Abstract
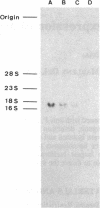
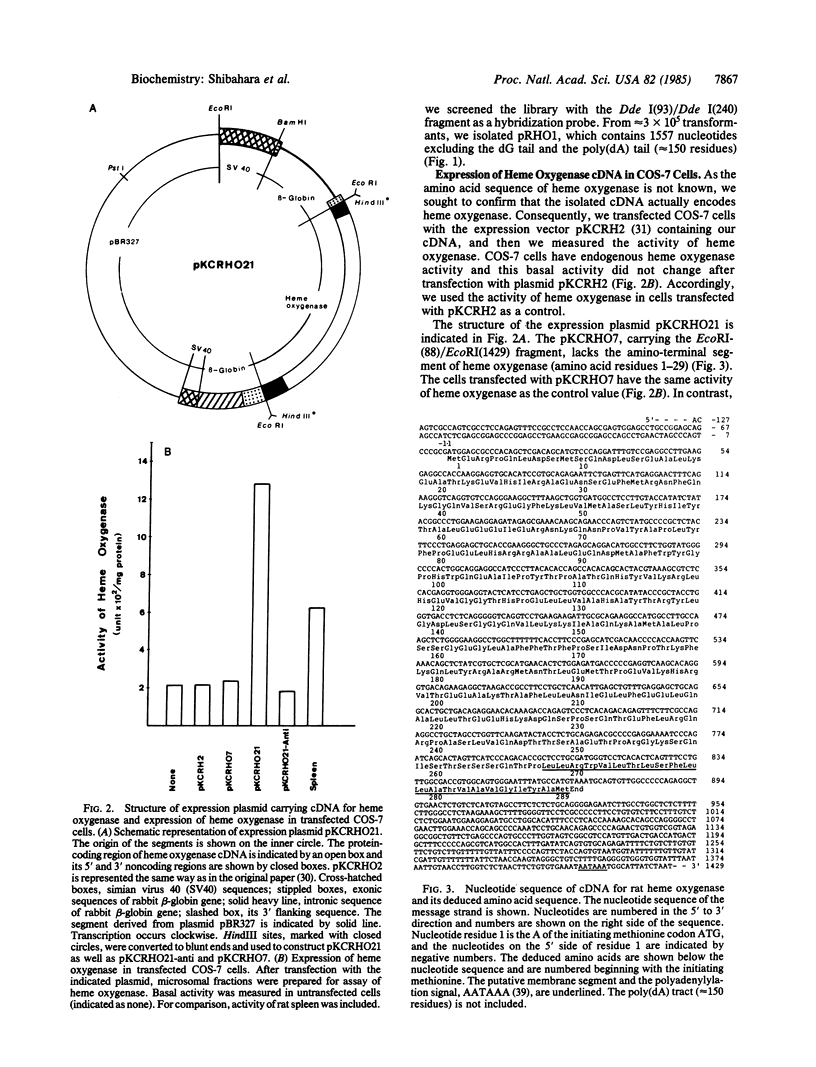
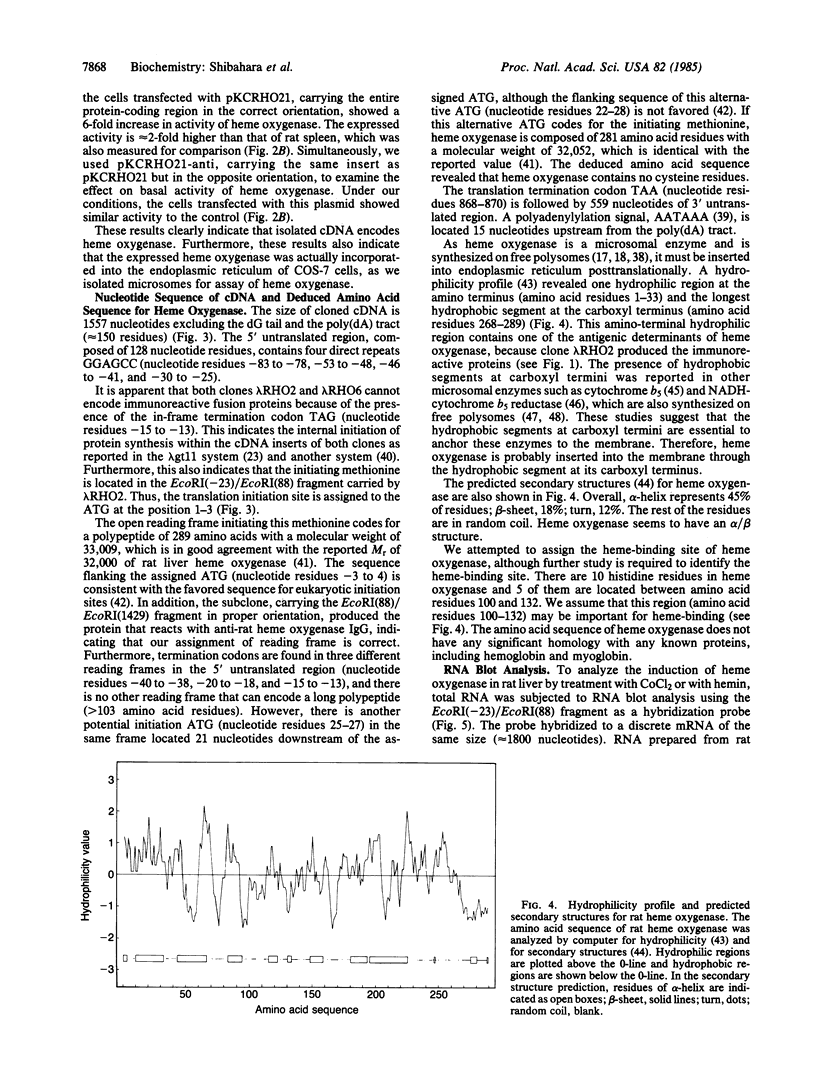
Two cDNA clones for rat heme oxygenase have been isolated from a rat spleen cDNA library in lambda gt11 by immunological screening using a specific polyclonal antibody. One of these clones has an insert of 1530 nucleotides that contains the entire protein-coding region. To confirm that the isolated cDNA encodes heme oxygenase, we transfected monkey kidney cells (COS-7) with the cDNA carried in a simian virus 40 vector. The heme oxygenase was highly expressed in endoplasmic reticulum of transfected cells. The nucleotide sequence of the cloned cDNA was determined and the primary structure of heme oxygenase was deduced. Heme oxygenase is composed of 289 amino acids and has one hydrophobic segment at its carboxyl terminus, which is probably important for the insertion of heme oxygenase into endoplasmic reticulum. The cloned cDNA was used to analyze the induction of heme oxygenase in rat liver by treatment with CoCl2 or with hemin. RNA blot analysis showed that both CoCl2 and hemin increased the amount of hybridizable mRNA, suggesting that these substances may act at the transcriptional level to increase the amount of heme oxygenase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Cytochrome P-450 heme and the regulation of hepatic heme oxygenase activity. Arch Biochem Biophys. 1976 Sep;176(1):91–102. doi: 10.1016/0003-9861(76)90144-2. [DOI] [PubMed] [Google Scholar]
- Chen J. J., London I. M. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981 Oct;26(1 Pt 1):117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dabney B. J., Beaudet A. L. Increase in globin chains and globin mRNA in erythroleukemia cells in response to hemin. Arch Biochem Biophys. 1977 Feb;179(1):106–112. doi: 10.1016/0003-9861(77)90092-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gemsa C., Woo C. H., Fudenberg H. H., Schmid R. Erythrocyte catabolism by macrophages in vitro. The effect of hydrocortisone on erythrophagocytosis and on the induction of heme oxygenase. J Clin Invest. 1973 Apr;52(4):812–822. doi: 10.1172/JCI107245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guzelian P. S., Elshourbagy N. A. Induction of hepatic heme oxygenase activity by bromobenzene. Arch Biochem Biophys. 1979 Aug;196(1):178–185. doi: 10.1016/0003-9861(79)90564-2. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D. N., Maniatis G. M. Haemin promotes rapid neurite outgrowth in cultured mouse neuroblastoma cells. Nature. 1978 Jul 27;274(5669):372–374. doi: 10.1038/274372a0. [DOI] [PubMed] [Google Scholar]
- Ishizawa S., Yoshida T., Kikuchi G. Induction of heme oxygenase in rat liver. Increase of the specific mRNA by treatment with various chemicals and immunological identity of the enzymes in various tissues as well as the induced enzymes. J Biol Chem. 1983 Apr 10;258(7):4220–4225. [PubMed] [Google Scholar]
- JANDL J. H., GREENBERG M. S., YONEMOTO R. H., CASTLE W. B. Clinical determination of the sites of red cell sequestration in hemolytic anemias. J Clin Invest. 1956 Aug;35(8):842–867. doi: 10.1172/JCI103338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Sato R., Sakakibara R., Wada H. Reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase: location of the hydrophobic, membrane-binding region at the carboxyl-terminal end and the masked amino terminus. Biochemistry. 1978 Jul 11;17(14):2839–2834. doi: 10.1021/bi00607a020. [DOI] [PubMed] [Google Scholar]
- Mishina M., Kurosaki T., Tobimatsu T., Morimoto Y., Noda M., Yamamoto T., Terao M., Lindstrom J., Takahashi T., Kuno M. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984 Feb 16;307(5952):604–608. doi: 10.1038/307604a0. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Pearson D., Altus M. S., Reich E. cDNA and gene nucleotide sequence of porcine plasminogen activator. Nucleic Acids Res. 1984 Dec 21;12(24):9525–9541. doi: 10.1093/nar/12.24.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Frey A. B., Guenthner T. M., Oesch F., Sabatini D. D., Kreibich G. Studies on the biosynthesis of microsomal membrane proteins. Site of synthesis and mode of insertion of cytochrome b5, cytochrome b5 reductase, cytochrome P-450 reductase and epoxide hydrolase. Eur J Biochem. 1982 Feb;122(2):393–402. doi: 10.1111/j.1432-1033.1982.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J., Gerard C. Primary structure of the membranous segment of cytochrome b5. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3725–3729. doi: 10.1073/pnas.74.9.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimstone N. R., Engel P., Tenhunen R., Seitz P. T., Marver H. S., Schmid R. Inducible heme oxygenase in the kidney: a model for the homeostatic control of hemoglobin catabolism. J Clin Invest. 1971 Oct;50(10):2042–2050. doi: 10.1172/JCI106697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimstone N. R., Tenhunen R., Seitz P. T., Marver H. S., Schmid R. The enzymatic degradation of hemoglobin to bile pigments by macrophages. J Exp Med. 1971 Jun 1;133(6):1264–1281. doi: 10.1084/jem.133.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rachubinski R. A., Verma D. P., Bergeron J. J. Synthesis of rat liver microsomal cytochrome b5 by free ribosomes. J Cell Biol. 1980 Mar;84(3):705–716. doi: 10.1083/jcb.84.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Sautner D. Induction of globin mRNA accumulation by hemin in cultured erythroleukemic cells. Cell. 1976 Aug;8(4):513–520. doi: 10.1016/0092-8674(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Yoshida T., Kikuchi G. Induction of heme oxygenase by hemin in cultured pig alveolar macrophages. Arch Biochem Biophys. 1978 Jun;188(2):243–250. doi: 10.1016/s0003-9861(78)80006-x. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Yoshida T., Kikuchi G. Intracellular site of synthesis of microsomal heme oxygenase in pig spleen. J Biochem. 1980 Jul;88(1):45–50. [PubMed] [Google Scholar]
- Shibahara S., Yoshida T., Kikuchi G. Mechanism of increase of heme oxygenase activity induced by hemin in cultured pig alveolar macrophages. Arch Biochem Biophys. 1979 Oct 15;197(2):607–617. doi: 10.1016/0003-9861(79)90285-6. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Ross M. E., Marver H. S., Schmid R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970 Jan 20;9(2):298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Purification and properties of heme oxygenase from rat liver microsomes. J Biol Chem. 1979 Jun 10;254(11):4487–4491. [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Reaction of the microsomal heme oxygenase with cobaltic protoporphyrin IX, and extremely poor substrate. J Biol Chem. 1978 Dec 10;253(23):8479–8482. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]