Abstract
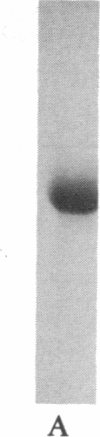
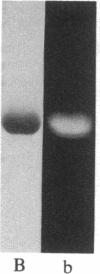
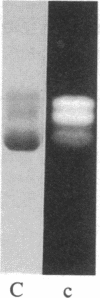
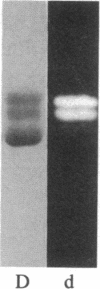
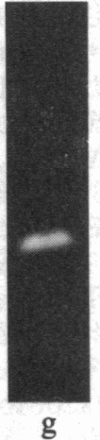
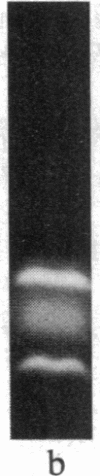
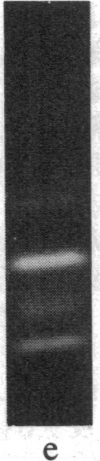
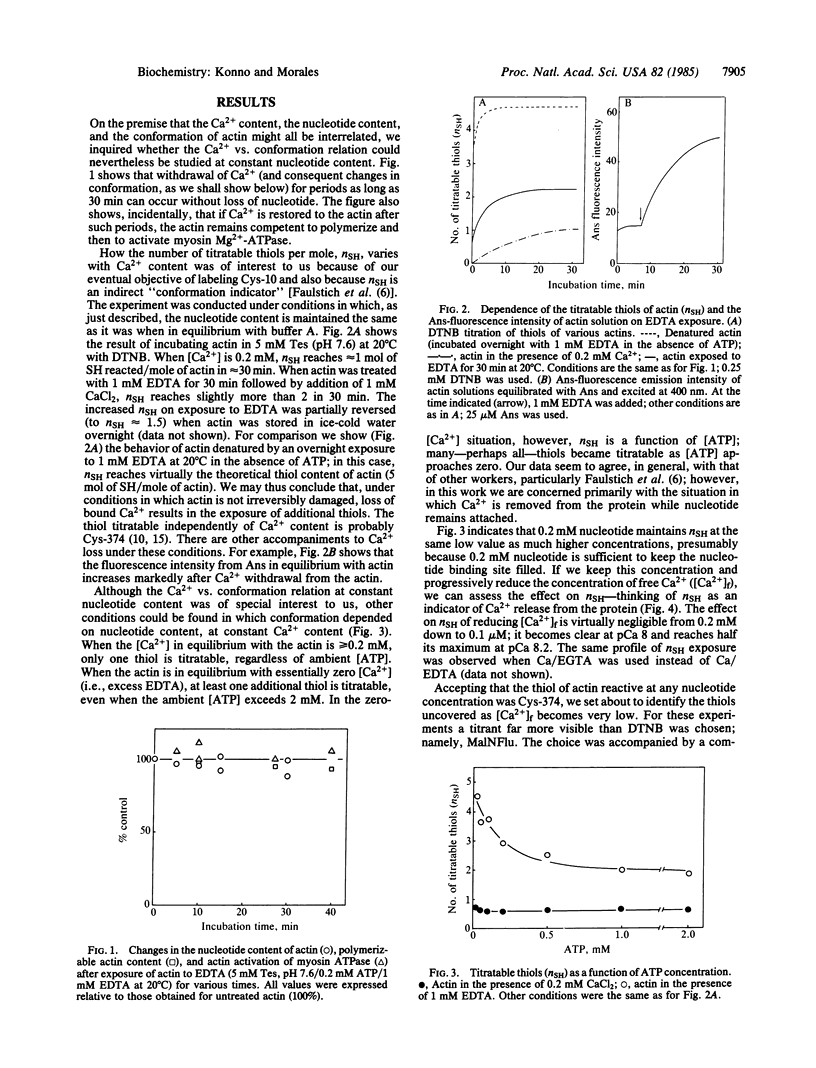
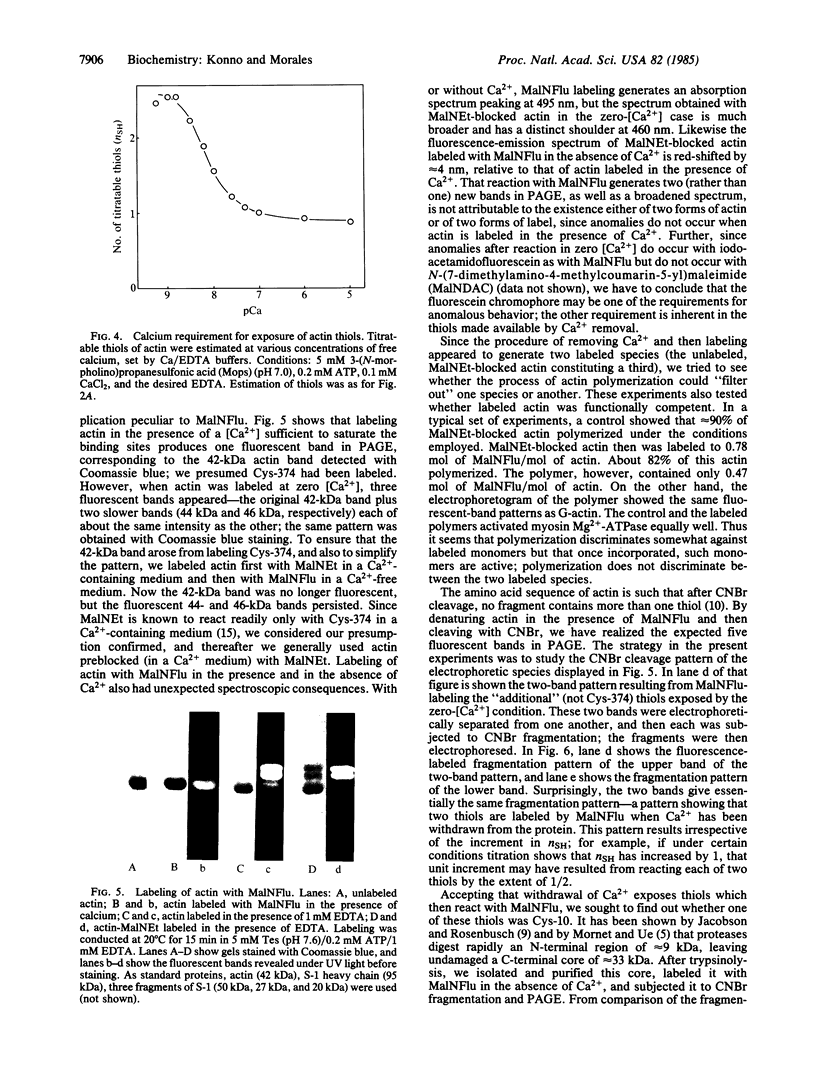
The removal of bound metal ions from G-actin uncovered two thiols, Cys-10 and Cys-257. The uncovering of these thiols requires a free calcium concentration lower than 10 nM. Therefore, participation of one or both thiols in Ca2+ binding is suggested. Actin labeled with N-(5-fluoresceinyl)maleimide in the absence of calcium moves as a doublet in NaDodSO4/PAGE. It is suggested that two conformers are induced by metal removal and labeling.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock D. F. Examination of the intracellular ionic environment and of ionophore action by null point measurements employing the fluorescein chromophore. J Biol Chem. 1983 May 25;258(10):6380–6389. [PubMed] [Google Scholar]
- Botts J., Takashi R., Torgerson P., Hozumi T., Muhlrad A., Mornet D., Morales M. F. On the mechanism of energy transduction in myosin subfragment 1. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2060–2064. doi: 10.1073/pnas.81.7.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani Y., Patchornik A. Cyanylation of sulfhydryl groups by 2-nitro-5-thiocyanobenzoic acid. High-yield modification and cleavage of peptides at cysteine residues. Biochemistry. 1974 Jan 1;13(1):1–11. doi: 10.1021/bi00698a001. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Merkler I., Blackholm H., Stournaras C. Nucleotide in monomeric actin regulates the reactivity of the thiol groups. Biochemistry. 1984 Apr 10;23(8):1608–1612. doi: 10.1021/bi00303a004. [DOI] [PubMed] [Google Scholar]
- Frieden C., Lieberman D., Gilbert H. R. A fluorescent probe for conformational changes in skeletal muscle G-actin. J Biol Chem. 1980 Oct 10;255(19):8991–8993. [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. ATP binding to a protease-resistant core of actin. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2742–2746. doi: 10.1073/pnas.73.8.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H. The reaction of a positively-charged N-ethylmaleimide derivative with actin and myosin subfragment-1. Int J Biochem. 1981;13(7):871–873. doi: 10.1016/0020-711x(81)90109-9. [DOI] [PubMed] [Google Scholar]
- Kasai M., Oosawa F. The exchangeability of actin-bound calcium with various divalent cations. Biochim Biophys Acta. 1968 Apr 9;154(3):520–528. doi: 10.1016/0005-2795(68)90012-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mornet D., Ue K. Proteolysis and structure of skeletal muscle actin. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3680–3684. doi: 10.1073/pnas.81.12.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. A., Estes J. E. Detection of conformational changes in actin by proteolytic digestion: evidence for a new monomeric species. J Mol Biol. 1976 Jul 15;104(4):777–792. doi: 10.1016/0022-2836(76)90181-9. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Sutoh K. Actin-actin and actin-deoxyribonuclease I contact sites in the actin sequence. Biochemistry. 1984 Apr 24;23(9):1942–1946. doi: 10.1021/bi00304a009. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry. 1983 Mar 29;22(7):1579–1585. doi: 10.1021/bi00276a009. [DOI] [PubMed] [Google Scholar]
- Takashi R. Fluorescence energy transfer between subfragment-1 and actin points in the rigor complex of actosubfragment-1. Biochemistry. 1979 Nov 13;18(23):5164–5169. doi: 10.1021/bi00590a021. [DOI] [PubMed] [Google Scholar]
- Waechter F., Engel J. The kinetics of the exchange of G-actin-bound 1: N6-ethenoadenosine 5'-triphosphate with ATP as followed by fluorescence. Eur J Biochem. 1975 Sep 15;57(2):453–459. doi: 10.1111/j.1432-1033.1975.tb02320.x. [DOI] [PubMed] [Google Scholar]