Abstract
Polybrominated diphenyl ether BDE-47 (2,2′,4,4′-tetrabromodiphenyl ether) is a thyroid hormone disruptor in mice; hepatic induction of various metabolic enzymes and transporters has been suggested as the mechanism for this disruption. Utilizing Car −/− and Pxr −/− mice as well as human primary hepatocytes, here we have demonstrated that BDE-47 activated both mouse and human nuclear receptor constitutive activated/androstane receptor (CAR). In mouse livers, CAR, not PXR, was responsible for Cyp2b10 mRNA induction by BDE-47. In human primary hepatocytes, BDE-47 was able to induce translocation of YFP-tagged human CAR from the cytoplasm to the nucleus andCYP2B6 and CYP3A4 mRNAs expressions. BDE-47 activated human CAR in a manner akin to the human CAR ligand CITCO (6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime) in luciferase-reporter assays using Huh-7 cells. In contrast, mouse CAR was not potently activated by BDE-47 in the same reporter assays. Furthermore, human pregnane X receptor (PXR) was effectively activated by BDE-47 while mouse PXR was weakly activated in luciferase-reporter assays. Our results indicate that BDE-47 induces CYP genes through activation of human CAR in addition to the previously identified pathway through human PXR.
KeyWords: PBDE, CAR, PXR, xenobiotic receptor.
Polybrominated diphenyl ethers (PBDEs) have been used as flame retardant chemicals for various products worldwide. PBDEs consist of a family of 209 possible congeners, identified by different bromination configurations of the core two-ring structure. PBDEs produced on an industrial scale are mixtures of multiple congeners. Among the major product groups of PBDE, penta-BDE (traded under brand names such as DE-71 and Bromkal70-5DE), were used as additives to the polyurethane foams found in upholstered furniture, mattresses, and carpet cushions. One congener designated as BDE-47 (2,2′,4,4′-tetrabromodiphenyl ether) accounts for roughly 40% (w/w) of commercial penta-BDE (LaGuardia et al., 2006). In numerous studies using model animals, PBDEs are suggested to have various adverse effects on human health affecting thyroid hormone concentration, behavior, learning ability, memory, and brain development (Birnbaum and Staskal, 2004; Dingemans et al., 2011; Kodavanti et al., 2010; Richardson et al., 2008; Szabo et al., 2009). Penta-BDE and octa-BDE production in the United States were voluntarily stopped at the end of 2004 because of these health concerns. However, around the world there are still many commercial products manufactured before the voluntary ban that contain penta and octa-BDE. Recent studies with human specimens measured the total content of BDE chemicals and BDE-47 in breast milk and serum and suggest that internal human exposure to these chemicals has been roughly one order of magnitude higher in North America than in Europe or Asia (Frederiksen et al., 2009).
Multiple pathways for possible human exposure to PBDEs are known. Despite the ban in 2004, bioaccumulations along the food chain have been observed and human intake of these chemicals from diet is suggested to be increasing (Frederiksen et al., 2009). Exposure to house dust contaminated with PBDEs in debris from furniture and carpet materials accounted for 82% of the overall estimated intake of PBDEs in the United States (Lorber, 2008). Inhalation of contaminated air is another exposure pathway, since PBDEs were reported to leak into air from various products and have been detected in air collected from households and near production sites (Birnbaum and Staskal, 2004). Serum PBDE levels in toddlers showed association with house dust and hand-to-mouth activity (Stapleton et al., 2012). Furthermore, infants have an additional exposure route through breast milk intake. A recent publication suggested lactational exposure to PBDEs may increase activity/impulsivity behaviors in early childhood (Hoffman et al., 2012).
Constitutive activated/androstane receptor (CAR) and pregnane X receptor (PXR) belong to the nuclear hormone receptor superfamily and were both found to be xenobiotic sensing receptors in 1998 (Honkakoski et al., 1998; Kliewer et al., 1998). Over the past 15 years, many research reports established their functions in induction of enzymes and transporters in phase I, II, and III xenobiotic metabolism, and hence their roles in detoxification/elimination of xeno- and endobiotics (Honkakoski et al., 2003; Klaassen and Slitt, 2005; Timsit and Negishi, 2007; Tolson and Wang, 2010). Both receptors have broad specificity; activating chemicals that include therapeutic drugs, steroids, dietary substances, herbal medicines, and environmental chemicals (Chang, 2009; DeKeyser et al., 2009, 2011; Hernandez et al., 2009; Honkakoski et al., 2003; Sueyoshi et al., 2011; Yeung et al., 2008). Furthermore, studies have found that various physiological processes, such as liver energy metabolism, tumor promotion, cell proliferation and migration, apoptosis, and bone mineralization are affected by modulation of the activity of either or both CAR and PXR. Their mechanisms for this broad range of pathways includes modulating expression of many genes and interacting with numerous cellular factors (Dong et al., 2009; Gao and Xie, 2010; Kamino and Negishi, 2012; Kodama et al., 2004; Kodama and Negishi, 2011; Konno et al., 2010; Nakamura et al., 2007; Wada et al., 2009; Yamamoto et al., 2004, 2010). Consequently, these receptors are now considered to be major players in liver physiology and pathology and candidates for intervention by drug treatments for various ailments (Dong et al., 2009; Kakizaki et al., 2011; Wada et al., 2009; Yamazaki et al., 2007, 2011). Thus, unforeseen activation of these receptors by environmental chemicals might cause a broad range of effects on human health.
In previous studies, disposition of BDE-47 in mice has been measured (Staskal et al., 2005, 2006b). BDE-47 was present in high concentrations throughout adipose and skin tissues because of their lipophilicity. Lower distribution was found in other tissues including the liver. Furthermore, the results in these publications indicate that BDE-47 induces CYP2B genes in mouse liver, which suggested to us that BDE-47 might do so by activation of CAR. Here we tested CAR-null mice (CAR−/−) and their wild-type littermates for the effect of BDE-47 on CYP2B expression. We show that whereas BDE-47 induced CYP2B in wild-type CAR mice, induction was absent in null animals. Similar experiments using PXR wild-type and null mice showed induction in both genotypes. We conclude that BDE-47 activates CAR but not PXR in the mouse. By contrast, BDE-47 activated both CAR and PXR in human primary hepatocytes. These findings lead us to speculate that BDE-47, and possibly other PDBEs, up-regulate CAR and PXR pathways in humans, through which these compounds could affect human responses to xenobiotics including many therapeutic drugs.
MATERIALS AND METHODS
Animals.
The CAR and PXR null mice with C3H/HeNCrlBR (C3H) genetic background used in this report were previously reported (Nakamura et al., 2007; Yamamoto et al., 2004). Mice were kept in 12:12h dark/light cycle and fed with NIH-31 diet ad libitum. BDE-47 (10–100mg/kg body weight) was administered as a single oral dose to 8 weeks old C3H wild type male mice via gavage using corn oil (300 µl/mouse) as the vehicle. For CAR and PXR null mice and their littermate male wild type mice (C3H genetic backgournd, 7–9 weeks old), 50mg/kg body weight BDE-47 was administered as a single oral dose via gavage using the same volume of corn oil. Mice were sacrificed 24h after BDE-47 administration for liver RNA preparation. All animal procedures were approved by the Animal Ethics Committee at NIEHS, NIH (Animal Care and Use Committee Assurance #A4149-1).
Liver microsome preparation and Western blotting.
Each mouse liver (1g) was homogenized in 10ml homogenization buffer (10mM Tris–HCl (pH 7.6), 10mM EDTA, and 0.15M KCl). Homogenate was centrifuged at 9000 × g for 10min and supernatant was further centrifuged at 10,0000 × g for 1h. Pellets were washed with homogenization buffer and protein in the final pellet was applied for SDS–PAGE separation using 10% gel. Western blotting was performed for mouse proteins using anti-Cyp2b10, anti-Cyp3 (group specific antibody reacting Cyp3a11 and several mouse Cyp3a enzymes (Santa Cruz), and anti-Calnexin (Abcam) antibodies. For human CYP enzyme expression analysis, whole cell extracts were employed and Western blotting anlaysis was performed with anti-CYP2B6 (Santa Cruz), anti CYP3A4 (Millipore), and anti-β actin (Sigma–Aldrich).
Chemicals.
BDE-47 (MW = 485.8) was synthesized in >99% purity (based on relative peak area determined by gas chromatography with flame ionization detection as shown in Supplementary Figures 1 and 2) by bromination of diphenyl ether (Norström et al., 1976; Oern et al., 1996). Androstenol (5 alpha-androst-16-en-3 alpha-ol) was purchased from Steraloids. All other chemicals used were from Sigma–Aldrich.
Human primary hepatocyte isolation and culture.
Human liver tissues were obtained by qualified medical staff after donor consent and prior approval from the Institutional Review Board at the University of Maryland, School of Medicine. Hepatocytes were isolated from human liver specimens by a modification of the two-step collagenase digestion method as described previously (LeCluyse et al., 2005; Li et al., 2009). We observed no cytotoxicity of BDE-47 on human primary hepatocytes based on its cell morphology, total RNA yield and total protein yield at current treatment conditions.
RNA and protein expression in human primary hepatocytes.
Hepatocytes were seeded at 1.5×106 cells/well in six-well BioCoat plates and cultured in sandwich format as described previously (Li et al., 2012). Hepatocytes were maintained in complete William’s E Medium for 36h before treatment with vehicle control (0.1% DMSO), phenobarbital (1mM), CITCO (1 µM), rifampicin (10 µM), or BDE-47 at concentrations of 5, 10, 25, and 50 (µM) for 24h. Total RNA was harvested and analyzed by real-time PCR. Also, CYP enzyme expression levels were analyzed by Western blotting using whole cell extracts.
Translocation of Ad/EYFP-hCAR in human primary hepatocytes.
Fluorescent-tagged human CAR (Ad/EYFP-hCAR) was used as described previously (Li et al., 2009). This construct consists of enhanced yellow fluorescent protein linked to the N-terminus of hCAR and introduced into cells using an adenovirus vector. Hepatocyte cultures in 24-well BioCoat plates were cultured in Williams’ E medium containing Ad/EYFP-hCAR at a multiplicity of infection of 50 for 12h before treatment with vehicle control (0.1% DMSO), PB (1mM), or BDE-47 (10 and 50 µM). After 8h of incubation, treated cells were subjected to confocal microscopy analysis with a Nikon (Tokyo, Japan) C1-LU3 instrument based on an inverted Nikon Eclipse TE2000 microscope. The subcellular localization of hCAR was visualized and quantitatively characterized as nuclear, cytosolic, or mixed (nuclear + cytosolic) expression by counting at least 100 Ad/EYFP-hCAR-expressing hepatocytes from each group. We used primary hepatocytes from two independent donors and representative results are shown in this article.
Quantitative RT PCR analysis of mRNA.
Total mouse liver RNA was isolated with Trizol reagent (Invitrogen) and human primary hepatocyte RNA was isolated with RNeasy mini kit (Qiagen). Reverse-transcription was performed using High Capacity cDNA Archive kits for RNA (Applied Biosystems). Quantitative real-time PCR (qPCR) was carried out with the ABI prism 7000 or 7900HT sequence detection system (Applied Biosystems). For Cyp2b10, Cyp3a11, and Cyp1a1, pre-synthesized probes from Applied Biosystems, Mm00456591_m1, Mm00731567, and Mm00487218_m1 were used, respectively. For normalization of gene expressions among individual mice, Actb gene expressions determined with TaqMan mouse ACTB endogenous control reagent (Applied Biosystems) were utilized. For CYP2B6, CYP3A4, and GAPDH mRNA detection, as previously reported (Wang et al., 2013)., the following primers were used: CYP2B6, 5′-AGACGCCTTCAATCCTGACC-3′ (forward) and 5′-CCTTCACCAAGACAAATCCGC-3′ (reverse); CYP3A4, 5′-GT GGGGCTTTTATGATGGTCA-3′ (forward) and 5′-GCCTCAGATTTCTCACC AACACA-3′ (reverse); and GAPDH, 5′-CCCATCACCATCTTCCAGGAG-3′ (forward) and 5′-GTTGTCATGGATGACCTTGGC-3′ (reverse). Induction values (fold over control) were calculated according to the 2ΔΔCt method, where ΔC t represents the differences in cycle threshold numbers between the target gene and GAPDH, and ΔΔC t represents the relative change in these differences between the control and treatment groups.
Cell culture and reporter gene assay.
Huh7 cells (JCRB cell bank, Osaka, Japan) were cultured in minimum essential medium supplemented with 10% fetal bovine serum, 2mM L-glutamine, penicillin (100 units/ml) and streptomycin (100 μg/ml). Plasmids were transfected into Huh7 cells using Fugene6 (Roche) according to the manufacturer’s instructions. Plasmids used were NR1×5/pGL3-tk (Kawamoto et al., 1999), XREM/pGL3 (originally reported as p3A4-362(7836/7208ins)) (Goodwin et al., 1999), phRL-tk (Promega), mCAR/pCR3 (Sueyoshi et al., 1999), hCAR/pCR3 (Ueda et al., 2002), mPXR/pCR3, and hPXR/pCR3 (Yeung et al., 2008). Transfected cells were kept for 24h, then BDE-47 was added to the medium and cells were incubated for an additional 24h. Luciferase reporter activities were measured using Dual Luciferase Assay System (Promega) and firefly luciferase activities were normalized with Renilla luciferase activities. We observed no cytotoxicity of BDE-47 on Huh-7 cells based on viability analysis employing MTT assay at current treatment conditions.
Statistical analysis.
Statistical analysis was performed by one-sided exact Mann–Whitney tests for the mRNA expression differences or luciferase reporter activity differences between vehicle and BDE-47 treated cells and mice.
RESULTS
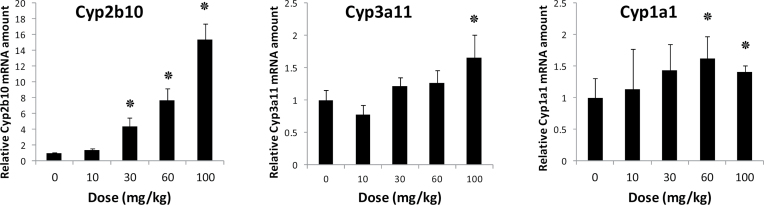
First, the effect of BDE-47 on the expression level of several mouse liver CYP genes was examined. Mice were treated with graded doses of BDE-47 (0, 10, 30, 60, 100mg/kg). Twenty-four hours after treatment, mice were sacrificed and CYP mRNA quantified by qPCR as shown in Figure 1. BDE-47 induced the Cyp2b10 gene dose dependently. In contrast, Cyp3a11 was weakly induced only at the highest dose whereas Cyp1a1 was not induced at all. The results in Figure 1 prompted us to examine the potential role of xenobiotic nuclear receptors CAR and PXR, since these nuclear receptors are known to be responsible for the gene activation of many CYP genes in response to xenobiotic compounds.
FIG. 1.
Dose-dependent induction of CYP mRNAs by BDE-47 in mouse livers. C3H/HeNCrlBR mice received a dose-dependent gastric administration of BDE-47 or vehicle as described in the methods section. qPCR was employed to measure Cyp2b10, Cyp3a11, and Cyp1a1 mRNAs in the livers. mRNA levels were determined relative to β-actin mRNA levels and were expressed by using the levels of vehicle-treated mice as one. Numbers represent mean ± SD (n = 3). ٭p < .05.
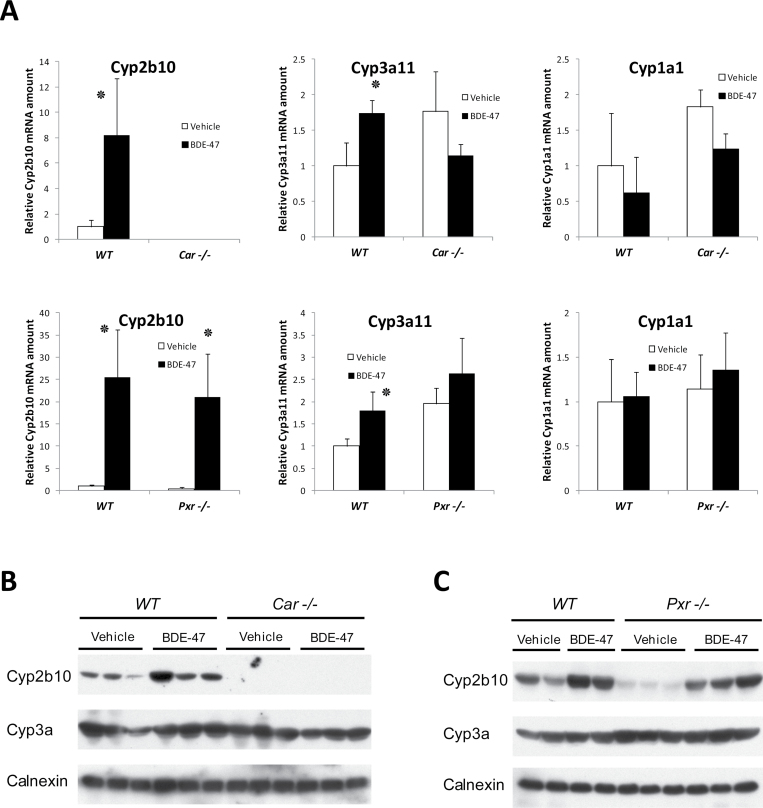
We used CAR and PXR wild-type and null mice to examine the role of CAR and PXR in mediating BDE-47 effects (Fig. 2A). Single administration of BDE-47 (50mg/kg) induced Cyp2b10 strongly in wild type mice while no induction was observed in CAR null mice. In PXR null mice, Cyp2b10 was induced to almost the same level of wild type mice. Cyp3a11 was induced weakly in wild type mice, but no induction was observed in CAR or PXR null mice. Cyp2b10 and Cyp3a protein expression levels in the microsome fractions in Car null and Pxr null and their littermate wild type mice were examined as in Figures 2B and 2C by Western blotting analysis. Cyp2b10 protein bands are increased by BDE-47 in WT and Pxr null mice in similar way. In contrast, no Cyp2b10 bands were observed in Car null mice with or without BDE-47 treatment. Anti-Cyp3a, which reacts with several Cyp3a enzymes including Cyp3a11, detected single band of protein from each sample. The intensities of the detected bands are essentially the same among the samples from any genetic conditions with or without BDE-47 treatment. These results revealed the critical roles of CAR in Cyp2b10 induction by BDE-47 in mouse liver.
FIG. 2.
Induction of CYP mRNAs and proteins by BDE-47 in the livers of Car−/− and Pxr−/− mice. A, Car −/−, Pxr −/− and their respective wild type mice received gastric administration of BDE-47 (50mg/kg body weight) for 24h. RNAs were prepared from livers from these mice and subjected to qPCR as described in the methods section. CYP mRNA levels were determined relative to β-actin mRNA levels and expressed by using the levels of vehicle-treated mice as one. Cyp2b10 mRNA in Car −/− mice were not detected (n.d.). Numbers represent mean ± SD (n = 3). ٭p < .05 and for vehicle treated group versus BDE-47 treated group. B and C, Mouse liver microsome protein (25 µg/lane) prepared form Car−/− (n = 3) and Pxr−/− (n = 3) mice was analyzed by Western blotting to find protein expression levels of Cyp2b10, and Cyp3a. Mice are treated as in A and microsome fractions were prepared and Western blotting was performed as in Methods section. Calnexin antibody was used for internal control for microsome proteins.
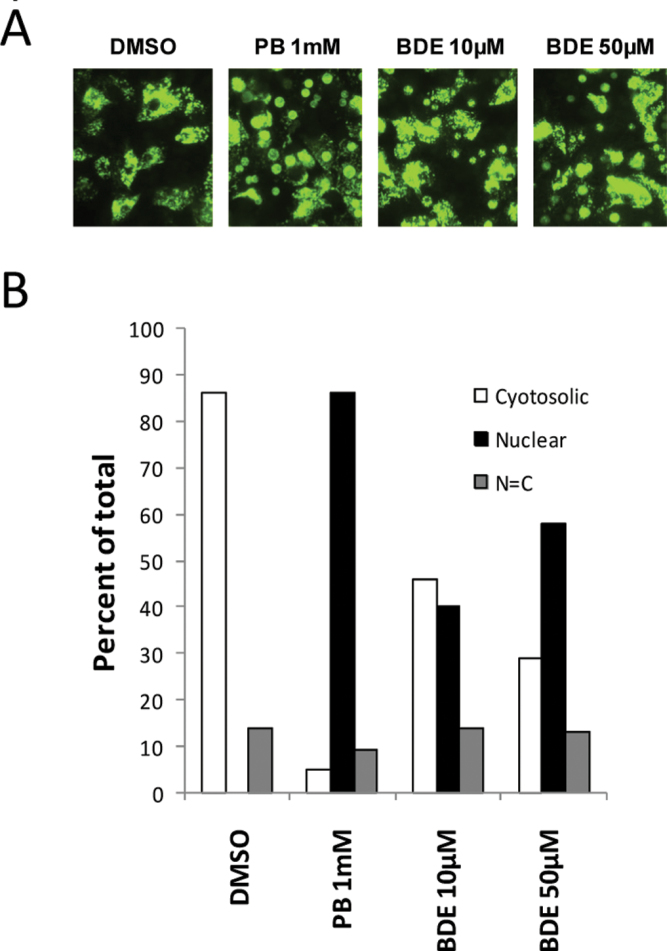
Next we examined the effect of BDE-47 on human CAR intracellular localization in human primary hepatocytes as shown in Figure 3. We transfected human primary hepatocytes with an adenovirus construct expressing YFP-hCAR fusion protein. We then treated the cells with PB (1mM) or BDE-47 (10 and 50 µM). Representative images for YFP fusion protein localization by confocal microscopy are shown in Figure 3 panel A. We counted cells to categorize YFP-fusion protein localization in cytoplasm, nucleus or mixed (localized in both cytoplasm and nucleus). The results shown in panel B indicate that PB treatment caused translocation of YFP-hCAR into the nucleus in almost 90% of cells. This PB effect was well in accordance with our results reported previously (Li et al., 2009). BDE-47 at concentrations of 10 and 50 µM showed roughly 40% and 60% nuclear localization, respectively. Thus, at these concentrations BDE-47 caused translocation of YFP-hCAR into the nucleus of human primary hepatocytes, although the efficacy of BDE-47 was less than that of PB under these experimental conditions.
FIG. 3.
Induced nuclear accumulation of CAR in BDE-47-treated human primary hepatocytes. A, Human primary hepatocytes (donor HL-40) were infected with Ad/EYFPhCAR as described in the methods section. Twelve hours after infection, cells were treated with DMSO or BDE-47 (10 and 50 µM). Images of YFP tagged hCAR were visualized in human primary hepatocytes with a confocal microscope. B, After over 100 cells were counted for each treatment, the intracellular localization of YFP-hCAR was categorized into three groups: cytoplasmic (C), nuclear (N), and both cytoplasmic and nuclear (N = C).
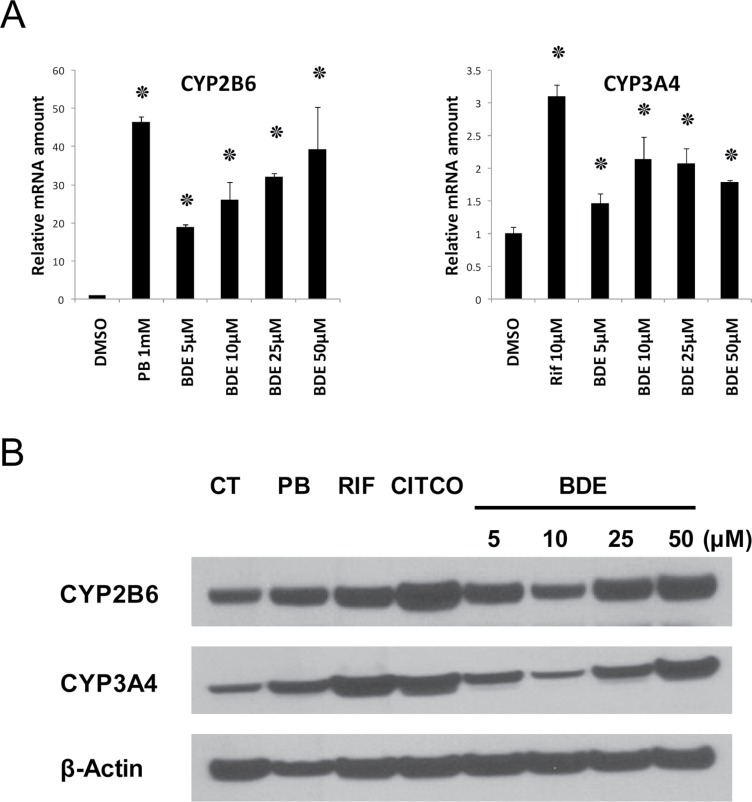
We then tested these human hepatocytes for the ability of BDE-47 to induce expression of CYP2B6, which is a CAR target gene in human liver (Fig. 4). CYP2B6 mRNA was induced in a dose-dependent manner. Induction was statistically significant down to the lowest concentration tested, 5 µM. At the highest dose tested (50 µM) CYP2B6 was induced approximately 40-fold to almost the same level as that seen using the canonical CAR activator PB (1mM). In contrast, CYP3A4 was less effectively induced; less than 2.5-fold at maximum. There was no dose dependency in this gene induction of BDE-47 with concentrations over 10 µM (Fig. 4). The inductions were significant but somewhat weaker than the human PXR activator rifampicin (10 µM). Western blotting analysis in Figure 4B shows indeed BDE-47 as low as 5 µM can increase CYP2B6 protein levels in human primary hepatocytes despite the effect was not prominent compared to the mRNA induction. CYP3A4 protein levels were also induced in higher concentration of BDE-47 and the dose dependency was more noticeable in protein levels than mRNA expressions. The reason why we observe slight decrease of CYP2B6 and CYP3A4 protein levels in 10 µM treated hepatocytes are not clear at this point and may need future analysis.
FIG. 4.
Induction of CYP mRNAs and proteins by BDE-47 in human primary hepatocytes. A, Human primary hepatocytes (donor HL-40) were treated with DMSO, phenobarbital (PB), rifampicin (Rif), and BDE-47 (at four concentrations ranging from 5 to 50 µM) for 24h. CYP mRNA levels were determined relative to the levels of GAPDH mRNA and expressed using the levels in DMSO-treated hepatocytes as one. Numbers represent mean ± SD (n = 3). ٭p < .05 . B, Human primary hepatocytes (donor HL-82) were treated as the same way as for mRNA preparations. Whole cell extracts and Western blotting was performed as described in the Methods section to detect CYP2B6 and CYP3A4 protein expression levels in the extracts (25 µg/lane). β-actin used internal control for whole cell extracts.
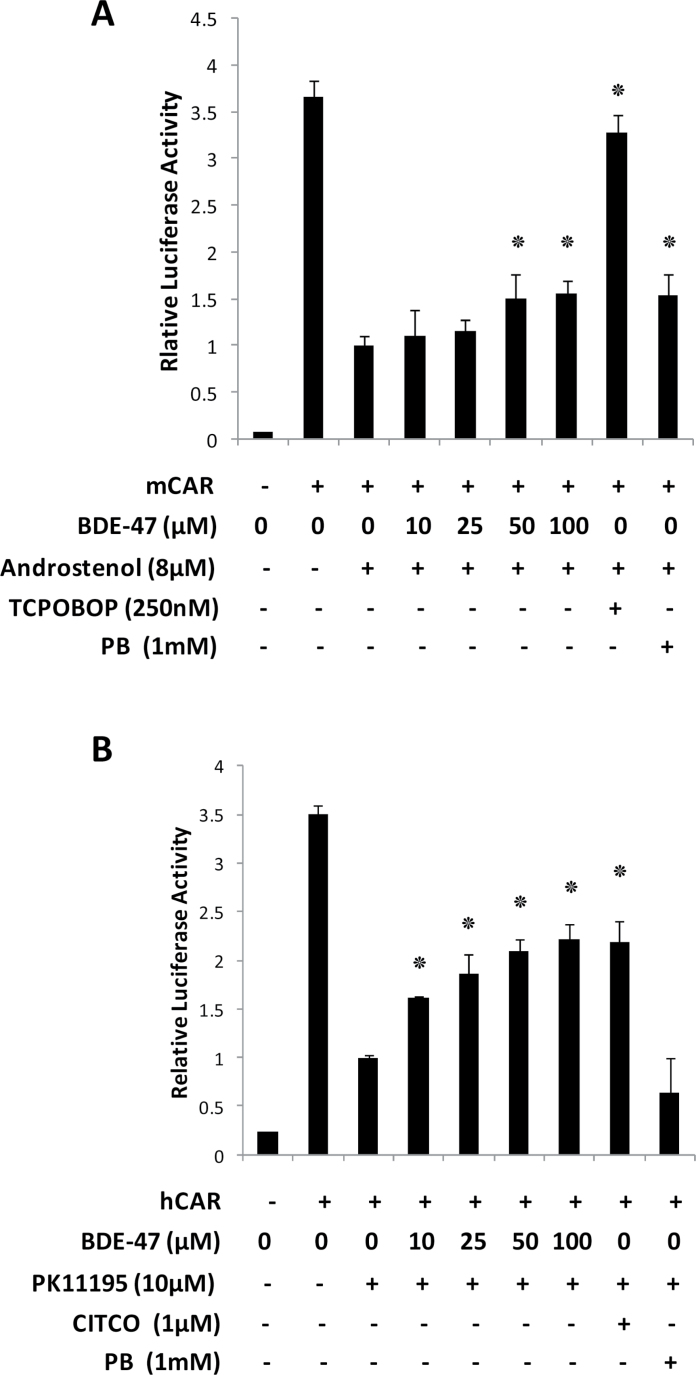
To further characterize the molecular mechanism of CAR activation by BDE-47, CAR-responsive reporter gene activation was examined in Huh-7 cells as shown in Figure 5. Huh-7 cells were co-transfected with the reporter construct (NR1×5/pGL3-tk) together with either mCAR or hCAR expression vectors. As previously reported (Honkakoski et al., 1998; Sueyoshi et al., 1999; Ueda et al., 2002), both mCAR and hCAR exhibited strong activation of the reporter gene without any chemical treatments. This high basal activity was suppressed with CAR antagonists androstenol (Sueyoshi et al., 1999) and PK11195 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide) (Li et al., 2008) for mCAR and hCAR, respectively. Activation by other compounds was then tested by adding them simultaneously with the antagonists. Activity is expressed as -fold induction relative to the level seen for the antagonist alone.
FIG. 5.
Activation of luciferase reporter gene by mCAR and hCAR. NR1×5-luciferase reporter plasmid was co-transfected with mCAR (A) or hCAR (B) expression vector into Huh-7 cells. Twenty-four hours after transfection, cells were treated with DMSO or the indicated concentrations of BDE-47 for another 24h in the presence of antagonistis for mCAR (androstenol) and hCAR (PK11195). Luciferase activities were determined as described in the methods section. The relative luciferase activities were calculated by considering antagonists (androstenol for mCAR (A) and PK11195 for hCAR (B)) treated cells with CAR transfection as one. Numbers represent mean ± SD (n = 3). ٭p < .05.
As shown in Figure 5A, unliganded mouse CAR induced luciferase reporter activity could be suppressed by about 75% by the addition of androstenol. Increasing concentrations of BDE-47 increased reporter expression in a concentration-dependent fashion, which was statistically significantly elevated at 50 µM. The mCAR agonist TCPOBOP (1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene, 3,3′,5,5′-Tetrachloro-1,4-bis(pyridyloxy)benzene) activated the reporter 3.4-fold. In this experiment PB, which activates CAR by a different mechanism, activated the reporter 1.5-fold.
The same experiments were carried out under the same conditions except that human CAR was used and the antagonist was hCAR-specific PK11195 as shown in Figure 5B. Similar results were observed for the hCAR experiment: a concentration-dependent activation of the reporter was seen. The hCAR system was even more sensitive to BDE-47 than mCAR was; statistically-significant activation was observed at 10 µM. BDE-47 at the highest concentration reached the same level of activation as 1 µM CITCO, an authentic hCAR activator ligand (Maglich et al., 2003). PB (1mM) did not activate the reporter in this assay condition. To find lower limit of BDE-47 concentration in this assay for hCAR activation, the exactly same format experiment using from 0.5 to 5 µM concentration of BDE-47 was performed as in Supplementary Figure 3. As low as 1 µM of BDE-47 induced the reporter gene significantly.
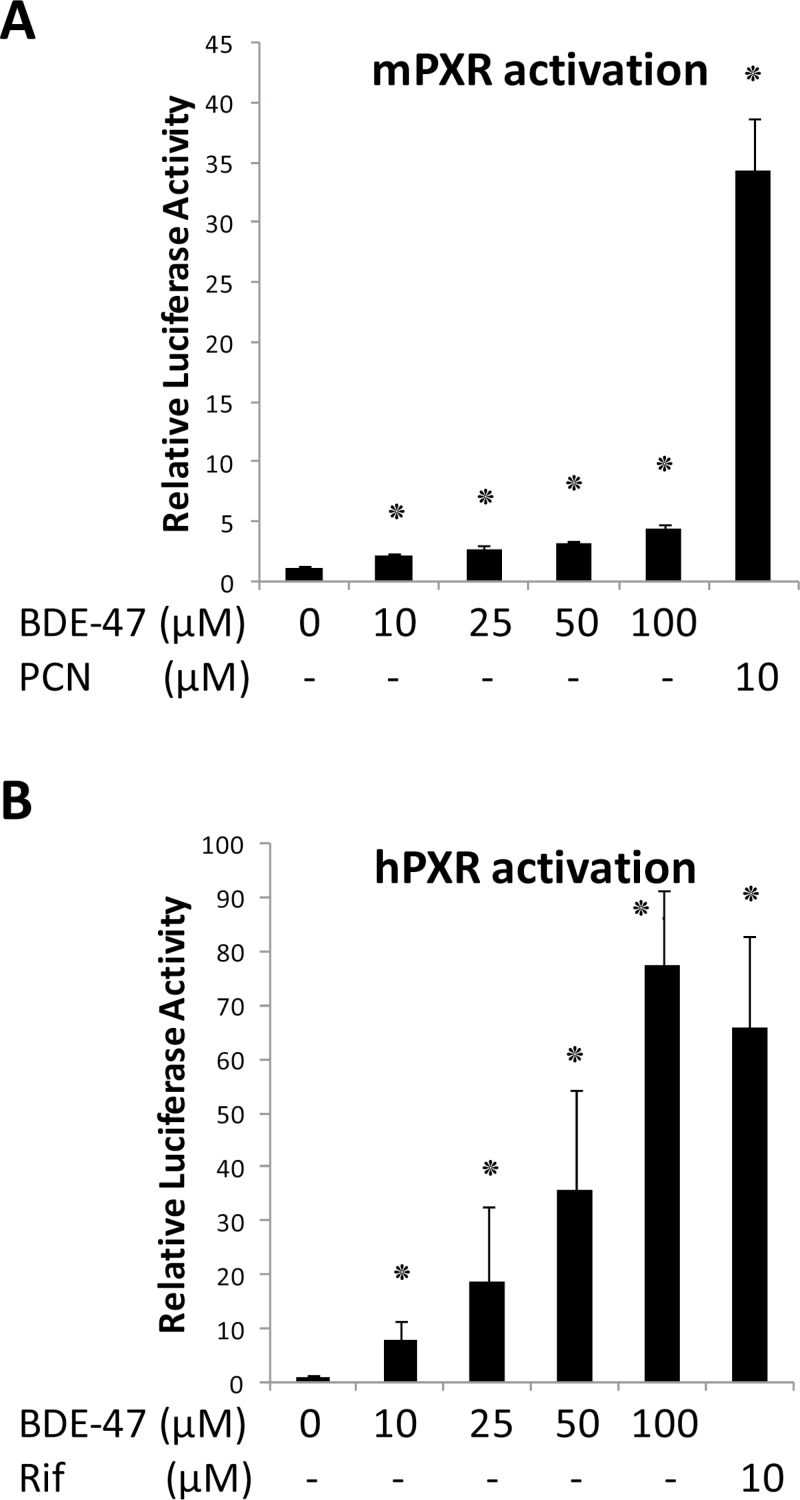
Next, we examined BDE-47 effect on human and mouse PXR activity in reporter gene assays in Huh7 cells transfected with mPXR or human PXR (Fig. 6). BDE-47 treatment caused weak, but statistically significant, activation of the mPXR reporter, even at the lowest concentration tested (10 µM). However, the efficacy of this activation was only about 15% of the level achieved using the mPXR ligand agonist PCN. In contrast, BDE-47 treatment resulted in robust activation of hPXR, with a 10- and 80-fold increase in reporter activity using 10- and 100-µM BDE-47, respectively (Fig. 6B). The activation with 10-µM BDE-47 was comparable to that of 10 µM rifampicin. These results suggest that BDE-47 is a activator of both hPXR and mPXR. Potency of activation by BDE-47 was more prominent with hPXR compare to mPXR.
FIG. 6.
Activation of luciferase reporter gene by mPXR and hPXR. XREM reporter plasmid was co-transfected with mPXR or hPXR expression vector into Huh-7 cells. Twenty-four hours after transfection, cells were treated with DMSO or the indicated concentrations of BDE-47 for another 24h. Luciferase activities were determined as described in the methods section. The relative luciferase activities were calculated by considering vehicle-treated cells as one. Numbers represent mean ± SD (n = 3). ٭p < .05.
DISCUSSION
In previous reports, BDE-47 was shown to induce CYP genes in mouse liver (Richardson et al., 2008; Staskal et al., 2005). CYP gene induction spectra in mouse liver suggested a role for nuclear receptor CAR in these effects (Richardson et al., 2008). In this study, we observed that a single administration of BDE-47 induced Cyp2b10 gene expression in a dose-dependent manner in mouse liver. Using gene KO mice, Cyp2b10 induction was shown to be CAR-dependent in mice. Furthermore, CYP2B6 induction in human primary hepatocytes by BDE-47 suggested that this chemical activates human CAR as well. We confirmed this hypothesis using a gene reporter assay utilizing a CAR-responsive sequence linked to a luciferase reporter gene. The results show that hCAR was able to be activated by BDE-47 in Huh7 cells.
Overall the results in this article clearly establish that BDE-47 activates nuclear receptor CAR to induce genes in human primary hepatocytes as well as in vivo in mice. In addition, these results also suggest that the mode of activation for CAR by BDE-47 may be different between species. Although CAR is indispensable for the activation of the Cyp2b10 gene in mouse liver by BDE-47 (Fig. 2), mCAR was not effectively activated in the gene reporter assay shown in Figure 5. Results using human primary hepatocytes showed CAR translocation into nuclei as well as CYP2B6 gene induction by BDE-47. These observations are consistent with the gene reporter assay results in Figure 5 which show effective activation of hCAR by BDE-47. Collectively, these results are possibly explained by that BDE-47 is a ligand activator of hCAR. Minimum activation of mouse CAR in reporter assays and effective activation of CAR in mouse liver by BDE-47 is similar to the pattern seen with PB-induced CAR activation. CAR was recently shown to be activated by PB through EGFR receptor activity modulation and eventual de-phosphorylation at a threonine residue in DNA binding domain (Mutoh et al.,2009, 2013). Future ligand-competition binding studies and detailed mechanistical studies will be needed to address molecular mechanisms of BDE-47 activation of human and mouse CAR.
Both mPXR and hPXR are activated by BDE-47 in reporter assays (Fig. 6). However, the assay results also showed a large difference in the efficacy of BDE-47 in activating these two receptors as compared to their respective positive controls. A similar difference between mPXR and hPXR activation in a BDE-47 reporter assay was reported previously (Pacyniak et al., 2007). Overall, CAR seems to be the major receptor for BDE-47 in mice while both CAR and PXR are activated by this chemical in humans. One limitation of our results concerning hCAR activation by BDE-47 is that our study employed only the CAR1 variant among the many reported variant forms of hCAR. Recent reports about di(2-ethylhexyl) phthalate (DEHP), a common plasticizer, activation of CAR 2 variants (DeKeyser et al., 2009, 2011) prompts further detailed analysis of receptor specificity. In the future, evaluation of CAR variant specificities against many PBDE congeners will be necessary.
CYP2B6 and CYP3A4 metabolize 3–15% and 50% of therapeutic drugs, respectively (Wang and Tompkins, 2008). Thus, if CYP2B6 and CYP3A4 genes are induced in human liver as we observed in human primary hepatocytes by BDE-47, it may affect human responses to numerous therapeutic drugs. Furthermore, not only BDE-47, but other congeners such as BDE-99 and BDE-209 have been reported to induce genes including CYP3A4 in human primary hepatocytes (Stapleton et al., 2009) and to activate human PXR (Pacyniak et al., 2007). Therefore individuals who have chronic exposure to PBDEs may have different responses to therapeutic drug treatments from those who have low or no exposure. Furthermore, many environmental chemicals (DeKeyser et al., 2009, 2011), natural products (Huang et al., 2004; Sueyoshi et al., 2011; Yeung et al., 2008)or even some growth factors (Mutoh et al., 2013)can affect both CAR and PXR or either one of these. Thus human response to BDE-47 should be assessed in the context of complex interactions among those factors.
We found 5-µM BDE-47 induced the CYP2B6 gene in human primary hepatocytes (Fig. 4) and 1-µM BDE-47 activated human CAR in luciferase reporter gene assays (Supplementary Figure S3). These concentrations of BDE-47 may not be very far from the highest possible human liver exposure in the North American population. The following amounts of BDE-47 were found as the highest values among human specimens: in adult blood, 1,388ng/g lipid (Morland et al., 2005), in toddler blood 350ng/g lipid (Stapleton et al., 2012), in adult adipose tissue 2,720ng/g lipid (Johnson-Restrepo et al., 2005). The molar concentration of BDE-47 at 2,720ng/g is approximately 5.5 µM if this were a water based solution. In autopsy liver samples, Meironyte Guvenius et al., (2001) found comparable amounts of BDE-47 in liver lipids compared to adipose tissue. In a mouse toxicokinetics study, the liver had 5–15 times less disposition of BDE-47 compared to adipose tissue between 24h and 21 days after the administration of a single 1mg/kg oral dose (as compared to contents in wet tissues) (Staskal et al., 2005). Thus the individual who has 5.5-µM BDE-47 in his/hers adipose tissue could have a liver BDE-47 exposure level in the sub µM to µM range and this is well within or close to our experimental conditions using primary hepatocytes or luciferase reporter assays with human liver derived culture cells.
A single 30mg/kg oral dose of BDE-47 induced Cyp2b10 in mouse liver as shown in Figure 1. After a single oral dose of 1mg/kg, BDE-47 generated a 1,662ng/g disposition peak in liver tissue after 3h (Staskal et al., 2005) which decreases to 355ng/g 24h after the dosing. Thus peak mouse liver exposure levels in our experimental condition may increase up to 100-µM BDE-47 if we calculate in the same way as we estimated that of human liver exposure on the basis that disposition in the liver proportionally increases up to the dose we employed. Therefore, in our experimental conditions, mouse liver was exposed in short pulses of high concentrations of BDE-47. In previous studies, four consecutive oral doses of 3mg/kg induced liver PROD activity and those of 10mg/kg increased Cyp2b10 mRNA expression levels (Staskal et al., 2005, 2006b). In a 10-day exposure study, in which 1mg/kg daily consecutive dosing employed, no PROD activity induction was observed (Staskal et al., 2006a). From these data we can estimate acute liver disposition around 10 µM is the lower limit to give Cyp2b10 induction and this is well consistent with our in vitro experiment results. At this point, information about actual human liver chronic disposition levels is limited. Given that semi chronic exposure results suggest bioaccumulation of BDE-47 (Staskal et al., 2006a) in rodents and other chronic conditions like fatty liver condition may affect BDE-47 partition in the liver in humans, the above mentioned highest observed 5.5-µM BDE-47 in human adipose tissue is close enough to the lower limit of Cyp2b10 induction in mice and warrants our study. Physiologically based pharmacokinetic models for chronic exposure to BDE-47 in rodents were developed (Emond et al., 2010) and development of those in humans is also underway. Once these are established, a better dosing scheme for rodents to mimic human chronic exposure conditions will be developed and these animal model results may be re-evaluated.
While we were preparing this manuscript, a report was published showing that BDE-47 was predominantly metabolized by CYP2B6 and converted into potentially toxic metabolites (Feo et al., 2012). Thus BDE-47 induces CYP2B6 in human liver and induced CYP2B6 metabolizes BDE-47. This reciprocal relationship between BDE-47 and CYP2B6 would make toxicokinetics of BDE-47 more complex in human liver than in animal models. In contrast to human CYP2B6, CYP2B1, which is a CAR regulated gene in rats, is suggested to play only a minor role in BDE-47 metabolism (Erratico et al., 2011). Many CYP450s which are not under the regulation of either CAR or PXR are involved in BDE-47 metabolism in rats based on results using rat liver microsome and recombinant CYP450s (Erratico et al., 2011). Therefore, in humans chronic exposure to BDE-47 may amplify the toxic effects of the substance as compared to the single dose exposure. In contrast, this type of reciprocal toxic amplification may not happen in rats.
The information in this report will assist in understanding the effects of PBDE chemicals on human health. Currently PBDEs are ubiquitous in the environment, and further studies of their potential for drug–drug interactions in humans are warranted. The finding here emphasizes a strong rationale for invoking both CAR and PXR in liver pathophysiology involving PDBE congeners.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
This study was supported by the Intramural Research Program of the NIH , National Institute of Environmental Health Sciences (NIEHS), Zo1Es71005-01, and National Cancer Institute (NCI) ZIA BC 011476. L.L and H.W. were supported by NIH grant DK061652. The BDE-47 was provided by the Synthesis Core of the Iowa Superfund Research program, funded by NIEHS/NIH grant ES013661. BDE-47 purity was analyzed by Izabela Kania-Korwel. Statsitical anlalysis evalulation was done by Grace Kissling at NIEHS. We thank Yin Li, Wendy Jefferson, and Bill Schrader for their critical reading of the manuscript.
REFERENCES
- Birnbaum L. S., Staskal D. F. (2004). Brominated flame retardants: Cause for concern? Environ. Health Perspect. 112, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. K. (2009). Activation of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by herbal medicines. AAPS J. 11, 590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeyser J. G., Laurenzana E. M., Peterson E. C., Chen T., Omiecinski C. J. (2011). Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol. Sci. 120, 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeyser J. G., Stagliano M. C., Auerbach S. S., Prabhu K. S., Jones A. D., Omiecinski C. J. (2009). Di(2-ethylhexyl) phthalate is a highly potent agonist for the human constitutive androstane receptor splice variant CAR2. Mol. Pharmacol. 75, 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans M. M., van den Berg M., Westerink R. H. (2011). Neurotoxicity of brominated flame retardants: (In)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ. Health Perspect. 119, 900–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Saha P. K., Huang W., Chen W., Abu-Elheiga L. A., Wakil S. J., Stevens R. D., Ilkayeva O., Newgard C. B., Chan L., et al. (2009). Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc. Natl. Acad. Sci. U.S.A. 106, 18831–18836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond C., Raymer J. H., Studabaker W. B., Garner C. E., Birnbaum L. S. (2010). A physiologically based pharmacokinetic model for developmental exposure to BDE-47 in rats. Toxicol. Appl. Pharmacol. 242, 290–298 [DOI] [PubMed] [Google Scholar]
- Erratico C. A., Moffatt S. C., Bandiera S. M. (2011). Comparative oxidative metabolism of BDE-47 and BDE-99 by rat hepatic microsomes. Toxicol. Sci. 123, 37–47 [DOI] [PubMed] [Google Scholar]
- Feo M. L., Gross M. S., McGarrigle B. P., Eljarrat E., Barcelo D., Aga D. S., Olson J. R. (2012). Biotransformation of BDE-47 to potentially toxic metabolites is predominantly mediated by human CYP2B6. Environ. Health Perspect. 121, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M., Vorkamp K., Thomsen M., Knudsen L. E. (2009). Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 212, 109–134 [DOI] [PubMed] [Google Scholar]
- Gao J., Xie W. (2010). Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab. Dispos. 38, 2091–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B., Hodgson E., Liddle C. (1999). The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol. Pharmacol. 56, 1329–1339 [DOI] [PubMed] [Google Scholar]
- Hernandez J. P., Mota L. C., Baldwin W. S. (2009). Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr. Pharmacogenomics Person. Med. 7, 81–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Adgent M., Goldman B. D., Sjodin A., Daniels J. L. (2012). Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ. Health Perspect. 120, 1438–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P., Sueyoshi T., Negishi M. (2003). Drug-activated nuclear receptors CAR and PXR. Ann. Med. 35, 172–182 [DOI] [PubMed] [Google Scholar]
- Honkakoski P., Zelko I., Sueyoshi T., Negishi M. (1998). The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 18, 5652–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Zhang J., Moore D. D. (2004). A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J. Clin. Invest. 113, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B., Kannan K., Rapaport D. P., Rodan B. D. (2005). Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ. Sci. Technol. 39, 5177–5182 [DOI] [PubMed] [Google Scholar]
- Kakizaki S., Takizawa D., Tojima H., Horiguchi N., Yamazaki Y., Mori M. (2011). Nuclear receptors CAR and PXR: Therapeutic targets for cholestatic liver disease. Front. Biosci. 16, 2988–3005 [DOI] [PubMed] [Google Scholar]
- Kamino H., Negishi M. (2012). The nuclear receptor constitutive active/androstane receptor arrests DNA-damaged human hepatocellular carcinoma Huh7 cells at the G2/M phase. Mol. Carcinog. 51, 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Sueyoshi T., Zelko I., Moore R., Washburn K., Negishi M. (1999). Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 19, 6318–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D., Slitt A. L. (2005). Regulation of hepatic transporters by xenobiotic receptors. Curr. Drug Metab. 6, 309–328 [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Moore J. T., Wade L., Staudinger J. L., Watson M. A., Jones S. A., McKee D. D., Oliver B. B., Willson T. M., Zetterstrom R. H., et al. (1998). An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92, 73–82 [DOI] [PubMed] [Google Scholar]
- Kodama S., Koike C., Negishi M., Yamamoto Y. (2004). Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 24, 7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S., Negishi M. (2011). Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J. Biol. Chem. 286, 3570–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti P. R., Coburn C. G., Moser V. C., MacPhail R. C., Fenton S. E., Stoker T. E., Rayner J. L., Kannan K., Birnbaum L. S. (2010). Developmental exposure to a commercial PBDE mixture, DE-71: Neurobehavioral, hormonal, and reproductive effects. Toxicol. Sci. 116, 297–312 [DOI] [PubMed] [Google Scholar]
- Konno Y., Moore R., Kamiya N., Negishi M. (2010). Nuclear xenobiotic receptor PXR-null mouse exhibits hypophosphatemia and represses the Na/Pi-cotransporter SLC34A2. Pharmacogenet. Genomics 20, 9–17 [DOI] [PubMed] [Google Scholar]
- LaGuardia M. J., Hale R. C., Harvey E. (2006). Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ. Sci. Technol. 40, 6247–6254 [DOI] [PubMed] [Google Scholar]
- LeCluyse E. L., Alexandre E., Hamilton G. A., Viollon-Abadie C., Coon D. J., Jolley S., Richert L. (2005). Isolation and culture of primary human hepatocytes. Methods Mol. Biol. 290, 207–229 [DOI] [PubMed] [Google Scholar]
- Li H., Chen T., Cottrell J., Wang H. (2009). Nuclear translocation of adenoviral-enhanced yellow fluorescent protein-tagged-human constitutive androstane receptor (hCAR): A novel tool for screening hCAR activators in human primary hepatocytes. Drug Metab. Dispos. 37, 1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chen T., Stanton J. D., Sueyoshi T., Negishi M., Wang H. (2008). The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol. Pharmacol. 74, 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Sinz M. W., Zimmermann K., Wang H. (2012). An insulin-like growth factor 1 receptor inhibitor induces CYP3A4 expression through a pregnane X receptor-independent, noncanonical constitutive androstane receptor-related mechanism. J. Pharmacol. Exp. Ther. 340, 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M. (2008). Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 18, 2–19 [DOI] [PubMed] [Google Scholar]
- Maglich J. M., Parks D. J., Moore L. B., Collins J. L., Goodwin B., Billin A. N., Stoltz C. A., Kliewer S. A., Lambert M. H., Willson T. M., et al. (2003). Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 278, 17277–17283 [DOI] [PubMed] [Google Scholar]
- Meironyte Guvenius D., Bergman A., Noren K. (2001). Polybrominated diphenyl ethers in Swedish human liver and adipose tissue. Arch. Environ. Contam. Toxicol. 40, 564–570 [DOI] [PubMed] [Google Scholar]
- Morland K. B., Landrigan P. J., Sjodin A., Gobeille A. K., Jones R. S., McGahee E. E., Needham L. L., Patterson D. G., Jr. (2005). Body burdens of polybrominated diphenyl ethers among urban anglers. Environ. Health Perspect. 113, 1689–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S., Osabe M., Inoue K., Moore R., Pedersen L., Perera L., Rebolloso Y., Sueyoshi T., Negishi M. (2009). Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR1I3). J Biol Chem. 284, 34785–34792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S., Sobhany M., Moore R., Perera L., Pedersen L., Sueyoshi T., Negishi M. (2013). Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci. Signal. 6, ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Moore R., Negishi M., Sueyoshi T. (2007). Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J. Biol. Chem. 282, 9768–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norström Å., Andersson K., Rappe C. (1976). Major components of some brominated aromatics used as flame retardants. Chemosphere 5, 255–261 [Google Scholar]
- Oern U., Eriksson L., Jakobsson E., Bergman A. (1996). Synthesis and characterization of polybrominated diphenyl ethers—Unlabeled and radiolabeled tetra-, penta- and hexabromodiphenyl ethers. Acta Chem. Scand. 50, 802–807 [Google Scholar]
- Pacyniak E. K., Cheng X., Cunningham M. L., Crofton K., Klaassen C. D., Guo G. L. (2007). The flame retardants, polybrominated diphenyl ethers, are pregnane X receptor activators. Toxicol. Sci. 97, 94–102 [DOI] [PubMed] [Google Scholar]
- Richardson V. M., Staskal D. F., Ross D. G., Diliberto J. J., DeVito M. J., Birnbaum L. S. (2008). Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol. Appl. Pharmacol. 226, 244–250 [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Eagle S., Sjodin A., Webster T. F. (2012). Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 120, 1049–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Kelly S. M., Pei R., Letcher R. J., Gunsch C. (2009). Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ. Health Perspect. 117, 197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal D. F., Diliberto J. J., Birnbaum L. S. (2006a). Impact of repeated exposure on the toxicokinetics of BDE 47 in mice. Toxicol. Sci. 89, 380–385 [DOI] [PubMed] [Google Scholar]
- Staskal D. F., Diliberto J. J., DeVito M. J., Birnbaum L. S. (2005). Toxicokinetics of BDE 47 in female mice: Effect of dose, route of exposure, and time. Toxicol. Sci. 83, 215–223 [DOI] [PubMed] [Google Scholar]
- Staskal D. F., Hakk H., Bauer D., Diliberto J. J., Birnbaum L. S. (2006b). Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol. Sci. 94, 28–37 [DOI] [PubMed] [Google Scholar]
- Sueyoshi T., Green W. D., Vinal K., Woodrum T. S., Moore R., Negishi M. (2011). Garlic extract diallyl sulfide (DAS) activates nuclear receptor CAR to induce the Sult1e1 gene in mouse liver. PloS ONE 6, e21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T., Kawamoto T., Zelko I., Honkakoski P., Negishi M. (1999). The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J. Biol. Chem. 274, 6043–6046 [DOI] [PubMed] [Google Scholar]
- Szabo D. T., Richardson V. M., Ross D. G., Diliberto J. J., Kodavanti P. R., Birnbaum L. S. (2009). Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol. Sci. 107, 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit Y. E., Negishi M. (2007). CAR and PXR: The xenobiotic-sensing receptors. Steroids 72, 231–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson A. H., Wang H. (2010). Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv. Drug Deliv. Rev. 62, 1238–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A., Kakizaki S., Negishi M., Sueyoshi T. (2002). Residue threonine 350 confers steroid hormone responsiveness to the mouse nuclear orphan receptor CAR. Mol. Pharmacol. 61, 1284–1288 [DOI] [PubMed] [Google Scholar]
- Wada T., Gao J., Xie W. (2009). PXR and CAR in energy metabolism. Trends Endocrinol. Metab. 20, 273–279 [DOI] [PubMed] [Google Scholar]
- Wang H., Tompkins L. M. (2008). CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr. Drug Metab. 9, 598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Li L., Yang H., Ferguson S. S., Baer M. R., Gartenhaus R. B., Wang H. (2013). The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood 121, 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Moore R., Flavell R. A., Lu B., Negishi M. (2010). Nuclear receptor CAR represses TNFalpha-induced cell death by interacting with the anti-apoptotic GADD45B. PloS ONE 5, e10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Moore R., Goldsworthy T. L., Negishi M., Maronpot R. R. (2004). The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 64, 7197–7200 [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Kakizaki S., Horiguchi N., Sohara N., Sato K., Takagi H., Mori M., Negishi M. (2007). The role of the nuclear receptor constitutive androstane receptor in the pathogenesis of non-alcoholic steatohepatitis. Gut 56, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y., Moore R., Negishi M. (2011). Nuclear receptor CAR (NR1I3) is essential for DDC-induced liver injury and oval cell proliferation in mouse liver. Lab. Invest. 91, 1624–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E. Y., Sueyoshi T., Negishi M., Chang T. K. (2008). Identification of Ginkgo biloba as a novel activator of pregnane X receptor. Drug Metab. Dispos. 36, 2270–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.