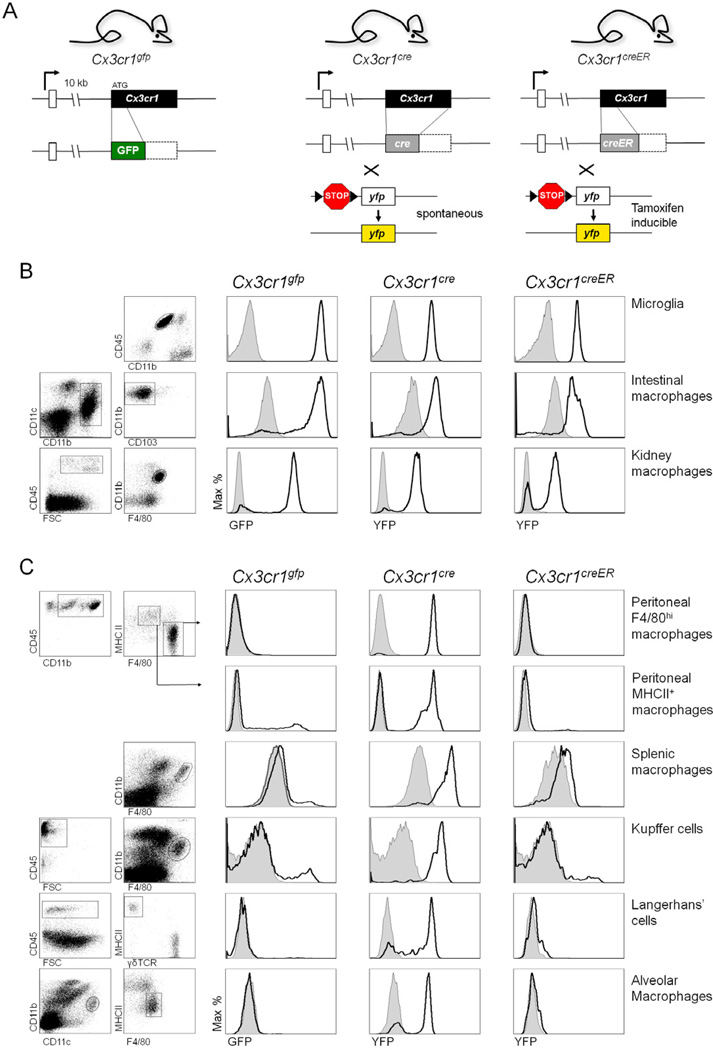
Figure 1. Reporter gene expression profile of macrophage populations in Cx3cr1gfp/+, Cx3cr1cre/+:R26-yfp and Tamoxifen-treated Cx3cr1creER/+:R26-yfp mice.
(A) Schematic of modified Cx3cr1 loci. The Cx3cr1cre and Cx3cr1creER mice were crossed to R26-yfp mice, in which irreversible induction of YFP expression is induced upon Cre recombinase expression and activation, respectively.
(B & C) Flow cytometric analysis of CX3CR1+ (B) and CX3CR1 (C) mononuclear phagocyte populations of Cx3cr1gfp/+, Cx3cr1cre/+:R26-yfp and Cx3cr1creER/+:R26-yfp mice. Cx3cr1creER/+:R26-yfp mice were treated for 4 weeks with Tamoxifen prior to analysis. Results are representative of 6 mice per group.
Recent studies have highlighted the differential origins of discrete peripheral macrophage and DC compartments in adult mice (Geissmann et al., 2010). Given the broad expression of the Cx3cr1 gene within the mononuclear phagocyte system we decided to perform a side-by-side comparison of the reporter gene activation patterns in Cx3cr1gfp, Cx3cr1cre and Cx3cr1creER mice, the latter crossed to R26-yfp reporter animals (Srinivas et al., 2001) (Supplemental Fig. 1). Macrophage populations from Cx3cr1gfp and Cx3cr1cre:R26-yfp mice fell into two categories. Cells, such as microglia, intestinal and renal macrophages express the CX3CR1 chemokine receptor (Jung et al., 2000; Niess et al., 2005; Soos et al., 2006) hence found labelled in both Cx3cr1gfp and Cx3cr1cre:R26-yfp mice (Fig. 1B). A second group of cells ceased to express the chemokine receptor but obviously originated from CX3CR1+ precursors, indicated by an absence and presence of label in Cx3cr1gfp and Cx3cr1cre:R26-yfp mice, respectively. This included peritoneal macrophages, liver Kupffer cells, splenic and lung macrophages as well as epidermal Langerhans’ cells (Fig. 1C). Coexistence of the respective CX3CR1-positive cells and CX3CR1-negative cells that acquired the label during their past in steady state tissues was confirmed by the histological examination of triple transgenic Cx3cr1gfp:Cx3cr1cre:R26-rfp mice, which harboured differentially labelled cells, as exemplified for RFP-positive CD11bint F4/80hi Kupffer cells and GFP-positive CD11bhi F4/80int perivascular macrophage’s in the liver (Fig. 2A i). Moreover, the notion that CX3CR1-negative CD11bint F4/80hi Kupffer cells derive from cells that previously expressed CX3CR1 was corroborated by the fact that CD11bint F4/80hi Kupffer cells were found to be GFP+ in fetal livers, but GFP− in adult livers of Cx3cr1gfp mice (Fig. 2Aii). Since in Cx3cr1cre:R26-yfp mice Cre recombinase is constitutively active, the CX3CR1+ precursors establishing the label of CX3CR1-negative tissue macrophages could be associated with prenatal development, or represent adult precursors, such as MDPs or monocytes (Fogg et al., 2006; Jung et al., 2000). To distinguish between these two scenarios, we took advantage of Cx3cr1creER:R26-yfp mice, in which Cre activity can be regulated by drug administration and hence induced after birth. One month of continuous tamoxifen exposure of adult animals resulted in the efficient reporter gene expression in CX3CR1+ resident macrophages and microglia of Cx3cr1creER:R26-yfp mice (Fig. 1B). Moreover, it yielded substantial excision of the LoxP-flanked STOP cassette from the R26-STOP-YFP loci, and hence YFP expression, in the monocyte compartment (see below). However, no reporter gene expression was detected in the CX3CR1− macrophage populations of the tamoxifen-treated Cx3cr1creER:R26-yfp mice signifying that no monocytes had entered the pool.
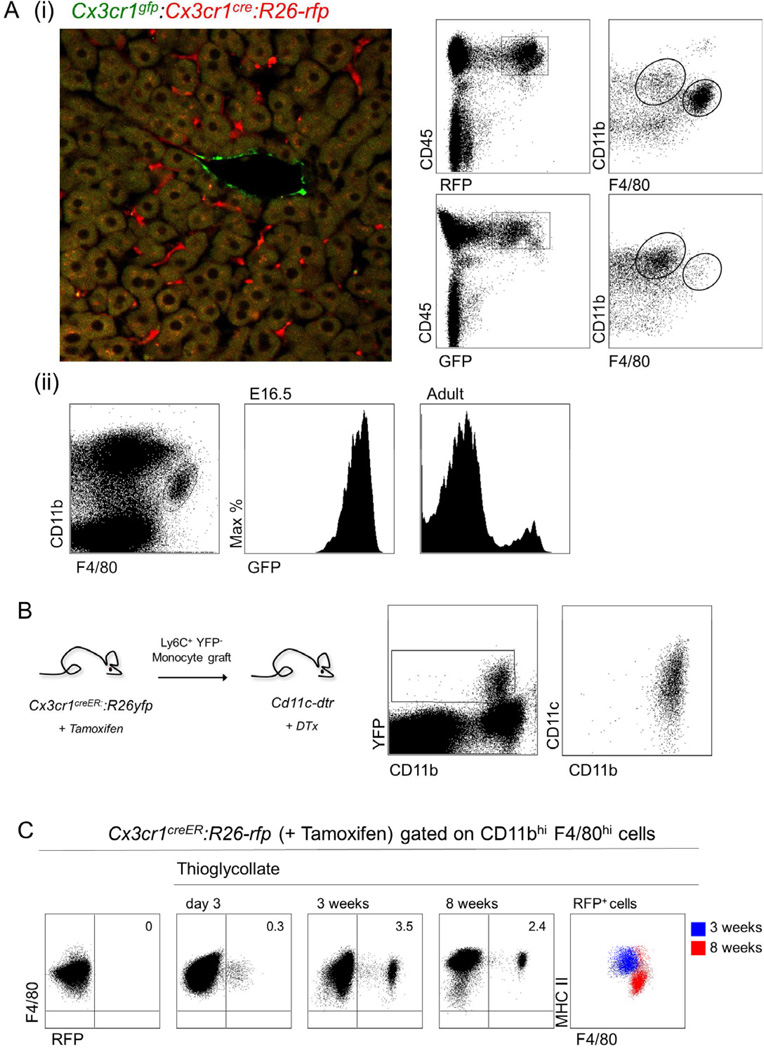
Figure 2. Dual origins of macrophages.
(A) (i) Flow cytometric and histological analysis of livers obtained from Cx3cr1cre/+:R26-rfp:Cx3cr1gfp/+ mice. In these mice GFP expression acts as a direct reporter for CX3CR1 expression, while RFP expression is controlled by Cre recombinase. (ii) Analysis of fetal liver and adult liver and Kupffer cells of Cx3cr1gfp/+ mice.
(B) Flow cytometric analysis of the intestinal lamina propria of DTx-treated Cd11c-dtr mice that received 7 days earlier a Ly6C+ YFP monocyte graft isolated from tamoxifen-treated Cx3cr1creER/+:R26-yfp mice. Results are representative of two experiments involving 3 mice per group.
(C) Flow cytometric analysis of peritoneal lavages of Cx3cr1creER/+:R26-rfp mice administered a single tamoxifen gavage (5 mg) one day following an intra-peritoneal thioglycollate injection. Rightmost graph shows phenotypic shift of monocyte-derived cells between three weeks and 8 weeks. Results are representative of two independent experiments involving 3 mice per group.
To formally establish that descendants of monocytes from tamoxifen-treated animals can be detectable according to reporter gene expression after an extended period of time in a tissue context, we resorted to an adoptive transfer strategy focusing on intestinal resident macrophages which we previously established to be Ly6C+ monocyte-derived (Varol et al., 2009). Specifically, Ly6C+ YFP− BM monocytes isolated from tamoxifen-treated mice were adoptively transferred into diphtheria toxin-treated [CD11c-DTR > WT] BM chimeras (Varol et al., 2009). As seen in Fig. 2B, grafted cells gave rise to YFP+ lamina propria macrophage’s Notably, as the recipients were not treated with tamoxifen, the rearrangement of the R26-STOP-YFP loci must have occurred in the donor animals. The fact that monocyte-derived cells could be detected in tamoxifentreated adult Cx3cr1creER:R26-yfp mice according to their reporter expression was further corroborated by analysing mice challenged intraperitoneally with thioglycollate, which led to the appearance of RFP+ cells in the peritoneal cavities 72 hrs after treatment (Fig. 2C). Interestingly, following the resolution of this response cells derived from the monocyte infiltrate persisted for up to two months after challenge. This was associated with a phenotypic shift to MHC II lo F4/80hi cells suggesting the integration of the monocyte-derived cells into the resident macrophage pool (Fig 2C).
Collectively, the absence of label in resident CX3CR1− macrophage populations of tamoxifen-treated Cx3cr1creER:R26-yfp mice establishes that there is no on-going steady state contribution of monocytes to Kupffer cells or peritoneal, splenic and lung macrophages and that these cells derive prenatally originating either independent from the yolk sac or fetal liver cells and subsequently maintaining themselves through longevity and limited self-renewal.
Characterization of Myeloid Precursors and Monocytes in Cx3cr1cre:R26-yfp and Cx3cr1creER:R26-yfp mice
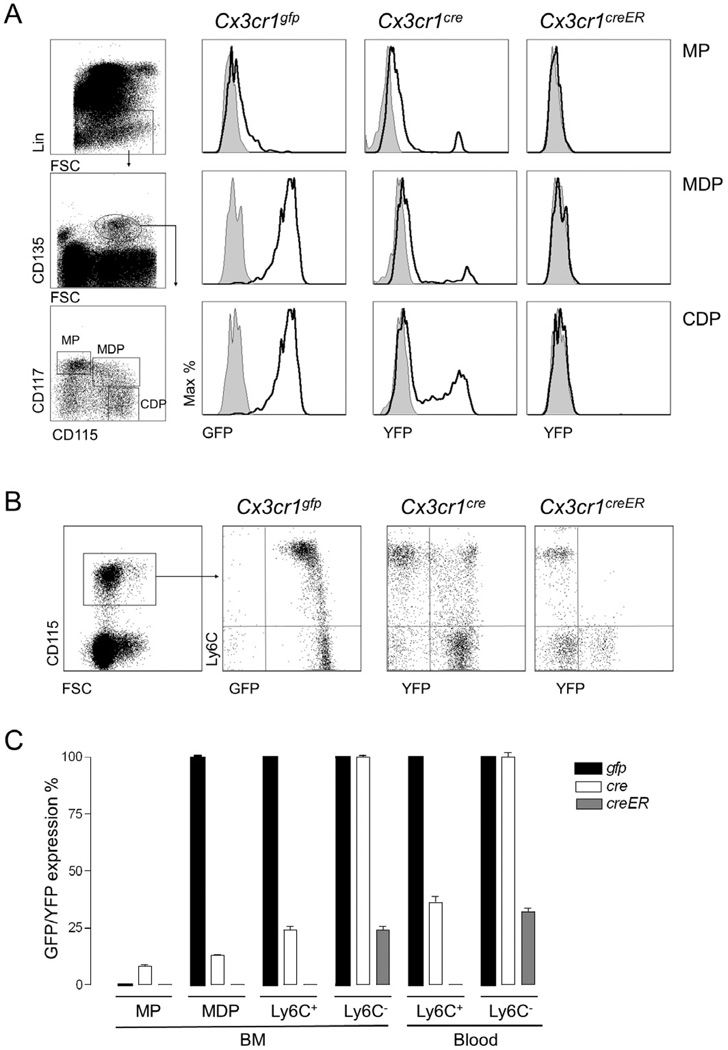
CX3CR1 chemokine receptor expression commences with the first dedicated mononuclear phagocyte precursor, the MDP, and expression is subsequently maintained in DC-committed precursors (CDPs, preDCs) and monocytes (Fogg et al., 2006; Liu et al., 2009; Onai et al., 2007). All these cell populations are consequently homogeneously marked by reporter gene expression in Cx3cr1gfp mice (Fig. 3A). Interestingly however, reporter gene expression was absent from MDPs of Cx3cr1cre:R26-yfp mice; rather YFP+ cells first appeared at the CDP and Ly6C+ monocyte stage (Fig. 3). This delay of YFP expression compared to Cx3cr1gfp mice may be due to the time restraint imposed by the required rearrangement for STOP cassette excision prior to reporter gene expression in Cx3cr1cre:R26-yfp animals. In Cx3cr1creER:R26-yfp mice, where reporter activation requires the nuclear translocation of the CreER protein prior to rearrangement, YFP expression was found to be even further restricted. Thus, in tamoxifen-treated Cx3cr1creER:R26-yfp mice reporter gene expression was absent from CDPs and Ly6C+ monocytes (Fig. 3B). Only Ly6C− monocytes - and only a fraction of them - expressed YFP even after extended tamoxifen treatment (Fig. 3B). The differential appearance of the YFP label in Ly6C+ and Ly6C− monocyte subsets indicated distinct temporal requirements for their creation, supporting the notion of their sequential generation (Sunderkotter et al., 2004; Varol et al., 2007,; Yrlid et al., 2006). Notably, key surface markers recently reported for the two monocytes subsets (Ingersoll et al., 2010), displayed gradual alterations between Ly6C+ YFP−, Ly6C− YFP− and Ly6C− YFP+ cells in Cx3cr1creER:R26-yfp mice (Supplemental Fig. 2A). Furthermore, comparison of expression of these markers by Cx3cr1cre:R26-yfp monocyte subsets also demonstrated gradual changes from Ly6C+ YFP−, to Ly6C+ YFP+ and Ly6C− YFP+ cells (Supplemental Fig. 2B). Taken together, reporter gene expression kinetics in Cx3cr1cre and Cx3cr1creER:R26-yfp mice and the gradual acquisition of maturation markers in the monocyte subsets support the notion that Ly6C+ monocytes differentiate into Ly6C− monocytes in steady state.
Figure 3. Reporter gene expression profile of mononuclear phagocyte precursors and circulating monocytes.
(A & B) Flow cytometric analysis of different mononuclear phagocyte populations, precursors (A) and blood monocytes (B) obtained from Cx3cr1gfp/+, Cx3cr1cre/+:R26-yfp and Cx3cr1creER/+:R26-yfp mice. Cx3cr1creER/+:R26-yfp mice were treated for 4 weeks with Tamoxifen prior to analysis. Results are representative of 4–6 mice per group.
(C) Bar graph summarizing frequencies of GFP or YFP+ cells in indicated mononuclear phagocyte populations of Cx3cr1gfp/+, Cx3cr1cre/+:R26-yfp and Cx3cr1creER/+:R26-yfp mice. Mean ± SEM are performed with n=4–6 mice per group.
Ly6C− monocytes derive from Ly6C+ monocytes independently in BM and blood
With the definition of MDPs as in vivo monocyte precursors (Varol et al., 2007), the question arose whether these cells give rise to both CX3CR1int Ly6C+ and CX3CR1hi Ly6C− monocytes or whether the two monocyte subsets are part of a developmental sequence (Sunderkotter et al., 2004; Varol et al., 2007; Yrlid et al., 2006). While adoptively-transferred Ly6C+ CX3CR1int monocytes isolated from BM can give rise to Ly6C− CX3CR1hi monocytes in recipient mice (Varol et al., 2007)., these grafts could have arguably been contaminated with immature BM precursors. To exclude this possibility, we isolated CX3CR1GFP/+ Ly6C+ monocytes from the splenic reservoir (Swirski et al., 2009) and grafted them into congenic WT mice (Supplemental Fig. 3A). Recipients were sacrificed at various time points following adoptive transfer and subjected to flow cytometry analysis. One day after transfer grafted cells were detectable in recipient blood, BM and spleen as Ly6C+ CX3CR1/GFPint cells (Supplemental Fig. 3B). However, corroborating our earlier study (Varol et al., 2007), by day 3 grafted splenic Ly6C+ monocytes had quantitatively differentiated into Ly6C− CX3CR1/GFPhi cells.
Next, we compared the differentiation potential of Ly6C+ YFP− and Ly6C+ YFP+ monocytes of Cx3cr1cre:R26-yfp mice. Isolated monocyte subsets were grafted into congenic WT recipient mice. Analysis over a three-day period revealed a number of sequential steps required for Ly6C+ monocytes to convert. Ly6C+ YFP− monocytes transformed from being YFP-negative to cells expressing the YFP reporter prior to converting into Ly6C− monocytes, authenticating the notion that Ly6C+ monocytes are the precursors of Ly6C− monocytes and our earlier assumption that acquisition of the YFP label by monocytes is a matter of time (Supplemental Fig. 4).
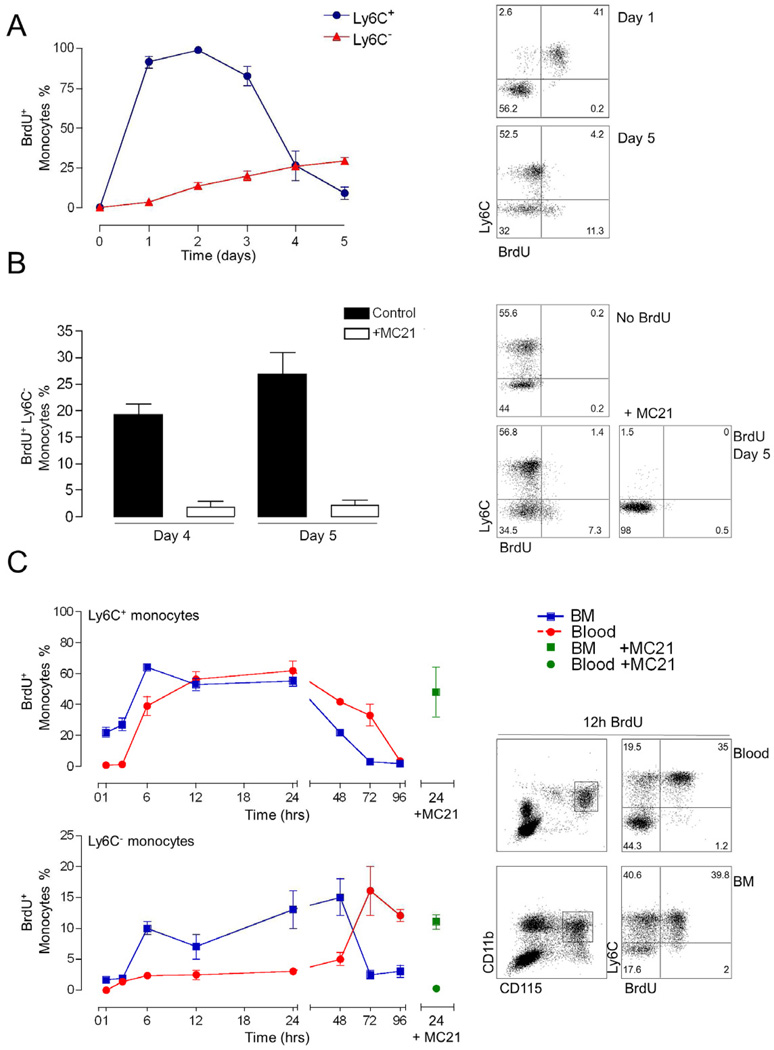
To further investigate the interrelation of blood monocytes and determine their turnover rates, we resorted to a 5-bromo-2'-deoxyuridine (BrdU) pulsing regime. Specifically, mice were dosed with three intraperitoneal (i.p.) injections of 2 mg BrdU (3 hrs apart) and subsequently monitored over a 5-day period for the presence of the thymidine analog in the genome of the monocyte subsets. This low dose of BrdU is unlikely to be mitogenic (Takizawa et al., 2011). Since only a minor subset of monocytic cells is in cell cycle, BrdU is expected to be incorporated into dividing monocyte precursors, such as MDPs. One day following the first BrdU injection, all circulating Ly6C+ monocytes were BrdU-positive (Fig. 4A). In contrast, no Ly6C− blood monocytes had incorporated label by day 1, indicating that these cells are not recently generated from a dividing precursor. The fraction of BrdU+ cells in the Ly6C− compartment gradually increased to around one third by day 5 (Fig. 4A), whereas the Ly6C+ monocytes lost the label by day 5. Fitting an exponential trendline from day 2, i.e. the peak of BrdU label, to day 5, we calculated the steady state half-live of the Ly6C+ blood monocytes to be 0.843 days (20 hrs) (r2=0.932). The sequential acquisition of the BrdU label by the blood monocyte subsets suggested that it was incorporated into monocyte precursors that differentiated into Ly6C+ monocytes and subsequently moved with time to Ly6C− monocytes. To corroborate this assumption we took advantage of a cell ablation strategy targeting Ly6C+ monocytes based on the injection of the CCR2 antibody MC21 (Bruhl et al., 2007). MC21 treatment of BrdU-pulsed mice on day 1 efficiently ablated CCR2+ Ly6C+ monocytes and spared as reported CCR2− Ly6C− cells (Fig. 4B). Interesting though and supporting the notion of a precursor/product relationship of the two monocyte subsets, removal of BrdU-labelled Ly6C+ monocytes also prevented accumulation of the BrdU label in the Ly6C− monocyte population (Fig. 4B).
Figure 4. Monocyte subset dynamics.
(A & B) Flow cytometric analysis of Ly6C+ and Ly6C− blood monocyte subsets pulsed three times with 2 mg BrdU 3 hrs apart and monitored over 5 days. (B) Mice were treated with the anti-CCR2 antibody MC21 and BrdU incorporation by Ly6C− blood monocyte was analysed.
(C) Analysis of time course of BrdU incorporation of Ly6C+ and Ly6C− monocytes in blood (red) and BM (blue) following a single 2 mg pulse of BrdU; BrdU incorporation by monocytes in mice treated with the MC21 antibody (green). Mean ± SEM are performed with n=3–4 mice per group.
To refine our analysis and extend it to monocytes residing in BM, C57BL/6 animals were injected with a single dose of BrdU (2 mg) (Fig. 4C). Analysis of blood monocytes confirmed the sequential appearance of the BrdU label in Ly6C+ and Ly6C− monocytes, respectively. Ly6C+ monocytes in the BM were labeled before Ly6C+ blood monocytes consistent with their generation from MDPs in the BM (Varol et al., 2007). BM-resident Ly6C− cells were found to incorporate BrdU well before the blood Ly6C− monocytes, though still delayed when compared to BM Ly6C+ monocytes. This suggests that Ly6C− BM monocytes are generated in the BM, and Ly6C− blood monocytes are generated in the blood. In line with this notion, MC21 treatment that ablates Ly6C+ blood but not Ly6C+ BM monocytes did not impair labelling of BM Ly6C− monocytes (Fig. 4C).
Collectively, these data establish that Ly6C+ monocytes are precursors of Ly6C− cells. Moreover, the limited acquisition of BrdU label in the Ly6C− monocytes supports the earlier notion based on adoptive transfer experiments (Geissmann et al., 2003) that these cells have an extended circulation half life as compared to Ly6C+ monocytes. Finally, differential label acquisition by Ly6C− BM and blood monocytes suggest that these compartments are established, and in steady state maintained, independent of each other.
Ly6C+ blood monocytes are obligatory precursors of steady state Ly6C− monocytes
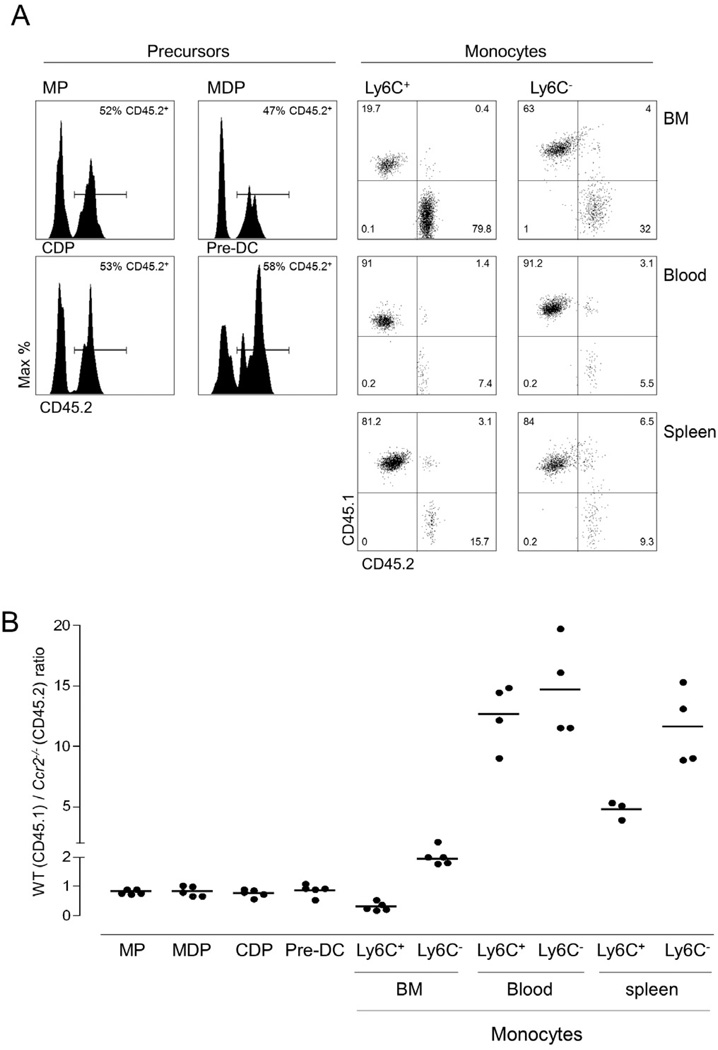
Among monocytes, membrane expression of the chemokine receptor CCR2 is restricted to Ly6C+ cells and a prerequisite for these cells to exit from the BM (Serbina and Pamer, 2006; Shi et al., 2011). Accordingly, Ly6C+ monocytes are reported to be selectively reduced in the circulation of CCR2 deficient mice (Serbina and Pamer, 2006). If Ly6C+ blood monocytes are precursors of Ly6C− monocytes, the impairment of Ly6C+ cell compartment would be expected to impinge on Ly6C− monocytes. To probe this point more rigorously we analyzed monocyte subset distributions in a competitive setting by generating mixed chimeras, with BM isolated from CCR2-proficient (CD45.1) and CCR2-deficient (CD45.2) Cx3cr1gfp/+ mice. Analysis of the BM of the respective chimeras eight weeks following irradiation revealed that the two genotypes were equally prevalent amongst MPs and MDPs, as well as DC precursors (CDPs and pre-DCs) (Fig. 5A, B). BM-resident CCR2 mutant Ly6C+ monocytes were found to accumulate, presumably as a result of their impaired BM exit (Serbina and Pamer, 2006), whereas BM-resident mutant Ly6C− monocytes were moderately dominated by CD45.1+ WT cells (Fig. 5B). As expected from the reported phenotype of Ccr2−/− mice (Serbina and Pamer, 2006), in the blood Ly6C+Ccr2−/− monocytes (CD45.2) were outcompeted by their CD45.1+ WT counterpart (Fig. 5A), but CCR2 deficiency also compromised the Ly6C− blood monocytes, with mutant cells under-represented in both compartments (Fig. 5A, B). The competitive disadvantage of both Ly6C+ and Ly6C− CCR2-deficient monocytes was also evident in the spleen (Fig. 5B), although it was less pronounced for Ly6C+ cells, which after having entered the splenic pool might be trapped due to impaired mobilization.
Figure 5. Impaired BM exit of Ly6C+ blood monocyte affects the Ly6C− cell compartment.
(A) Flow cytometric analysis of mixed [Ccr2−/−:Cx3cr1gfp/+ (CD45.2) / Ccr2+/+:Cx3cr1gfp/+ (CD45.1) > wt] BM chimeras. Distribution of cells within the mononuclear compartment were analysed for percentage of CD45.2+ cells. Cell definitions for gating: MP (Lin CD135+ CD115 CD117+) MDP (Lin CD135+ CD115+ CD117+ CDP (Lin CD135+ CD115+ CD117) PreDC (Lin CD11cint MHCIICD135+ CD172a CX3CR1+) monocyte (CD11b+ CD115+). Results are representative of 6 analyzed mice.
(B) Analysis of mixed [Ccr2−/−:Cx3cr1gfp/+ (cd45.2) / Ccr2+/+:Cx3cr1gfp/+ (CD45.1) > wt] BM chimeras. Representative result of two experiments involving 3–5 mice per group.
Collectively, this establishes that the generation of Ly6C− CX3CR1hi monocytes in steady state critically depends on CCR2 proficient Ly6C+ CX3CR1int monocytes as immediate precursors. Moreover, in order to serve as Ly6C− CX3CR1hi monocyte progeny these cells have to exit from the BM to the circulation.
Ly6C+ blood monocytes negatively control the life span of Ly6C− monocytes
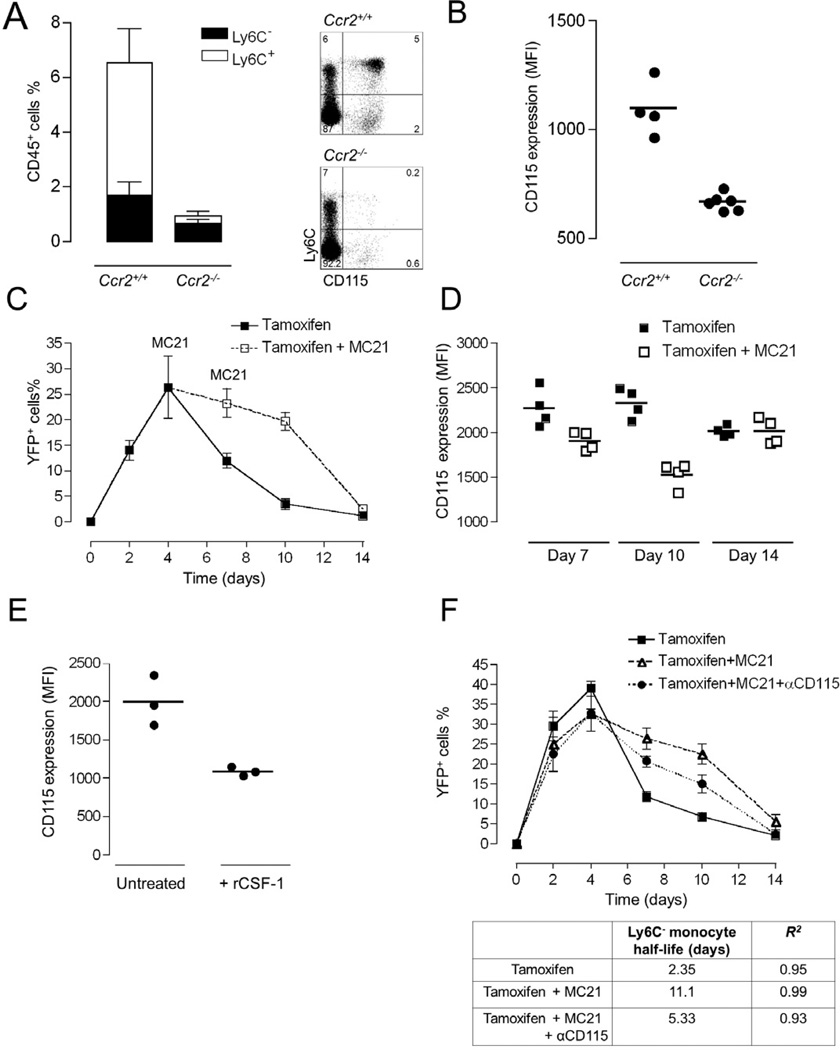
The fact that Ccr2−/− mice harbour circulating Ly6C− CX3CR1hi monocytes despite their impaired Ly6C+ monocyte compartment (Qu et al., 2004) seemingly contradicts the notion that the latter cells require Ly6C+ monocytes as precursors. Indeed analysis of the frequencies of the two monocyte subsets in Ccr2−/− Cx3cr1gfp mice, confirmed the presence of a sizable Ly6C− CX3CR1hi monocyte population, albeit drastically reduced as compared to Ccr2+/+:Cx3cr1gfp littermates (Fig. 6A). Interestingly, Ly6C− blood monocytes of Ccr2−/− mice had down-modulated their receptors for the macrophage growth factor, CSF-1, detected with a CD115 antibody (Fig. 6B). One could postulate that in Ccr2−/− mice, Ly6C−CX3CR1hi monocytes might compensate for the diminished replenishment from Ly6C+ monocytes by extending their life span.
Figure 6. Prevalence of Ly6C+ blood monocytes determines the circulation half-life of Ly6C− blood cells.
(A) Analysis of Ly6C+ and Ly6C− blood monocyte subsets of Ccr2+/+ Cx3cr1gfp/+ and Ccr2−/− Cx3cr1gfp/+ mice. Mean ± SEM are performed with n=4–6 mice per group.
(B) Mean fluorescent intensities of CD115 expression on Ly6C− monocytes analyzed in (A). Mean ± SEM are performed with n=4–6 mice each.
(C) Flow cytometric analysis of blood of Cx3cr1creER/+:R26-yfp mice treated by tamoxifen gavage to induce excision of the STOP cassette from R26-YFP loci. Ly6C− blood monocytes were analysed over a 2 week period for reporter gene expression. Mice were left untreated or treated with the CCR2 antibody MC21. Representative result of two experiments involving 3 mice per group.
(D) Mean fluorescent intensities of CD115 expression on Ly6C− YFP+ monocytes analyzed in (D). Mean ± SEM are performed with n=3 mice per group.
(E) Mean fluorescent intensities of CD115 expression on Ly6C− monocytes of mice that received an injection of recombinant CSF-1. Mean ± SEM are performed with n=3 mice each.
(F) Flow cytometric analysis of blood of tamoxifen treated Cx3cr1creER/+:R26-yfp mice. Ly6C− blood monocytes were analysed over a 2 week period for reporter gene expression. Mice were left untreated, treated with the anti-CCR2 antibody, or treated with a combination of MC21 and anti-CSF-1 R. Table summarizes half-lives of Ly6C− blood monocytes in the time window from 4 to 10 days, as determined by exponential trendline fitting.
To directly investigate thispossibility we took advantage of our fate mapping system. A single 5 mg tamoxifen gavage of Cx3cr1creER:R26-yfp mice induced in their CX3CR1+ cells the nuclear translocation of Cre recombinase and rearrangement of the YFP reporter locus, resulting in a discrete label of Ly6C− monocytes peaking by day 4 after gavage (Fig. 6C). This label disappeared with time, and exponential trendline fitting from day 4 onwards yielded a calculated steady state half-life of Ly6C− blood monocytes of 2.2 days (r2=0.996). If this half-live is critically determined by input from CCR2-expressing Ly6C+ monocytes, then ablation of the latter using the anti-CCR2 regimen should affect it. Indeed, as seen in Fig. 6C, MC21 treatment resulted in persistence of YFP-labelled Ly6C− monocytes in tamoxifen-treated Cx3cr1creER:R26-yfp mice extending their half-life from 2.2 days to 11 days (r2=0.992). Once Ly6C+ monocytes were restored, after cessation of MC21 treatment, Ly6C− cells regained their steady-state half-life and the population hence lost its YFP label. The depletion of CCR2-expressing Ly6C+ monocytes was associated with a substantial down-modulation of detectable CD115 (CSF-1 receptor) on the Ly6C− monocytes reminiscent of the situation in Ccr2−/− mice (Fig. 6B), which was restored following MC21 withdrawal (Fig. 6D). Down-modulation of the CSF-1 receptor from the cell surface could be a reflection of engagement by its ligand and indeed, administration of recombinant CSF-1 provided direct evidence that this can occur (Fig. 6E). Since myeloid cells themselves control the circulating availability of CSF-1 (Bartocci et al., 1987; Tushinski et al., 1982) depletion of CSF-1R expressing Ly6C+ monocytes in Ccr2−/− mice or MC21-treated animals could potentially alter the availability of the factor. While a blocking antibody against the receptor causes a substantial increase in circulating CSF-1 (MacDonald et al. 2010), we were not able to detect such an increase in the depleted animals (Supplemental Fig. 5). Nevertheless, to examine the function of CSF-1 in the extension of the circulation half-life of Ly6C− monocytes, we tested the impact of CSF-1 receptor neutralization by using an antibody shown previously to ablate these cells in a time-dependent manner (MacDonald et al. 2010). Specifically, we co-administered tamoxifen-treated Cx3cr1creER:R26-yfp mice with the MC21 regimen and CSF1R antibody. Interestingly and in support of a role of CSF-1 in the phenomenon, this protocol partially prevented the half-life extension of the YFP-labeled Ly6C− cells following depletion of Ly6C+ monocytes (Fig. 6F).
DISCUSSION
Here, we have reported a fate mapping analysis of the murine macrophage and monocyte compartment taking advantage of mice that harbour genes encoding a GFP reporter or Cre recombinases in their CX3CR1 loci. We have demonstrated that the major tissue macrophage populations in the liver, spleen, lung and peritoneal cavity are established prenatally during mouse development, and provided definitive support for the view that in the healthy adult organism tissue macrophages can be maintained independent of monocyte input. In addition, we have provided evidence that CX3CR1int Ly6C+ monocytes are in steady state an obligatory intermediate for the generation of CX3CR1hi Ly6C− monocytes and via their abundance control the circulation half-life of their progeny.
Recent studies have highlighted the existence of multiple pathways for the generation and maintenance of distinct mononuclear phagocyte subpopulations in the adult mouse (Ginhoux et al., 2010; Jakubzick et al., 2008; Varol et al., 2009). Accordingly, classical short-lived Flt3L-dependent DCs are continuously renewed by on-going haematopoiesis in the BM that feeds the periphery with dedicated DC precursors (Naik et al., 2007; Onai et al., 2007;(Birnberg et al., 2008; Karsunky et al., 2003; Naik et al., 2007; Onai et al., 2007; Waskow et al., 2008). The temporal tissue residence profiles of macrophages have remained less well defined (Hume, 2006; Hume et al., 2002). Macrophages residing in the murine intestinal lamina propria are reported to have an estimated half-life of three weeks (Jaensson et al., 2008). Moreover, using adoptive cell transfers, we and others provided evidence that these intestinal CX3CR1+ macrophages are continuously replenished from Ly6C+ monocytes (Bogunovic et al., 2009; Varol et al., 2009). However, the gut mucosa-associated tissue likely represents an exception, since it is constitutively exposed to the luminal microflora and its products, which probably cause tonic low-grade inflammation. Other macrophage populations such as alveolar macrophage’s have been reported to persist for years (Murphy et al., 2008). Notably however, previous studies on macrophage half-lives have been confounded by the fact that they involved irradiation regimens, which induce considerable damage to the tissue analyzed. Clear distinctions between inflammatory monocyte infiltrates and resident macrophages in these experimental setups might hence have been blurred. This complication was recently demonstrated in a series of studies that aimed to define the origins of brain microglia (Ginhoux et al., 2010).
Our comparative analysis of mice carrying a GFP reporter and conditional or constitutive active Cre recombinase genes in their Cx3cr1 loci, revealed that Kupffer cells, as well as lung, peritoneal and splenic macrophage’s are established before birth and remain in adulthood uncoupled from the steady state monocyte pool. Notably, our results are well in line with the recent demonstration that epidermal Langerhans' cells are derived from primitive macrophages and fetal liver cells, but that in adulthood this compartment maintains itself independent from monocyte input (Hoeffel et al., 2012). Congruent with these data, patients with autosomal dominant and sporadic monocytopenia present with an unaffected Langerhans' cell compartment (Bigley et al., 2011). What is more, our results are consistent with the recent evidence of the coexistence of two independent macrophage lineages in the mouse according to their differential dependence on the transcription factors PU.1 and c-myb (Schulz et al., 2012).
It is tempting to speculate that the reliance on resident macrophage populations established before birth represents an important design principle of tissue homeostasis ensuring robustness of the steady state. Accordingly, challenges such as injury and pathogen encounter will result in the recruitment of highly plastic monocytes, which critically, but transiently (Ajami et al., 2011) contribute to inflammation and its resolution until restoration of homeostasis. Although our study focuses on the steady state, we have shown that an inflammatory stimulus can recruit monocytes whose descendants can seemingly integrate into the resident serosal, peritoneal cavity macrophage compartment. Future studies will have to address whether these cells become functionally equivalent to the resident cells and whether this applies also to solid tissues.
Monocytes can be divided into two main subpopulations, defined in the human as CD14hi CD16+/−, CD14dim CD16hi cells and in the mouse as Ly6C+ CX3CR1int and Ly6C− CX3CR1hi cells (Geissmann et al., 2003; Palframan et al., 2001). Earlier studies have provided circumstantial evidence that the monocyte subpopulations of the mouse are a differentiation series (Sunderkotter et al., 2004; Varol et al., 2007; Yrlid et al., 2006). Here we have reported the use of a series of complementary non-invasive approaches to interrogate the steady state contribution of a Ly6C+ monocyte intermediate to the generation of Ly6C− monocytes. First, we observed distinct temporal requirements for the creation of the two monocyte subsets with delayed appearance of the YFP reporter label in Ly6C− monocytes in Cx3cr1Cre:R26-yfp and Cx3cr1creER:R26-yfp mice, as compared to Cx3cr1gfp mice. Secondly, we have shown that a BrdU pulse label moves with time from Ly6C+ monocytes to Ly6C− monocytes and ablation of the former cells abolishes appearance of the label in the latter. The data support earlier reports of an exceedingly short circulation half-life of Ly6C+ monocytes (Liu et al., 2007; Varol et al., 2007), that is with 0.8 days (19 hours) longer than, but comparable to that of short-lived neutrophils (11.4 hours) (Basu et al., 2002). Finally, we have shown using competitive mixed chimeras that CCR2, which is critical for the BM exit of Ly6C+ monocytes (Serbina and Pamer, 2006; Shi et al., 2011) is also required for the generation of Ly6C− monocytes. This strongly suggests that the majority, if not all blood Ly6C− monocytes are derived from CCR2+ Ly6C+ blood monocytes in the steady state. (Supplemental Fig. 1).
We have also demonstrated that Ly6C− monocytes are generated independently in situ in the bone marrow. Thus, Ly6C− BM monocytes acquired the BrdU label well before the Ly6C− blood monocytes and appearance of the BrdU label was unaffected by the MC21-mediated ablation of Ly6C+ blood monocytes. Moreover, also the differential dependence of Ly6C− BM and blood monocytes on CCR2 expression, as well as the resilience of BrdU incorporation of the latter to the MC21 regimen, suggest that these cells are generated in BM and blood, respectively. It remains to be shown whether Ly6C− BM monocytes arise like Ly6C− blood monocytes via a Ly6C+ intermediate or can be generated directly from MDPs, since the MC21 treatment spares Ly6C+ BM monocytes. The function of Ly6C− blood monocytes has been proposed to be to patrol and survey endothelial integrity (Auffray et al., 2007). When cultured in vitro with CSF-1 (M-CSF) or CSF-2 (GM-CSF) both Ly6C+ and Ly6C− monocytes can differentiate into macrophages and DCs, respectively (Jung, unpublished observation). However, while Ly6C+ monocytes are established in vivo precursors of intestinal macrophages, Ly6C− monocytes fail to do so (Varol et al., 2009). Moreover, reports demonstrating an in vivo potential of these cells to act as resident mononuclear phagocyte precursors are scarce (Landsman and Jung, 2007; Nahrendorf et al., 2007). Paradoxically, in humans the supposed functional equivalent, the CD14− CD16+ non-classical monocytes, is normally much less abundant but increases in response to chronic inflammatory stimuli (Ziegler-Heitbrock et al., 2010). In light of our present findings we propose that Ly6C− blood monocytes represent the homeostatic default product of short-lived Ly6C+ monocytes in the blood that surveys endothelial integrity (Auffray et al., 2007), as terminally differentiated blood-resident macrophages.
Our study has revealed that the life span of Ly6C− blood monocytes is affected by the prevalence of Ly6C+ blood monocytes, their direct precursors. Thus, whereas these cells display in un-manipulated steady state a half-life of 2 days, the latter is extended to 11 days when the Ly6C+ precursors are ablated. Prolonged neutralization of the CSF-1 receptor has been shown to result in the selective loss of Ly6C− blood monocytes (MacDonald et al., 2010). Conversely, ablation of CD115+ Ly6C+ blood monocytes could result in an increase of plasma CSF-1 promoting survival of Ly6C− blood monocytes. Interestingly, CD115 levels were found down-modulated on Ly6C− blood monocytes of CCR2−/− mice. Moreover, expression of CD115 was also reduced in mice in which Ly6C+ blood monocytes were ablated, but CD115 was rapidly restored upon withdrawal of the MC21 treatment. Extension of the half-life in response to the MC21 treatment was partially impaired by concomitant neutralization of the CSF-1 receptor suggesting that limited CSF-1 availability in the serum is associated with this phenomenon and that Ly6C+ monocytes constitute a sink for this critical survival factor. Interestingly, CSF-1 has been reported to induce the NOR1 (Pei et al., 2005), an orphan nuclear receptor and member of the steroid thyroid receptor family, which also comprises Nur77 (Nr4A) that was recently implicated in the control of Ly6C− monocyte survival (Hanna et al., 2011).
While the exact molecular pathway underlying the regulation remains to be elucidated, our study establishes that Ly6C+ monocytes are not only obligatory steady state precursors of Ly6C− monocytes, but also restrict the life span of their otherwise long-lived progeny. Conversely, the unique ability of Ly6C− monocyte to extend their circulation half-life could be a mechanism to ensure robust maintenance of a critical Ly6C− population size, potentially required for efficient surveillance of endothelial integrity (Auffray et al., 2007), even under conditions when Ly6C+ monocytes are withdrawn from their precursor task to sites of inflammation.
Collectively, we have provided here a fate mapping study of murine mononuclear phagocytes, focusing on macrophages and monocytes. Our results highlight the co-existence of distinct tissue-resident macrophage populations in the mammalian organism, including cellular compartments that are established during development from primitive macrophages or fetal liver cells, but remain during health independent from monocyte input. Within the monocyte compartment, we have established Ly6C+ blood monocytes as obligatory precursor cells of Ly6C− blood monocytes and reveal that the life span of these cells can be dynamically regulated to maintain a stable Ly6C− blood monocyte compartment in case of Ly6C+ blood monocyte shortage. Full appreciation of the monocyte and macrophage complexity in the mammalian organism will require future studies exploring the existence of distinctive molecular and functional characteristics of these distinct populations that might pave the way for rational strategies to manipulate mononuclear phagocytes for therapeutic purposes.
EXPERIMENTAL PROCEDURES
Mice
The following 6 to 10 week-old mouse strains were used: Cx3cr1gfp/+ mice (Jung et al., 2000); Pgk-cre mice (Lallemand et al., 1998), Ccr2−/− mice (Boring et al., 1997); Ccr2−/−:Cx3cr1gfp/+ mice. To analyse recombinase activity of Cx3cr1cre or Cx3cr1creER mice, these animals were crossed to Rosa-26-yfp (Srinivas et al., 2001) or Rosa-26-rfp reporter mice (Luche et al., 2007). BM chimeras, animals were lethally irradiated and reconstituted with donor BM see online experimental procedures for further details. All mice studied were on C57BL/6 background, maintained under specific pathogen-free conditions and handled under protocols approved by the Weizmann Institute Animal Care Committee (IACUC) in accordance with international guidelines.
Generation of Cx3cr1cre and Cx3cr1creER mice
See online experimental procedures for further details
Tamoxifen treatment
Tamoxifen was administered either orally or administered s.c. See online experimental procedures for further details.
Isolation of tissue samples
Blood, BM and tissue samples were prepared as described previously (Yona et al., 2010). Single-cell suspensions were then stained and subsequently analysed by polychromatic flow cytometry. A detailed description of tissue mononuclear phagocyte isolation and antibody clones used throughout this study can be found on the supplemental online experimental procedures.
Isolation of monocytes for adoptive transfers
BM cells were harvested and mononuclear cells enriched by density gradient. Splenic monocytes were isolated by MACS enrichment using biotinylated anti-CD115 antibody and streptavidin-coupled magnetic beads (Miltenyi Biotec). Ly6Chi monocytes were identified as CD11b+ CD115+ and Gr-1+, cells were purified by high-speed cell sorting using a FACS Aria (Becton-Dickson). Sorted cells were resuspended and injected i.v. into congenic CD45.1 WT mice. See online experimental procedures for further details.
BrdU pulsing
Mice were treated with 3 injections of 2 mg BrdU (5-bromo-2-deoxyuridine; BD Pharmingen) i.p. 3 hours apart or a single 2 mg i.p injection. To assess BrdU incorporation, monocytes were stained, fixed and permeabilized according to the manufacturer's instructions prior to analysis by flow cytometry. See online experimental procedures for further details.
Antibody and cytokine treatment
Mice were treated with 150 µl of CCR2 mAb (clone: MC21) conditioned media i.p. To block CD115, mice were treated CD115 mAb (clone: M279, MacDonald et al. 2010) i.p. Recombinant M-CSF (PeproTech) (25µg/mouse) was given i.p. See online experimental procedures for further details.
Histology
Mice were anaesthetized and perfused with 30 ml of PBS, organs were excised and fixed in para-formaldehyde prior to being imbedded in OCT. Cryostatic sections, were post-fixed and stained. Analysis by confocal laser scanning microscopy was performed using a Zeiss LSM510 microscope. Image acquisition was processed with Zeiss LSM Image browser software. See online experimental procedures for further details.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Rebecca Haffner for guidance with the ES cell work and the staff of the Weizmann Animal facility for the excellent care. S.Y. was a recipient of a long-term FEBS fellowship, A. M. was a fellow of the Minerva foundation. This work was supported by the Leir Charitable Foundation, the Wolfson Family Charitable Trust, the Israel Science Foundation (ISF) and the Deutsche Forschungsgemeinschaft (DFG) Research Unit (FOR) 1336. S.J. is a Helmsley Scholar at the Crohn’s & Colitis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Bar-On L, Birnberg T, Lewis KL, Edelson BT, Bruder D, Hildner K, Buer J, Murphy KM, Reizis B, Jung S. CX3CR1+ CD8alpha+ dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 107:14745–14750. doi: 10.1073/pnas.1001562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci A, Mastrogiannis DS, Migliorati G, Stockert RJ, Wolkoff AW, Stanley ER. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:6179–6183. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- Bigley V, Haniffa M, Doulatov S, Wang XN, Dickinson R, McGovern N, Jardine L, Pagan S, Dimmick I, Chua I, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. The Journal of experimental medicine. 2011;208:227–234. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr., Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhl H, Cihak J, Plachy J, Kunz-Schughart L, Niedermeier M, Denzel A, Rodriguez Gomez M, Talke Y, Luckow B, Stangassinger M, Mack M. Targeting of Gr-1+,CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum. 2007;56:2975–2985. doi: 10.1002/art.22854. [DOI] [PubMed] [Google Scholar]
- Crofton RW, Diesselhoff-den Dulk MM, van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978;148:1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nature immunology. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. The Journal of experimental medicine. 2012 doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA. The mononuclear phagocyte system. Current opinion in immunology. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Hume DA, Gordon S. The correlation between plasminogen activator activity and thymidine incorporation in mouse bone marrow-derived macrophages. Opposing actions of colony-stimulating factor, phorbol myristate acetate, dexamethasone and prostaglandin E. Experimental cell research. 1984;150:347–355. doi: 10.1016/0014-4827(84)90578-0. [DOI] [PubMed] [Google Scholar]
- Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. Journal of leukocyte biology. 2002;72:621–627. [PubMed] [Google Scholar]
- Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood Monocyte Subsets Differentially Give Rise to CD103+ and CD103- Pulmonary Dendritic Cell Populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature reviews. Immunology. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in "knock-in" Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol. 2008;38:380–385. doi: 10.1165/rcmb.2007-0224RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–596. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. The Journal of experimental medicine. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushinski RJ, Oliver IT, Guilbert LJ, Tynan PW, Warner JR, Stanley ER. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. [Mononuclear phagocytic system: new classification of macrophages, monocytes and of their cell line] Bull World Health Organ. 1972;47:651–658. [PMC free article] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Hayhoe R, Avraham-Davidi I. Monocyte and neutrophil isolation and migration assays. Curr Protoc Immunol Chapter. 2010;14:14–15. doi: 10.1002/0471142735.im1415s88. Unit. [DOI] [PubMed] [Google Scholar]
- Yrlid U, Cerovic V, Milling S, Jenkins CD, Klavinskis LS, MacPherson GG. A distinct subset of intestinal dendritic cells responds selectively to oral TLR7/8 stimulation. Eur J Immunol. 2006;36:2639–2648. doi: 10.1002/eji.200636426. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.