Abstract
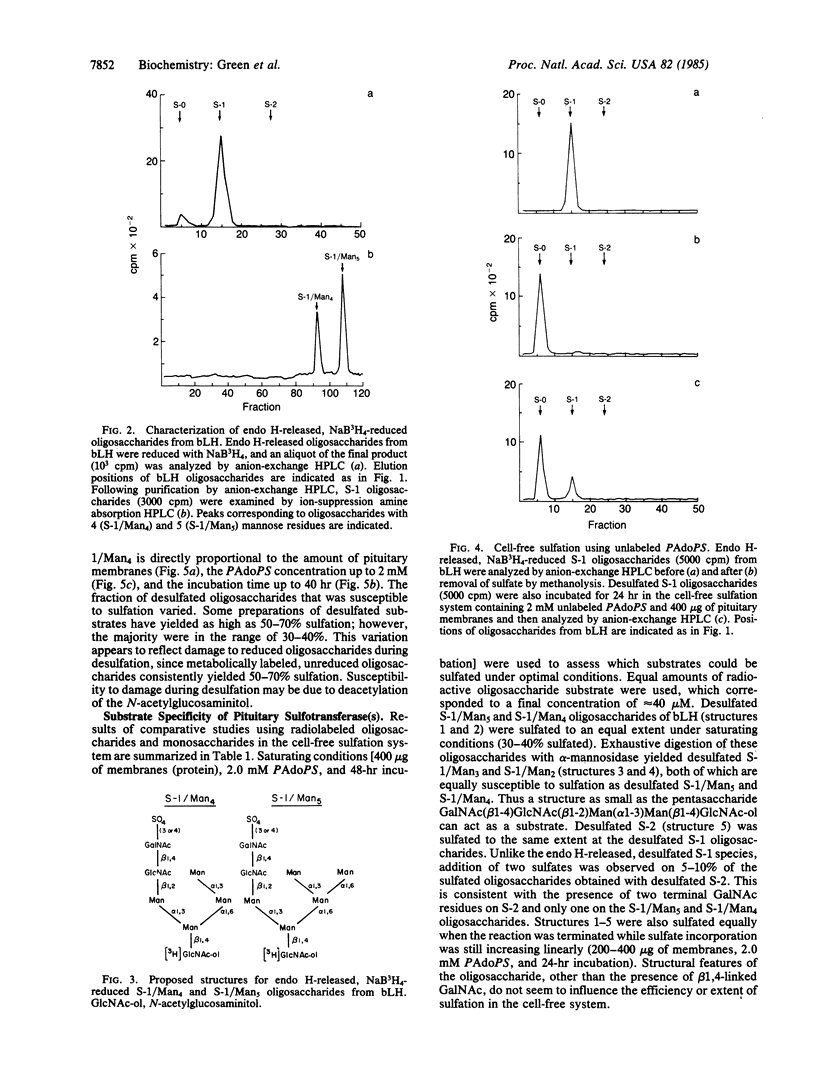
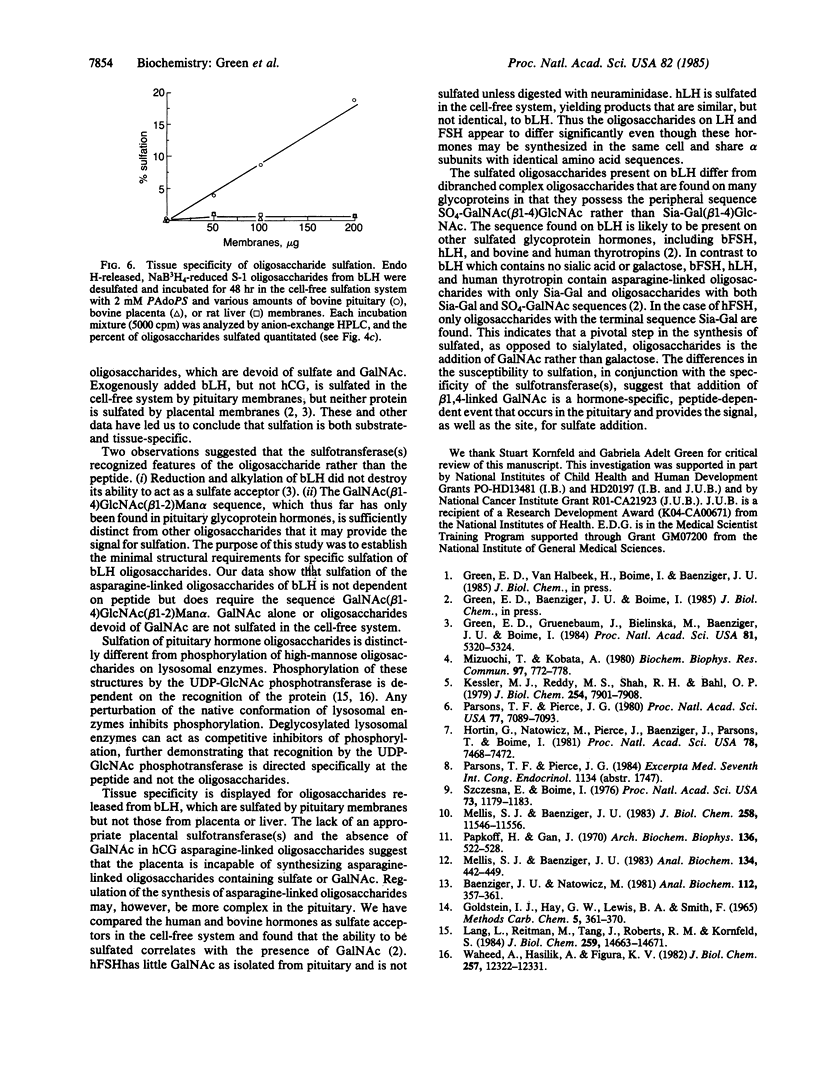
Human and bovine pituitary glycoprotein hormones (lutropin, follitropin, and thyrotropin) contain varying amounts of N-acetylgalactosamine and sulfate. The sulfate on asparagine-linked oligosaccharides of bovine lutropin (bLH) is present exclusively on GalNAc in the sequence GalNAc(beta 1-4)GlcNAc(beta 1-2)Man alpha. We have examined the structural requirements for sulfation of bLH oligosaccharides by using a reconstituted cell-free system. After cleavage from the protein, oligosaccharides containing the sequence GalNAc(beta 1-4)Glc-NAc(beta 1-2)Man alpha were sulfated by enzymes in pituitary membranes. Addition of one or two sulfates was observed, depending upon the number of GalNAc acceptor sites on the oligosaccharide. Neither GalNAc alone nor oligosaccharides devoid of GalNAc were sulfated. Membranes from placenta or liver did not sulfate oligosaccharides released from bLH, indicating that the sulfating activity is pituitary-specific. The lack of peptide dependence for sulfation, in conjunction with the oligosaccharide specificity, suggests that the sequence GalNAc(beta 1-4)GlcNAc(beta 1-2)Man alpha contains the recognition signal for the sulfotransferase(s).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Natowicz M. Rapid separation of anionic oligosaccharide species by high performance liquid chromatography. Anal Biochem. 1981 Apr;112(2):357–361. doi: 10.1016/0003-2697(81)90305-5. [DOI] [PubMed] [Google Scholar]
- Green E. D., Gruenebaum J., Bielinska M., Baenziger J. U., Boime I. Sulfation of lutropin oligosaccharides with a cell-free system. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5320–5324. doi: 10.1073/pnas.81.17.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortin G., Natowicz M., Pierce J., Baenziger J., Parsons T., Boime I. Metabolic labeling of lutropin with [35S]sulfate. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7468–7472. doi: 10.1073/pnas.78.12.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. J., Reddy M. S., Shah R. H., Bahl O. P. Structures of N-glycosidic carbohydrate units of human chorionic gonadotropin. J Biol Chem. 1979 Aug 25;254(16):7901–7908. [PubMed] [Google Scholar]
- Lang L., Reitman M., Tang J., Roberts R. M., Kornfeld S. Lysosomal enzyme phosphorylation. Recognition of a protein-dependent determinant allows specific phosphorylation of oligosaccharides present on lysosomal enzymes. J Biol Chem. 1984 Dec 10;259(23):14663–14671. [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Size fractionation of anionic oligosaccharides and glycopeptides by high-performance liquid chromatography. Anal Biochem. 1983 Oct 15;134(2):442–449. doi: 10.1016/0003-2697(83)90320-2. [DOI] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Structures of the oligosaccharides present at the three asparagine-linked glycosylation sites of human IgD. J Biol Chem. 1983 Oct 10;258(19):11546–11556. [PubMed] [Google Scholar]
- Mizuochi T., Kobata A. Different asparagine-linked sugar chains on the two polypeptide chains of human chorionic gonadotropin. Biochem Biophys Res Commun. 1980 Nov 28;97(2):772–778. doi: 10.1016/0006-291x(80)90331-9. [DOI] [PubMed] [Google Scholar]
- Papkoff H., Gan J. Bovine interstitial cell-stimulating hormone: purification and properties. Arch Biochem Biophys. 1970 Feb;136(2):522–528. doi: 10.1016/0003-9861(70)90224-9. [DOI] [PubMed] [Google Scholar]
- Parsons T. F., Pierce J. G. Oligosaccharide moieties of glycoprotein hormones: bovine lutropin resists enzymatic deglycosylation because of terminal O-sulfated N-acetylhexosamines. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7089–7093. doi: 10.1073/pnas.77.12.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. UDP-N-acetylglucosamine:lysosomal enzyme precursor N-acetylglucosamine-1-phosphotransferase. Partial purification and characterization of the rat liver Golgi enzyme. J Biol Chem. 1982 Oct 25;257(20):12322–12331. [PubMed] [Google Scholar]


