Abstract
Background
G-protein coupled receptor kinase 2 (GRK2) is a primary regulator of β-adrenergic signaling in the heart. GRK2 ablation ameliorates heart failure development but the cellular mechanisms have not been elucidated and are the aim of this study.
Methods and Results
Myocyte contractility, Ca2+ handling and excitation-contraction coupling were studied in isolated cardiomyocytes from wild-type (WT) and GRK2 knockout (GRK2KO) mice without (sham) or with myocardial infarction (MI). In cardiac myocytes isolated from unstressed WT and GRK2KO hearts, myocyte contractions and Ca2+ transients were similar but GRK2KO myocytes had lower sarcoplasmic reticulum (SR) Ca2+ content due to increased sodium-Ca2+ exchanger (NCX) activity and inhibited SR Ca2+ ATPase by local PKA mediated activation of PDE4 resulting in hypophosphorylated phospholamban. This Ca2+ handling phenotype is explained by a higher fractional SR Ca2+ release induced by increased L-type Ca2+ channel currents (ICa,L). After β-adrenergic stimulation, GRK2KO myocytes revealed significant increases in contractility and Ca2+ transients, which were not mediated through cardiac L-type Ca2+ channels (LTCCs), but through an increased SR Ca2+. Interestingly, post-MI GRK2KO mice showed better cardiac function than post-MI control mice, which is explained by an improved Ca2+ handling phenotype. The SR Ca2+ content was better maintained in post-MI GRK2KO myocytes than in post-MI control myocytes, due to better maintained ICa,L density and no increase in NCX in GRK2KO myocytes. An LTCC blocker, verapamil, reversed some beneficial effects of GRK2KO.
Conclusions
These data argue for novel differential regulation of ICa,L and SR load by GRK2. GRK2 ablation represents a novel beneficial Ca2+ handling phenotype resisting adverse remodeling after MI.
Keywords: calcium, experimental models heart failure, heart failure, Excitation Contraction Coupling, G-Protein Coupled Receptor Kinase 2
Introduction
Alterations in myocyte contractility and Ca2+ cycling are hallmarks of the progression of heart failure (HF). In failing cardiac myocytes, the SR Ca2+ content is decreased and the Ca2+ transient is decreased and prolonged, resulting in depressed myocyte contractility1. This is generally considered to be a consequence of increased sodium– Ca2+ exchanger (NCX) activity, reduced sarcoplasmic (SR) reticulum Ca2+ ATPase (SERCA2a) expression and activity (probably due to decreased phospholamban (PLB) phosphorylation), and increased PLB/SERCA2a ratio, as well as an augmented open probability of the ryanodine receptor (RyR), causing SR Ca2+ leak 2, 3. Alterations of the L-type Ca2+ channel (LTCC), the trigger for the Ca2+ induced-SR Ca2+ release, also contribute to the pathophysiological changes in Ca2+ homeostasis in failing myocytes4. In addition to these changes causing dysfunctional contractile performance, the rise of diastolic Ca2+ may increase the risk of arrhythmias and induce pathological cardiac remodeling5.
The increased activity of the sympathetic nervous system associated with HF is a compensation to normalize cardiac function by enhancing Ca2+ cycling and maximize contractile force through the β-adrenergic signaling pathway. In acute HF these changes can improve systemic perfusion, whereas in chronic HF the augmentation in catecholamines is associated with mortality6 and results in a down-regulation of β-adrenergic receptors (βARs) promoted by upregulated G protein-coupled receptor kinase 2 (GRK2). GRK2 is the primary GRK in the heart and a prototype regulator of βAR signaling7. We have previously identified GRK2 as a culprit in the progression of HF and GRK2 inhibition (by expression of its c-terminal domain, called βARKct) or gene silencing has rescued disparate models of HF8-10. Our recent study indicates the benefits of βARKct could be related to enhanced myocyte contractility by increasing LTCC currents and its responsiveness to β-adrenergic agonists11. However, the exact underlying cellular mechanisms for these beneficial effects in HF after βARKct expression or GRK2 ablation are not clearly defined. It is especially important to define these mechanisms since in light of the recent success of βAR blocker therapy in clinical HF management, the results with βARKct and GRK2 silencing appear paradox, as the major function of GRK2 in cardiac myocytes is to dampen βAR signaling, similar to βAR blockade. We have found that βARKct expression can cause a molecular remodeling of the cardiac βAR system with receptor up-regulation and improved βAR signaling, and a recent study with chronic AAV-mediated βARKct expression in a rat HF model showed that myocardial βAR changes are probably down-stream of neurohormonal lowering including reduction in sympathetic nervous system activity12. In this regard, the role of GRK2 inhibition must mechanistically go beyond resensitizing βARs and fully understanding GRK2-dependent signaling pathways might enlighten novel therapeutic targets.
To date, little is known about how GRK2 specifically alters cardiac myocyte function and Ca2+ cycling in normal and failing cardiac myocytes. The present study was designed to define the role of GRK2 and GRK2-dependent signaling in excitation-contraction coupling (ECC) in normal and diseased hearts. We used our previously characterized cardiac-specific GRK2 knockout mice10, 13 to study myocyte ECC coupling and Ca2+ homeostasis in cardiac myocytes from mice with or without post-MI ischemic HF. We demonstrate for the first time that loss of GRK2 induces a distinct Ca2+ handling phenotype: myocyte contractility and Ca2+ handling are normal even though the SR Ca2+ content is reduced because there is an increased LTCC activities and resulting increases in ICa,L with a compensatory increase in NCX activity. This Ca2+ handling phenotype brought about by GRK2 ablation is resistant to adverse Ca2+ handling remodeling after MI and leads to better cardiac function in GRK2 KO mice post MI.
Materials and Methods
Conditional mice bearing floxed GRK2 alleles were described previously10, 13. GRK2KO (αMHC-Cre × GRK2fl/fl) and WT (GRK2fl/fl) mice were maintained on a C57BL6 genetic background. All animal procedures and experiments were performed in accordance with the guidelines of the IACUC of Thomas Jefferson University. GRK2KO and WT mice were 8-10 weeks of age when entering the study. Unstressed normal mice and mice with coronary artery ligation (myocardial infarction (MI)) or sham-operation were studied. MI was induced by ligating the left anterior descending coronary artery at 2-3 mm below its origin as described previously10, 14 and animals were studied 28 days post-MI or sham-operation. For the verapamil study, mice were treated with verapamil starting 14 days post MI or sham-operation until the end of the study period (42 days post MI). Verapamil treated mice received oral supplementation of verapamil (Sigma-Aldrich, St. Louis, Missouri, USA) as described previously15 and were evaluated by echocardiography (ECHO). Cardiac myocytes were isolated cultured from animals. Myocyte Ca2+ transients and contractions, SR Ca2+ load, LTCC currents (ICa,L), and INCX were measured. Quantitative real-time polymerase chain reaction and Western blot analysis were done for gene expression assessment. Detailed description of experimental procedures is available in the expanded online-only data supplement.
Data are expressed as mean±SEM. An unpaired two tailed t-test or a one-way ANOVA and a two-way ANOVA (linear mixed effects model) were performed with SAS 9.3 for between-group comparisons followed by a post-hoc Bonferroni adjustment. For all tests, a P value < 0.05 was considered significant.
Results
Myocyte GRK2 ablation enhances adrenergic responsiveness of cellular contractility and Ca2+ transients
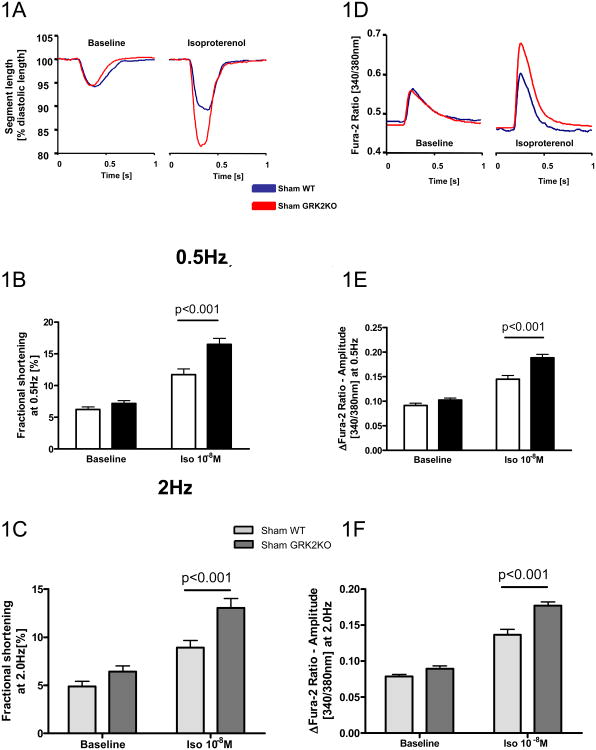
Our previous study has shown that GRK2 KO mice have comparable cardiac function as control mice at baseline (Supplemental Table 1) but their cardiac function responds to β-adrenergic stimulation better10. Here we determine the cellular mechanisms for this observation. Myocyte contraction and intracellular Ca2+ transients were recorded from WT and GRK2KO myocytes under baseline conditions and after βAR stimulation. Myocytes from both lines revealed a similar fractional shortening when paced at both 0.5Hz and 2Hz (Figure 1A-C). However, after isoproterenol (ISO), myocytes from GRK2 KO mice showed a significantly greater increase in fractional shortening (Figure 1A-C, Supplemental Table 2). In a good agreement, as shown in Figure 1D, at baseline, the characteristics and amplitude of the Ca2+ transients (as βFura-2 340/380nm ratio) were similar in both groups of myocytes. Stimulation with ISO mediated an anticipated increase in the amplitude of the Fura-2 ratio in WT cardiac myocytes and a greater increase in GRK2KO cardiac myocytes (Figure 1D-F, Supplemental Table 2). We observed no differences in the Ca2+ transient decay time at baseline and after ISO between groups.
Figure 1.
The loss of GRK2 in myocytes enhances the responsiveness of myocyte contraction and Ca2+ transients after bAR stimulation. A, Representative tracings of single myocyte contractions under basal conditions and stimulation with isoproterenol at 0.5 Hz. Averaged myocyte fractional shortening at 0.5 Hz (B) and 2.0 Hz (C) stimulation frequencies under basal conditions and stimulation with isoproterenol. D, Representative intracellular Ca2+ transients measured with Fura-2 [340/380nm ratio] under basal conditions and stimulation with isoproterenol at 0.5 Hz from the same cell as in A. Averaged Fura-2 ratio amplitude at 0.5 Hz (E) and 2.0 Hz (F) stimulation frequencies under basal conditions and isoproterenol. For measurements represented by B, C, E and F; a total of 48-63 cardiac myocytes from 3 different hearts were measured for each group; two-way ANOVA was used for B, C, E and F.
GRK2 silencing in myocytes enhances ECC efficiency
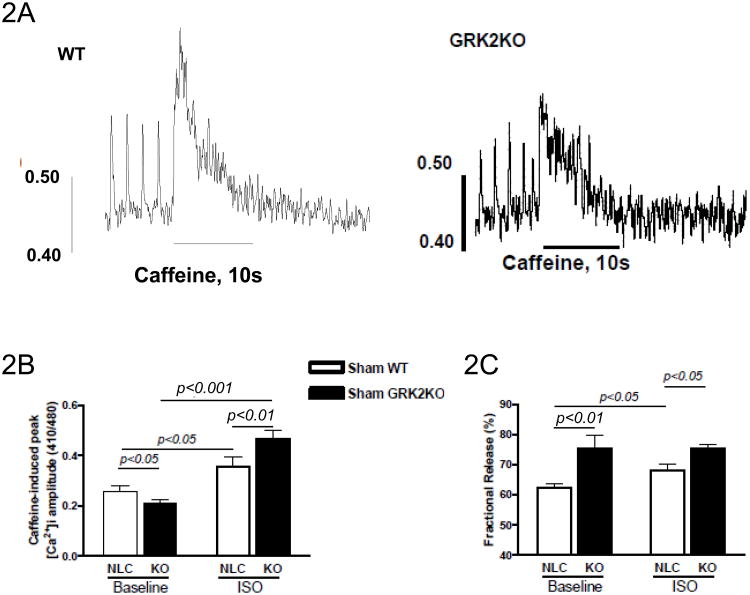
Myocyte contractility and Ca2+ transients are determined by Ca2+ release from the SR16. We measured SR Ca2+ content by caffeine spritz at baseline or after ISO stimulation. Figure 2A shows representative tracings of cytosolic Ca2+ transients (measured with indo-1 AM) induced by a rapid application of caffeine (caffeine-spritz) after 4 field stimulations in WT and GRK2KO cardiac myocytes to measure the SR Ca2+ load. At baseline SR Ca2+ load is less in GRK2 myocytes than in control myocytes (Figure 2B); in response to ISO, the SR Ca2+ load in cardiac myocytes from both WT and GRK2KO mice was significantly increased but GRK2KO myocytes had a greater increase (Figure 2B). Interestingly, a significantly higher fractional release of Ca2+ was observed in GRK2KO cardiac myocytes compared to WT myocytes at both baseline and after ISO (Figure 2C, Supplemental Table 2).
Figure 2.
GRK2 silencing in cardiac myocytes alters SR Ca2+ content and its regulation. A, Representative tracings of the caffeine-induced intracellular Ca2+ transients determined with Indo-1 in a control myocyte and a GRK2KO myocyte. B, Caffeine-induced peak intracellular Ca2+ amplitudes under baseline conditions and isoproterenol stimulation. C, Fractional release calculated as the ratio of peak Ca2+ concentration induced by field stimulation to peak Ca2+ concentration induced by caffeine. For measurements represented by figures B-C; a total of 16-29 cardiac myocytes from 3 different hearts were analyzed per group; two-way ANOVA with post hoc student t-test for B and C.
Loss of myocyte GRK2 enhances ICa,L by a local PKA-dependent mechanism but blunts its βAR responsiveness
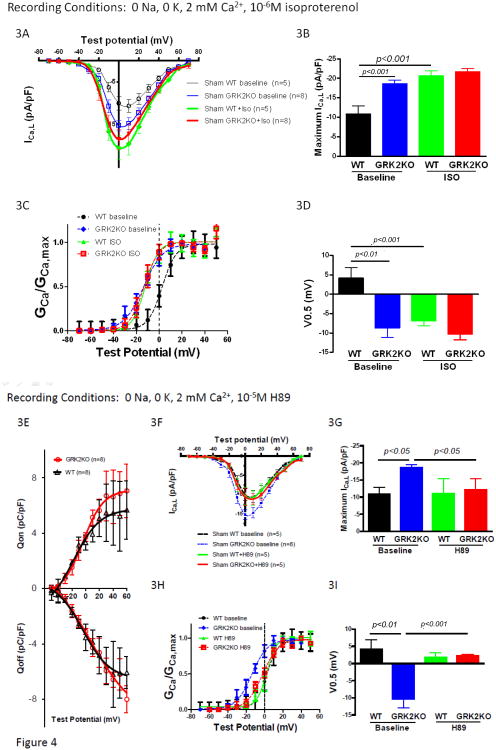
To explore the underlying cellular mechanisms for the reduced SR Ca2+ content while enhanced EC coupling efficiency in GRK2 KO myocytes, we measured the LTCC current (ICa,L) in GRK2 KO and control myocytes because ICa,L serves as both the trigger of Ca2+ release from the SR and the source for loading the SR.16. Peak ICa,L density at baseline was significantly greater in GRK2KO compared to WT cardiac myocytes (Figure 3A and B, Supplemental Table 2). When stimulated with a saturating dose of ISO (10-6M), peak ICa,L was increased to the same level in cardiac myocytes from both mouse lines (Figure 3A and B), suggesting that the LTCC density was similar in both groups4. The voltage-dependency of channel activation was shifted to more negative voltages in GRK2KO myocytes at baseline and ISO stimulation caused a significant leftward shift of activation in WT myocytes but not in GRK2KO myocytes (Figure 3C and D). These results imply that the LTCC in GRK2KO myocytes could be in such a high activity state that it loses responsiveness to β-adrenergic stimulation.
Figure 3.
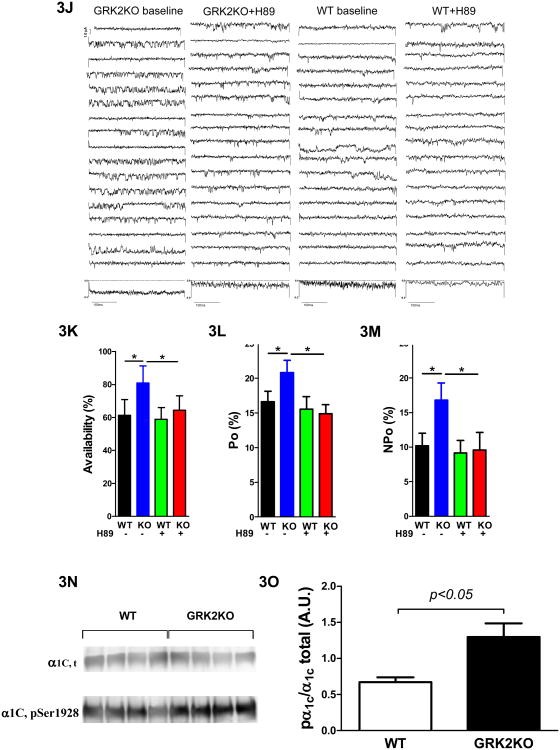
GRK2 silencing in myocytes increases basal ICa,L by enhancing PKA-dependent augmentation of LTCC channel activity but reduces its responsiveness to b-adrenergic stimulation. A, Current-voltage relationships of ICa,L (pA/pF) under basal conditions and isoproterenol stimulation. B, Peak ICa,L under basal conditions and isoproterenol stimulation. C, Voltage dependence of ICa,L activation at basal conditions and under isoproterenol stimulation. D, Voltage potentials at 50% ICa,L activation in WT and KO myocytes before and after ISO. E, Charge movement measurements in control and KO myocytes at baseline, showing no difference in available LTCCs on the surface membrane in KO and WT myocytes. F, The effect of H89 on the current-voltage relationships of ICa,L in sham WT control and KO myocytes. H89 significantly decreased ICa,L at most tested voltages in KO myocytes but not in WT myocytes. G, Effects of H89 on maximum ICa,L in WT and KO myocytes. H & I, Effects of H89 on V0.5 of ICa,L activation in WT and KO myocytes. J, Examples of single channel recordings of the LTCC in KO and WT myocytes before and after the treatment of H89. Sweeps were selected in an increment of 20, i.e., the sweeps shown were sweep 1, 20, 40, etc. The downward deflections were the openings of the channel. At the bottom of the raw sweeps were the ensemble average current of all 400 sweeps recorded. The availability (K), open probability in active sweeps (L) and the overall open probability (NPo shown in M) were significantly increased in KO myocytes but were normalized by H89. H89 did not have significant effects on these parameters of single LTCC activities in WT myocytes. N, Western blot of immunoprecipated total a1c and phosphorylated a1c at ser1928. O, Phosphorylation level of a1c in unstressed WT and GRK2KO hearts. n=5 animals/group, two-way ANOVA with post hoc t-test was used for comparison for A-M in this figure; t-test was used for O.
To explore the underlying mechanisms for these changes of ICa,L properties, we tested whether the increased ICa,L was due to increased available channels on the surface membrane and/or the increase of channel activities at single channel levels. Charge movement was used to quantitate the amount of available channels on the surface membrane of KO and control WT myocytes. Figure 3E shows that there was no significant difference in the charge movements induced by various depolarizing voltages, indicating that there was no significant alteration of LTCC density on the membrane and the increased whole-cell ICa,L could be due to the increased single channel activities. Immunoprecipitation of α1c, the pore forming subunit of the LTCC, from the same amount of proteins from GRK2KO and control hearts showed no difference in α1c expression (Figure 3N). These data suggest that the LTCC in GRK2KO myocytes must have higher than normal activity, which was supported by single channel recording of LTCC activities (Figure 3J-M). The availability (Figure 3K) and the open probabilities (Figure 3L and M) were significantly increased in GRK2KO myocytes, which could fully explain the increase in whole cell ICa,L in KO myocytes.
Enhanced phosphorylation of the LTCC by protein kinase A (PKA) may result in increased LTCC activity4, 17. Previously we have shown that βARs carries constitutive activation to activate PKA locally to phosphorylate the LTCC18. Here we tested if high LTCC activity was mediated by a PKA dependent mechanism. H89, a PKA specific inhibitor, was used and it normalized the current density and voltage-dependent activation of the LTCC in KO myocytes but had no significant effect on LTCCs in WT myocytes (Figure 3F-I). The single channel study further confirmed that the heightened LTCC activity in the KO myocytes was due to PKA activation because H89 also normalized the increased channel activity in KO myocytes (Figure 3J-M). The phosphorylation of α1c at Ser1928, a PKA site, was shown to be more in GRK2KO hearts than in control hearts (Figure 3N and O).
Loss of GRK2 in myocytes increases Na+/Ca2+ exchanger expression
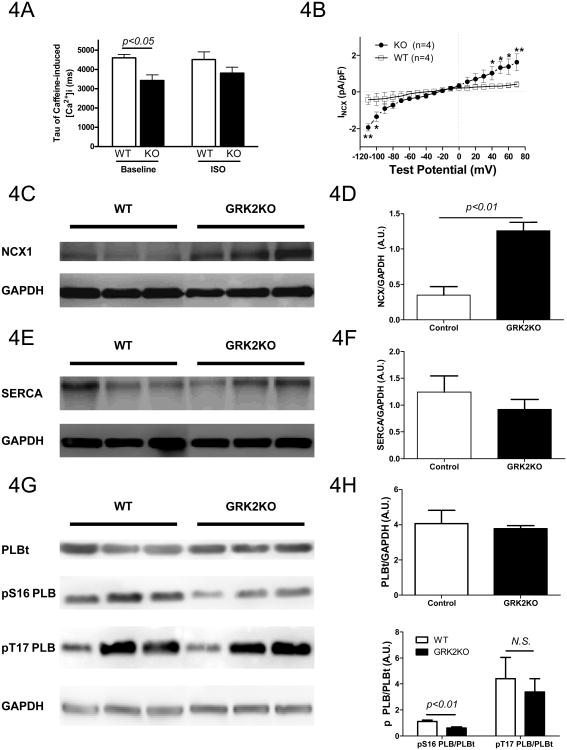
Since there is a decreased SR Ca2+ content with increased ICa,L in GRK2KO myocytes, there should be increased Ca2+ efflux out of GRK2KO myocytes. In ventricular myocytes, the major route of Ca2+ efflux is through the sodium-Ca2+ exchanger (NCX). One way to measure NCX activity is to examine the decay rate of caffeine-induced Ca2+ transients that can be fit by a single exponential decay equation19. The tau value was significantly smaller in the GRK2 KO myocytes than in control myocytes and ISO did not change these values (Figure 4A), suggesting that the NCX activity at baseline is increased in GRK2KO myocytes compared to WT myocytes, which was confirmed by direct measurement of NCX current (Figure 4B) and NCX protein expression (Figure 4C and D).
Figure 4.
GRK2 silencing in cardiac myocytes increased NCX expression, reduced PLB phosphorylation by PKA dependent PDE4 activation and enhanced responsiveness to ISO. A, Tau values of caffeine-induced intracellular Ca2+ transient decay, indicating increased NCX activity in GRK2KO myocytes; n=16-29 cardiac myocytes from 3 different hearts. B, NCX currents at baseline in control (WT) and GRK2KO myocytes; n=4 animals/group, two-way repeated ANOVA with post-hoc student t-test. C, Western blot of NCX proteins in WT and GRK2KO hearts. D, NCX expression normalized to GAPDH. E and F, SERCA expression in WT and GRK2KO hearts. G and H, PLB expression and phosphorylation at Ser16 (PKA site) and Thr17 (CaMK II site) in WT and GRK2KO hearts; n=5 animals/group D, F, and H; t-test for D, F, and H. I and J, PLB phosphorylation at Ser16 in response to different concentrations of ISO; n=5animals/group; data were analyzed by regression with repeated measures for J. K and L, Western blotting for pSer16-PLB and total PLB in WT and GRK2KO hearts stimulated with or without the selective PDE4 inhibitor rolipram; a total of 3-5 hearts were analyzed for each group; one-way ANOVA for L.
Loss of myocytes GRK2 decreases basal phospholamban (PLB) phosphorylation but increases its responsiveness to βAR stimulation
The loading of the SR with Ca2+ depends on the competition between the extrusion of Ca2+ out of the cell (mainly through NCX) and the resequestration of Ca2+ into SR by SERCA which is regulated by PLB16. Dephosphorylated PLB exerts a tonic inhibition on SERCA activity. For these reasons, we determined the expression level of SERCA and PLB as well as the phosphorylation level of PLB. The expression of SERCA and PLB was not significantly altered by silencing GRK2 (Figure 4E-H). However, the phosphorylation of PLB at Ser16, a PKA site, was significantly reduced in GRK2KO hearts but the phosphorylation of PLB at Thr17 site was not altered (Figure 4G and H). When myocytes were stimulated with the βAR agonist, ISO, a robust increase in the phosphorylation of PLB at Ser16 sites was observed in both WT and GRK2KO cardiac myocytes (Figure 4I and J). However, a significant leftward shift of the dose response curve was found in GRK2KO myocytes compared to WT cells (Figure 4J), demonstrating higher βAR sensitivity in cells isolated from unstressed GRK2KO mice.
To further clarify the mechanism responsible for the hypophosphorylation of PLB we examined whether PDE 4, which is activated by PKA, could play a mechanistic role. A PDE 4 specific inhibitor rolipram (10mg/kg BW, i.p.) was injected into unstressed WT and GRK2KO mice for 2 hours to allow it to take effect. Then the hearts were snap-frozen in liquid nitrogen and Western blotting for phospho-PLB and total PLB was performed. Interestingly, rolipram blunted the hypophosphorylation of PLB in GRK2KO myocytes (Figures 4K and L), which indicates that the proposed higher PKA activity in GRK2KO myocytes results in an increased local PDE4 activity causing hypophosphorylation of PLB.
Loss of myocyte GRK2 before MI prevents the development of heart failure and preserves myocytes contractility
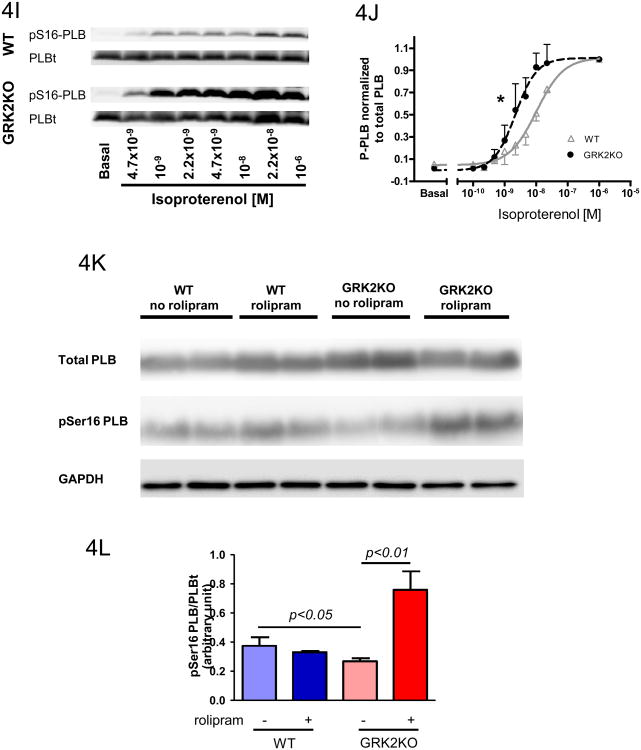
Our previous studies have shown that cardiac specific loss of GRK2 ameliorates the development of heart failure after MI10. In vivo cardiac function as assessed by echocardiography 28 days after MI showed that although sham GRK2KO mice were indistinguishable from sham WT mice, GRK2KO mice had significantly improved post-MI cardiac function and ventricular remodeling after the loss of myocyte GRK2 (Supplemental Table 1). Since this improved post-MI cardiac function was seen in GRK2KO mice with similar infarct sizes, the beneficial effects of GRK2 deficiency probably occurs at the myocyte level. Therefore, the function of cardiac myocytes isolated from WT and GRK2KO mice at 28 days after MI were determined. The basal fractional shortening (FS) and Ca2+ transient amplitudes in myocytes from GRK2KO mice post MI (GRK2KO MI) were almost normal compared to GRK2KO myocytes from mice not subject to MI and greater than those of WT cardiac myocytes post-MI (WT MI) at both pacing frequencies of 0.5Hz and 2Hz (Figure 5A, B, D and E, Supplemental Table 2). Furthermore, WT MI myocytes had a blunted functional response to ISO. In contrast, cardiac myocytes from GRK2KO mice post-MI displayed significantly improved β-adrenergic responses (Figure 5A, B, D and E). These results clearly show that the loss of GRK2 in cardiac myocytes can partially prevent pathological cellular mechanical and Ca2+ handling remodeling after MI, and provide a potential cellular mechanism for the benefits of GRK2 lowering or inhibition in the failing heart.
Figure 5.
Loss of GRK2 in cardiac myocytes ameliorates single cell contractility and Ca2+ handling post-MI. Measurements were obtained at 0.5Hz (A-C) or 2Hz (D-F) under basal conditions and isoproterenol stimulation (10-8M). Fractional shortenings (A & D), Fura-2 ratio amplitudes (B & E) representing the change of intracellular Ca2+ transient from baseline, and tau of Ca2+ transient decay (C & F) were shown. For all measurements a total of 60 cardiac myocytes from 6 different hearts were measured for each group; two-way ANOVA.
GRK2KO MI myocytes have preserved SR Ca2+ loading and ICa,L
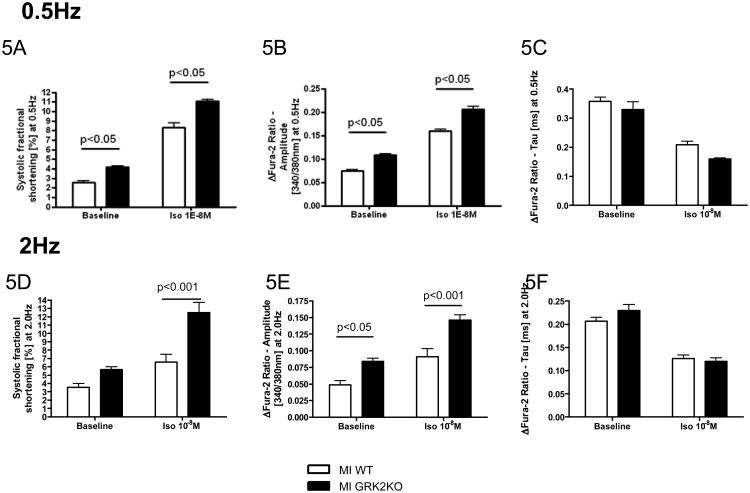
A decrease in the SR Ca2+ load is a contributing factor for the depressed myocyte contractility, a hallmark of HF. Although GRK2KO mice without MI having a lower SR Ca2+ load than WT mice without MI, the SR Ca2+ load in GRK2KO MI myocytes was not decreased as in WT MI myocytes (Figure 6A and B, Supplemental Table 2). Fractional Ca2+ release from the SR was higher in GRK2KO MI myocytes as well, explaining the preserved myocyte contractility (see above and Figure 6C). NCX-activity, as indirectly assessed by the decay constant tau of the caffeine-induced peak Ca2+ transient was normalized in post-MI GRK2KO myocytes, despite significant increases in NCX activity in infarcted WT mice consistent with severe heart failure (Figure 6D).
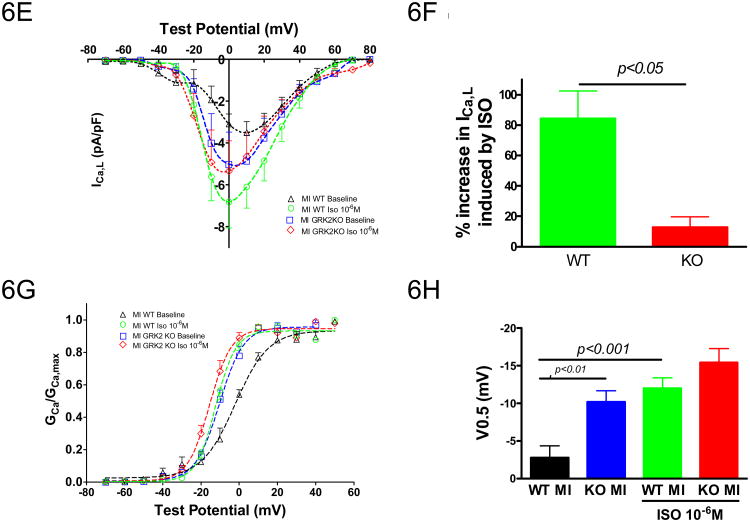
Figure 6.
Myocyte GRK2 silencing inhibits adverse Ca2+ handling remodeling post-MI. A, Representative tracings of the caffeine-induced intracellular Ca2+ transients determined with Indo-1; B, Amplitudes of Ca2+ transients elicited by field stimulation (0.5Hz) and caffeine-spritz; C, Fractional release calculated as the ratio of peak Ca2+ concentration induced by field stimulation to peak Ca2+ concentration induced by caffeine; D, Tau of caffeine-induced intracellular Ca2+ transients. n=14-23 cells from 4-5 animals/group, unpaired two-tailed t-test for B, two-way ANOVA for C and D; E, Current-voltage relationship of ICa,L (pA/pF) under basal conditions and isoproterenol stimulation in post-MI WT and KO myocytes; F, % increase in peak ICa,L by isoproterenol. G, Voltage dependence (G-V curve) of ICa,L activation at basal conditions and under isoproterenol stimulation. H, Voltages at 50% ICa,L activation; n=7 cells from 3-4 different animals/group; one-way ANOVA for E-H.
Improvements in intracellular Ca2+ transients and SR fractional Ca2+ release in the GRK2KO MI myocytes could result from changes in cardiac myocyte ICa,L. Although basal ICa,L amplitudes in both post-MI WT and GRK2KO myocytes were reduced compared to pre-MI values (see Figure 3A and B), peak ICa,L in post-MI myocytes were significantly greater with the loss of GRK2 (Figure 6E, Supplemental Table 2). When stimulated with ISO (10-6M), ICa,L in GRK2KO post-MI myocytes was only insignificantly increased (12.9±6.9%, n=4) but ICa,L in post-MI WT myocytes was significantly increased by 84.3±18.1% (n=7) (Figure 6F). However, after ISO, ICa,L amplitudes in myocytes were not different between the two mouse lines (Figure 6G and H), indicating similar LTCC density. The marked enhancement in basal ICa,L in the GRK2KO MI myocytes might contribute to the normalization of intracellular Ca2+ handling and improved cardiac myocyte contractility. Of interest, the ISO stimulation caused a significant leftward shift of voltage-dependency of channel activation in WT post-MI myocytes but not in GRK2KO myocytes probably because the activation of ICa,L in GRK2KO MI myocytes at baseline was already shifted to the left. After ISO stimulation the voltage-dependent activation of ICa,L was similar in both groups (Figure 6G and H).
Loss of myocyte GRK2 inhibits adverse cellular remodeling post-MI
Cardiac myocyte size at 28 days post-MI was assessed by measurements of myocyte capacitance. Myocytes isolated from GRK2KO MI mice had significantly smaller capacitance, indicating less myocyte hypertrophy and inhibition of adverse cellular remodeling compared to WT mice post-MI (GRK2KO MI 211±19pF vs. WT MI 311±35pF vs. WT Sham 178±19pF vs. KO Sham 188±15pF; p<0.05 between GRK2KO MI and WT MI).
Beneficial effects of myocyte GRK2 silencing is suppressed by the LTCC blocker verapamil
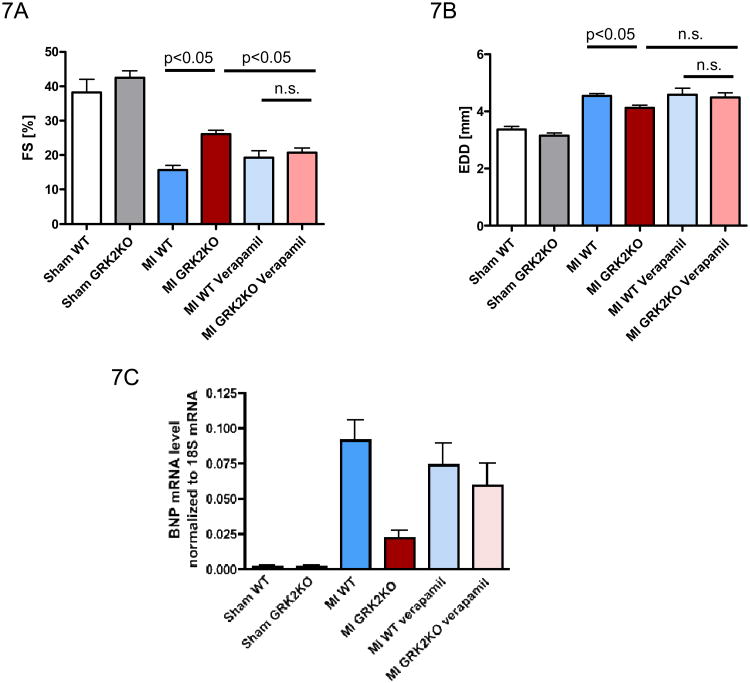
To determine if the beneficial Ca2+ effects seen post-MI after myocyte GRK2 lowering is mediated through the novel changes in LTCC function, we treated WT and GRK2KO mice with verapamil from 14 days till 42 days post-MI. Interestingly, echocardiography revealed that verapamil treatment negated some of the beneficial effects of GRK2 silencing on post-MI cardiac function while the LTCC blocker had minimal effects on post-MI WT mice (Figure 7A). Furthermore, cardiac BNP mRNA expression as a molecular marker of HF was significantly lower in post-MI GRK2KO mice compared to WT MI mice but was reversed by verapamil treatment to the level seen in post-MI WT mice (Figure 7C). It could be true that the absolute amount of Ca2+ current blocked by verapamil is more in GRK2 KO myocytes because the total LTCC density is higher in GRK2 KO MI myocytes and thus verapamil had a stronger effect in KO MI myocytes. In conclusion, it appears that the beneficial effects offered by a loss of GRK2 in cardiomyocytes are at least in part attributable to the upregulation of ICa,L.
Figure 7.
Beneficial effects of myocytes GRK2 lowering are reduced by the LTCC blocker verapamil. Echocardiographic measurements of fractional shortening [FS] (A) and left ventricular end diastolic diameter [EDD] (B) at 42 days post MI or sham operation. Mice were supplemented orally with verapamil starting 14 days post MI until the end of the study period (= 42 days post MI). n=6-8/sham group, n=15-25/MI group without verapamil, n=10-13/MI group with verapamil, one-way ANOVA. C, cardiac myocyte BNP mRNA levels 42 days post MI or sham operation; n=5-6/group for sham, n=8-9/group for MI; one-way ANOVA and unpaired two-tailed t-test.
Discussion
GRK2 is an important molecule in the heart not only as a primary regulator of adrenergic signaling but also claims an important role in the development of HF8-10. It is up-regulated during the early stage in injured myocardium, indicating that it participates in the progression of ventricular dysfunction and cardiomyopathy8-10. Our previous studies have shown that GRK2 silencing10 or inhibition by βARKct20 is able to improve cardiac function during HF progression after MI. However, the specific role of GRK2 in the regulation of normal and failing Ca2+ cycling has never been studied. In heart failure, myocyte Ca2+ cycling is deranged, and abnormalities include altered cardiac LTCC density and properties and reduced SR Ca2+ content due to decreased SERCA2a and increased NCX-activities. These changes result in reduced intracellular Ca2+ transients and depressed myocyte contractility1, 4. Our current study has revealed that GRK2 can influence myocyte Ca2+ homeostasis and its absence in cardiac myocytes10, 13 causes a novel Ca2+ handling phenotype that is resistant to cardiac function deterioration after MI. The benefits rendered by GRK2 silencing is associated with the differential regulation of sarcolemmal and SR Ca2+ handling by the β-adrenergic system.
1. A novel Ca2+ handling phenotype induced by GRK2 silencing
Although GRK2 plays an important role in regulating the β-adrenergic system, its KO does not affect basal cardiac10 and cardiomyocyte function. Myocyte loss of GRK2 did not change characteristics of basal intracellular Ca2+ transients and myocyte contractions. However, detailed characterization of myocyte Ca2+ handling have shown many differences in ECC coupling between GRK2KO and WT myocytes: (1) The SR Ca2+ content is reduced in GRK2 KO myocytes but Ca2+ transient and contraction in GRK2KO myocytes are normal because an increased fractional Ca2+ release from the SR; (2) Increased ICa,L ensures normal Ca2+ transients and cardiac myocyte contractility; (3) Decreased SR Ca2+ content in the face of increased ICa,L is due to increased Ca2+ efflux through the NCX and the inhibition of SERCA by hypophosphorylated PLB; (4) Increased ICa,L is possibly due to local increase in PKA activity. Most of these aspects of Ca2+ handling in GRK2 KO myocytes, except the greater than normal ICa,L and enhanced β-adrenergic regulation, have some similarity to these observed in failing myocytes21. These findings could imply that even in failing myocytes, some of the Ca2+ handling aspects could be more of adaptation.
2. Differential regulation of sarcolemmal and SR Ca2+ handling by the β-adrenergic system
Our data clearly show that there is a differential regulation of the LTCC and the PLB on the SR by the β-adrenergic system in GRK2 KO myocytes: at baseline, the LTCC is already in high activity mode probably due to high phosphorylation state of the channel but the LTCC loses its responses to β -adrenergic stimulation; in contrast, the PLB is in low phosphorylation state (hypophosphorylation) but it has enhanced responsiveness to β-AR stimulation. Our study indicates that the increased LTCC activity could be due to an increase in subsarcolemmal (local) PKA activation brought about by constitutive activity of the β-ARs. In normal cardiac physiology, GRK2 mediates the desensitization of β-ARs20. The loss of GRK2 prevents desensitization of β-ARs and thus likely promotes the accumulation of activated β-ARs and causes constitutive activity of β-ARs in GRK2 KO myocytes even after the isolation. The increase in LTCC activity induced by constitutive β1-ARs has been shown in cardiac β1-AR overexpression mice18. The high LTCC activity in GRK2KO myocytes blunts the responsiveness of the channel to β-adrenergic stimulation. Similar situations have been reported in myocytes with high basal LTCC activities17, 22. Recently, we have shown that overexpression of βARKct, an inhibitor of GRK2, in adult rat myocytes increases basal ICa,L as we have seen with GRK2 silencing. However, βARKct overexpression also enhanced the responses of ICa,L to ISO11, which contrasts to our findings with GRK2 KO. These results suggest a potentially different mechanism is involved in our current study and the βARKct study11 with the net effect (increased LTCC) being comparable. βARKct mainly reduces the inhibory Gβγ effect on the LTCC while in contrast, GRK2KO leads to a local increase in PKA, thereby activating the LTCC. The role of Gβγ in this setting was not specifically addressed, however, if Gβγ was released with the KO of GRK2 the inhibitory effect on the LTCC must be at least overcome by the PKA-dependent activation of the LTCC. An interesting experiment for future studies will indeed be the expression of βARKct in GRK2KO myocytes. This experiment will finally address the role of Gβγ in this setting but goes far beyond the scope of our current study. The use of different models (GRK2KO in mice in vivo for a relatively long period of time vs. βARKct expression in cultured rat ventricular myocytes11 in vitro for 24 hours) could also account for different mechanisms in mediating increased basal ICa,L and different responsiveness to ISO stimulation.
In contrast to the enhanced LTCC phosphorylation in GRK2 KO myocytes, the phosphorylation state of PLB on the SR is lower than normal and the responsiveness of PLB to β-adrenergic agonists is enhanced. The underlying mechanism is related to locally activated PDE4 by activated subsarcolemmal PKA since rolipram - a selective PDE4 inhibitor - blunted the hypophosphorylation of PLB in GRK2KO hearts. Subsarcolemmal PDEs are generally able to diffuse to the SR and thus, can limit local cAMP production and PKA activation. Our results mechanistically explain the differential regulation of sarcolemmal versus SR Ca2+ handling associated with the cardiac myocyte lowering of GRK2, which ultimately can improve cardiac function in HF models.
3. The novel Ca2+ handling phenotype induced by the loss of GRK2 resists to adverse remodeling in hearts post-MI
As summarized above, GRK2 KO induces a novel Ca2+ handling phenotype which maintains a normal myocyte contractility in a way less dependent on SR Ca2+ but more dependent on ICa,L. We also show that this type of Ca2+ handling in GRK2KO myocytes is more resistant to adverse remodeling induced by MI in that the SR Ca2+ content and its regulation by the βAR system, and ICa,L density are better preserved and NCX activity is not increased. The cellular processes responsible for better remodeling after MI in GRK2 KO mice are potentially due to a combination of increased ICa,L and normalized NCX activity resulting into reduced SR Ca2+ content at baseline. We suspect that the post-MI relatively unchanged and small NCX activity predisposes the GRK2KO MI myocyte to maintain an unchanged SR Ca2+ loading in contrast to WT mice. Overall, this combination renders cardiac myocytes less susceptible to SR Ca2+ overload, which is known to induce myocyte apoptosis and necrosis 24, 25. Importantly, the beneficial effects of GRK2 KO are negated by an LTCC antagonist, verapamil. Elevated SR Ca2+ content might also participate in myocyte hypertrophy 26. In this study, GRK2KO myocytes develop less cardiac myocyte hypertrophy post-MI. This could be due to the concomitant decrease of SR Ca2+ load induced by the loss of GRK2 expression in the cardiac myocyte. The enhanced β-adrenergic responsiveness in GRK2 KO myocytes may also contribute to the beneficial effects of GRK2 silencing.
These results obtained here explain our previous studies showing that the loss of GRK2 in cardiac myocytes reduces HF associated mortality and enhances global cardiac function post-MI10. The improvements associated with the loss of GRK2 expression and activity are in a large part attributable to the normalization of intracellular Ca2+ cycling and cardiac myocyte function.
Conclusions
In summary, our data provide novel and important insights into the role of GRK2 in normal and failing hearts. Loss of GRK2 in cardiac myocytes enhances ECC efficiency in the presence of a lower than normal SR Ca2+ loading condition and better β-adrenergic responsiveness in unstressed hearts. This enhancement of ECC occurs through differential regulation of sarcolemmal versus SR Ca2+ handling, with the net result being improved Ca2+ transients leading to the amelioration of the HF phenotype. This is seen at the myocytes level and also globally in vivo with improved cardiac function of GRK2KO mice post-MI. Our data revealed for the first time that the beneficial effects seen with a loss of myocyte GRK2 activity after MI were associated with marked amelioration of cardiac myocyte contractility and significant improvements in intracellular Ca2+ cycling effected by modulation of ICa,L. These effects seen at the myocytes level may also contribute to the beneficial effects seen in various HF models treated with the βARKct peptide as a GRK2 inhibitor 8, 9, 12. Further, GRK2 appears to induce novel regulatory modulation on the LTCC since currents were enhanced with a loss of GRK2. Overall, our current results mechanistically explain the beneficial effects of GRK2 silencing or inhibition after MI in the heart at the cellular and molecular levels and validates GRK2 as a potential target for HF prevention and treatment.
Supplementary Material
Clinical Perspective.
GRK2 is a molecular culprit in the development and progression of HF. We now provide the molecular basis for potential benefits of therapeutic strategies aiming at GRK2 inhibition. With our current study we demonstrate that loss of GRK2 in cardiac myocytes enhances ECC in unstressed hearts and in failing myocytes. We show that this enhancement of ECC occurs through differential regulation of sarcolemmal versus sarcoplasmic reticulum Ca2+ handling, with the net result being improved Ca2+ transients without SR Ca2+ overload leading to reversal of the HF phenotype. Further, we demonstrate that the beneficial effects seen with GRK2 inhibition in HF were associated with marked amelioration of cardiac myocyte contractility and significant improvements in intracellular Ca2+ cycling effected by modulation of the cardiac LTCC. Clear novelty is the compartmentalization of intracellular signaling by GRK2 and thereby differential regulation of Ca2+ signaling. Overall, our current results explain the beneficial effects of GRK2 knockdown in cardiac myocytes with intracellular changes in Ca2+ cycling being orchestrated to improve overall cardiac-myocyte contractility. Furthermore, our study demonstrates that this interplay between GRK2 and the LTCC might represent an attractive target to correct deranged Ca2+ cycling in the failing heart. Future gene therapeutic or pharmacological strategies will thus potentially directly target this interplay and might thus contribute to further improvements in clinical HF treatment.
Acknowledgments
Funding Sources: This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (Ra 1668/1-1 and Ra 1668/3-1 to Philip W. Raake). Xiongwen Chen, Yingxin Li, and Mingxin Tang were supported by NIH R01 HL088243 and American Heart Association AHA0730347N. Walter J. Koch is the W.W. Smith Professor of Medicine and this research was supported by NIH grants R37 HL61690, R01 HL56205, R01 HL085503 and P01 HL075443 (Project 2). Also Drs. Koch and Gao were supported through P01 HL091799 (Project 1 and Core B). The effort of Gerald W. Dorn II on this project was supported by NIH R01 HL87871 and Steven Houser was supported by NIH P01 HL091799 (Project 3).
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 2.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Altered cardiac myocyte ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 6.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 7.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 8.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ark1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci U S A. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, 2nd, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkers M, Weidenhammer C, Herzog N, Qiu G, Spaich K, von Wegner F, Peppel K, Muller OJ, Schinkel S, Rabinowitz JE, Hippe HJ, Brinks H, Katus HA, Koch WJ, Eckhart AD, Friedrich O, Most P. The inotropic peptide betaarkct improves betaar responsiveness in normal and failing cardiomyocytes through g(betagamma)-mediated l-type calcium current disinhibition. Circulation research. 2011;108:27–39. doi: 10.1161/CIRCRESAHA.110.225201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaarkct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW., 2nd Cardiac-specific ablation of g-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 14.Most P, Seifert H, Gao E, Funakoshi H, Volkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR, Jr, Eckhart AD, Feldman AM, Koch WJ. Cardiac s100a1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 15.Cohn RD, Durbeej M, Moore SA, Coral-Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J Clin Invest. 2001;107:R1–7. doi: 10.1172/JCI11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 17.Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single l-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 18.Foerster K, Kaeferstein T, Groner F, Engelhardt S, Matthes J, Koch WJ, Lohse MJ, Herzig S. Calcium channel function and regulation in beta 1- and beta 2-adrenoceptor transgenic mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:490–495. doi: 10.1007/s00210-004-0928-3. [DOI] [PubMed] [Google Scholar]
- 19.Tang M, Zhang X, Li Y, Guan Y, Ai X, Szeto C, Nakayama H, Zhang H, Ge S, Molkentin JD, Houser SR, Chen X. Enhanced basal contractility but reduced excitation-contraction coupling efficiency and beta-adrenergic reserve of hearts with increased cav1.2 activity. Am J Physiol Heart Circ Physiol. 299:H519–528. doi: 10.1152/ajpheart.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rengo G, Lymperopoulos A, Leosco D, Koch WJ. Grk2 as a novel gene therapy target in heart failure. Journal of molecular and cellular cardiology. 50:785–792. doi: 10.1016/j.yjmcc.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 22.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of cavbeta subunits, and lack of functional reserve, in protein kinase a modulation of cardiac cav1.2 channels. Circulation research. 2008;102:e54–64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 23.De Arcangelis V, Liu S, Zhang D, Soto D, Xiang YK. Equilibrium between adenylyl cyclase and phosphodiesterase patterns adrenergic agonist dose-dependent spatiotemporal camp/protein kinase a activities in cardiomyocytes. Mol Pharmacol. 2010;78:340–349. doi: 10.1124/mol.110.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A ca(2+)-dependent transgenic model of cardiac hypertrophy: A role for protein kinase calpha. Circulation. 2001;103:140–147. doi: 10.1161/01.cir.103.1.140. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. Journal of molecular and cellular cardiology. 50:460–470. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.