Abstract
Vitamin D has received a lot of attention recently as a result of a meteoric rise in the number of publications showing that vitamin D plays a crucial role in a plethora of physiological functions and associating vitamin D deficiency with many acute and chronic illnesses including disorders of calcium metabolism, autoimmune diseases, some cancers, type 2 diabetes mellitus, infectious diseases and cardiovascular disease. The recent data on vitamin D from experimental, ecological, case-control, retrospective and prospective observational studies, as well as smaller intervention studies, are significant and confirm the sunshine vitamin’s essential role in a variety of physiological and preventative functions. The results of these studies justify the recommendation to improve the general vitamin D status in children and adults by means of a healthy approach to sunlight exposure, consumption of foods containing vitamin D and supplementation with vitamin D preparations. In general, closer attention should therefore be paid to vitamin D deficiency in medical and pharmaceutical practice than has been the case hitherto.
Keywords: vitamin D, 25-hydroxyvitamin D, vitamin D deficiency, osteoporosis, gene expression, cardiovascular diseases, hypertension, diabetes mellitus, autoimmune diseases, degenerative brain disease, respiratory tract infection, atopic dermatitis, cancer, drugs
Introduction
Since the discovery of its antirachitic effect in the 1920s, the sunshine vitamin was for many years only seen in relation to its function in calcium and bone metabolism. A variety of research results from recent years have shown that vitamin D in its hormonally active form, 1α,25-dihydroxyvitamin D [1α,25(OH)2D; calcitriol] is not only a regulator of calcium and phosphate homeostasis, but has numerous extra-skeletal effects. These include the significant impact of the vitamin D hormone on the cardiovascular system, central nervous system, endocrine system and immune system as well as on cell differentiation and cell growth.1,2
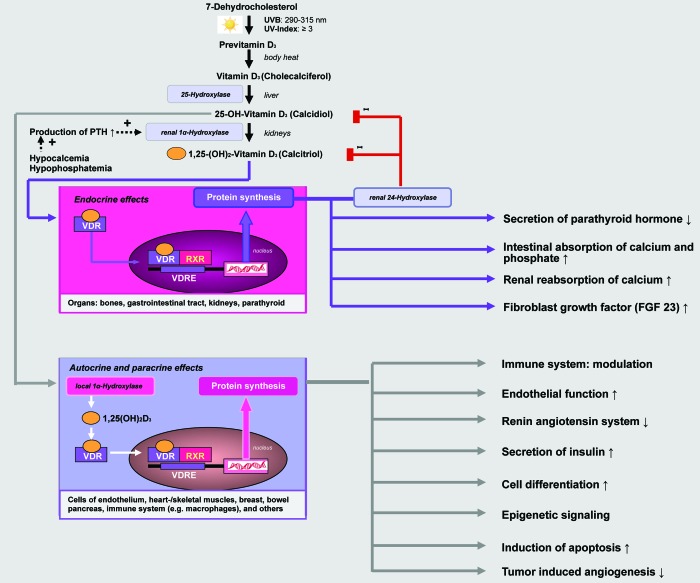
1α,25(OH)2D manifests its diverse biological effects (endocrine, autocrine, paracrine) by binding to the vitamin D receptor (VDR) found in most body cells (Fig. 1). Vitamin D receptors have been found in over 35 target tissues that are not involved in bone metabolism. These include endothelial cells, islet cells of the pancreas, hematopoietic cells, cardiac and skeletal muscle cells, monocytes, neurons, placental cells and T-lymphocytes. It is estimated that VDR activation may regulate directly and/or indirectly a very large number of genes (0.5–5% of the total human genome i.e., 100–1250 genes).4 The fact that the vitamin D receptor is expressed by many tissues results in the pronounced pleiotropic effect of vitamin D hormone.1-5
Figure 1. Vitamin D in its hormonally active form, 1α,25-dihydroxyvitamin D is not only a regulator of calcium and phosphate homeostasis, but has numerous nonskeletal functions effects. 1α,25(OH)2D manifests its diverse biological effects (endocrine, autocrine, paracrine) by binding to the vitamin D receptor (VDR) found in most body cells. It is estimated that VDR activation may regulate directly and/or indirectly more than 200 genes, including genes responsible for the regulation of cellular proliferation, differentiation, apoptosis, and angiogenesis.135
From Sunshine Vitamin to Sunshine Hormone
Vitamin D—the sunshine vitamin—is formed in the skin from 7-dehydrocholesterol (7-DHC) via the intermediate previtamin D3 and with the help of sunlight (UVB: 290–315 nm). Previtamin D3 is converted by body heat to vitamin D3 [cholecalciferol]. Excessive sunlight exposure degrades previtamin D3 and vitamin D3 to inactive photoproducts, thus preventing excessive production of the sunshine vitamin in the skin. The liver converts vitamin D3 via the enzyme 25-hydroxylase (25-OHase: CYP27A1, CYP2R1) into 25-hydroxyvitamin D [25(OH)D], also known as calcidiol. The mitochondrial CYP27A1 and microsomal CYP2R1 are the two major enzymes involved in the hydroxylation at C-25, although there are several CYP enzymes that show 25-hydroxylase (25-OHase) activity but with higher Km and lower Vmax. Serum 25(OH)D (1 ng/mL = 2,5 nmol/L) is the barometer for the medical laboratory evaluation of the vitamin D status.1-3
25(OH)D is then converted in the kidneys via the enzyme 25-hydroxyvitamin D-1-α-hydroxylase also known as cytochrome p450 27B1 (1-OHase: CYP27B1) into the metabolically active vitamin D hormone [1α,25(OH)2D]. This enzyme is also called renal 1-α-hydroxylase - since it occurs in the kidneys (→ endocrine effect). The renal synthesis of 1,25(OH)2D is regulated by several factors including serum phosphorus, calcium, fibroblast growth factor 23 (FGF-23), parathyroid hormone (PTH) and itself.3 Besides the kidneys, a multitude of tissues have a local 1-α-hydroxylase (1-OHase) including bone, placenta, prostate, keratinocytes, macrophages, T-lymphocytes, dendritic cells, several cancer cells, and the parathyroid gland. Depending on the availability of 25(OH)D and the amounts required, these cells can produce the biologically active vitamin D hormone with the help of their local 1-OHase (→ autocrine and paracrine effect). 1α,25(OH)2D is like the sex hormones (e.g., estradiol) and corticosteroids (e.g., cortisone), which are all steroid hormones.2,4,5 Via a feedback mechanism, the 1α,25(OH)2D level regulates the synthesis of 1α,25(OH)2D and reduces the synthesis and secretion of parathyroid hormone in the parathyroid glands (Fig. 1). 1α,25(OH)2D induces its own destruction by activating the 25-hydroxyvitamin D-24-hydroxylase (24-OHase: CYP24A1), which leads to the multistep catabolism of both 25(OH)D and 1α,25(OH)2D into biologically inactive, water-soluble metabolites including calcitroic acid.1,3
The Barometer of Vitamin D Health: 25-hydroxyvitamin D
According to current scientific knowledge, the serum 25(OH)D level should be between 30 and 100 ng/mL to avoid long-term negative health consequences. A 25(OH)D status between 40 and 60 ng/mL or 100 to 150 nmol/L is ideal.3 A pronounced vitamin D deficiency is present at 25(OH)D levels below 20 ng/mL, with levels between 21–29 ng/mL designated as moderate vitamin D deficiency, also referred to as vitamin D insufficiency. Vitamin D intoxication is only to be expected at levels of 25(OH)D > 150 ng/mL.3,6
Vitamin D deficiency is often accompanied with elevation in serum parathyroid hormone (PTH) levels. Evidence is increasing that PTH elevation may promote cardiovascular disease through diminished cardiac contractility, enhanced coronary risk, and cardiac valvular and vascular calcification. High PTH levels appear to be linked to the metabolic syndrome and are aligned with hyperlipidemia, decreased insulin sensitivity, and, perhaps, decreased insulin secretion. Increased PTH also is associated with neuroendocrine activation, increased sympathetic activity, and endothelial stress. PTH values provide useful clinical diagnostic and prognostic information in monitoring many chronic ailments such as heart and renal failure and multiple sclerosis.13 25(OH)D values of ≥40 ng/mL or 100 nmol/L are necessary to avoid an increase of parathyroid hormone (PTH) levels.1,3,4,6 However, in a recently published analysis of more than 312 962 paired PTH and 25(OH)D levels, no threshold level of 25(OH)D-dependent parathyroid hormone status was observed at which an increase of the 25(OH)D value suppresses the PTH increase, even at 25(OH)D levels >60 ng/mL. The high proportion of blood samples showing a vitamin D deficiency and secondary hyperparathyroidism was remarkable in this analysis.1,11 Active 1,25(OH)2D should not be measured to assess vitamin D status, since in the presence of a vitamin D deficiency it is often normal or even shows a compensatory increase due to elevated parathyroid hormone levels!3,6
North of the 35th parallel, the sun is not high enough in the sky from October to March to supply our skin with the necessary 290 to 315 nm UVB radiation. The flat angle of incidence of the sun is responsible for the low intensity of the sun’s rays. Germany is located between 47th and 55th parallels, i.e., in the northern hemisphere of the earth, at same level as Canada. This also explains why so many people, especially in the winter months, suffer from vitamin D deficiency [25(OH)D <20 ng/mL or 50 nmol/L]. The UV index can also be used to estimate sun-dependent vitamin D formation in the skin. With a UV index of less than 3, no vitamin D synthesis can take place in the skin.2,3 An App for the iPhone dminder.info provides the user anywhere on the planet information about how much vitamin D can be made in the skin during sun exposure. Vitamin D intake in the diet plays only a minor role in the vitamin D supply.1,2 Based on the results of recent studies, approximately 1 billion people worldwide are affected by a vitamin D deficiency [25-OH-D: <20 ng/mL] or a vitamin D insufficiency [25(OH)D: 21–29 ng/mL].3,14
Health Risk: Vitamin D Deficiency
According to recent studies, a vitamin D deficiency [serum 25(OH)D <20 ng/mL] is likely to be an important etiological factor in the pathogenesis of many chronic diseases. These include autoimmune diseases (e.g., multiple sclerosis, type 1 diabetes) inflammatory bowel disease (e.g., Crohn disease), infections (such as infections of the upper respiratory tract), immune deficiency, cardiovascular diseases (e.g., hypertension, heart failure, sudden cardiac death), cancer (e.g., colon cancer, breast cancer, non-Hodgkin’s lymphoma) and neurocognitive disorders (e.g., Alzheimer disease).5-11
The current results of the ESTHER study, a nationwide cohort study from the Saarland, involving about 10,000 women and men aged 50 to 74 years in whom the 25(OH)D status was ascertained, showed that a vitamin D deficiency significantly increased general and cardiovascular mortality over a follow up median of 9.5 years. The 25(OH)D levels and overall mortality showed a pronounced nonlinear inverse association with increased risk beginning at 25(OH)D levels below 75 nmol/l (<30 ng/ml). A vitamin D deficiency was also associated with significantly increased cancer mortality and a higher mortality rate for respiratory diseases.11
Bone, Muscle Metabolism, and Dental Health: Risk of Fractures, Falling and Caries
Vitamin D plays an important role in the calcium and phosphorus metabolism and helps ensure adequate levels of these minerals for metabolic functions and bone mineralization. 1α,25(OH)2D increases the efficiency of intestinal calcium absorption from 10–15% to 30–40% by interacting with the VDR-RXR and thereby promoting the expression of an epithelial calcium channel and a calcium-binding protein. Based on several animal experiments it has been estimated that 1α,25(OH)2D also increases the intestinal phosphorus absorption from 50–60% to approximately 80%.1,3 Vitamin D deficiency is a widespread medical condition that plays a major role in human bone health. A severe vitamin D deficiency [25(OH)D <10 ng/mL] results in rickets in children and osteomalacia in adults. The clinical symptoms of a severe vitamin D deficiency include, besides a mineralization disorder, a myopathy with proximal muscle weakness and muscle pain. The mineralization disorder osteomalacia can also cause bone pain and fractures. The secondary hyperparathyroidism by vitamin D deficiency results in an increased osteoclastic activity which removes matrix and mineral causing bone mineral loss, this can precipitate and exacerbate osteopenia and osteoporosis in adults. In older individuals vitamin D deficiency is associated with an increased risk of functional limitations, falling and fractures.14,15
Fracture susceptibility in the context of vitamin D deficiency has been primarily associated with defective mineralization of collagenous matrix and osteoclastic destruction of the bone. Recent research has shown that bone’s fracture resistance is influenced by toughening mechanisms at various hierarchical levels ranging from the nano- to the microstructure. The characteristic increase in osteoid-covered surfaces in vitamin D-deficient bone hampers remodeling of the remaining mineralized bone tissue. By spatially resolved synchrotron bone mineral density distribution analyses and spectroscopic techniques, it was observed that the bone tissue within the osteoid frame has a higher mineral content with mature collagen and mineral constituents, which are characteristic of aged tissue. In situ fracture mechanics measurements and synchrotron radiation micro-CT of the crack path have shown that a deficiency of vitamin D increases both the initiation and propagation of cracks by 22–31%. These data shed a new light on the impact of vitamin D deficiency in bone health and explains why a normal vitamin D status is essential to maintain bone's structural integrity.16
In the most recent meta-analysis original data on 30 011 study participants from 11 double-blind and randomized studies were pooled.17 The classic intent-to-treat analysis of 30,011 persons showed a statistically non-significant reduction of hip fractures by 10%. However, an analysis of the effect in relation to the vitamin D amount actually taken, showed a statistically significant reduction of hip fractures by 30% among the subjects taking the highest dosage (792 to 2000 IU vitamin D/day; median: 800 IU vitamin D/day) compared with the control group. In persons supplemented with less than 792 IU vitamin D per day, no statistically significant reduction of hip fractures was detectable. A similar dose-effect dependence was detected for all non-vertebral fractures. The subgroup analysis showed a significant reduction of fractures in all age groups, for elderly people living at home as well as those living in nursing homes with the highest vitamin D dosage.17 The results of a bone biopsy study of 675 presumed healthy adults aged 20 to 90 years, who died in an accident indicated a threshold level of 25(OH)D ≥75 nmol/L or ≥30 ng/mL as a target value for healthy bone metabolism at which further no mineralization disorder (osteomalacia) was detected.22
In addition to a positive effect on bone mineral density, vitamin D has an immediate restorative effect on the muscles, which can be explained in terms of a better influx of calcium into the muscle cells as well as a receptor-mediated stimulation of muscle protein synthesis.18,19 It may be that this additional effect is a crucial factor in the fracture reduction observed with vitamin D supplementation, since falling represents the primary risk factor for fractures. This is also supported by study results according to which a significant reduction in the risk of falling is already observed after 2–3 mo of vitamin D supplementation, indicating that the musculature responds quite rapidly to vitamin D intake, and fracture reduction is noticeable after only about 6 mo.20 In the reanalysis of a meta-analysis of 8 double-blind and randomized studies, which had been published in 2009, with high-quality detection of the factor falling, vitamin D demonstrated a benefit in all studies (OR = 0.73 [.62, 0.87]; P = 0.0004). The relevance of vitamin D dosing with respect to reduction of falls could also be confirmed: at the higher dose (700–1000 IU vitamin D/day), vitamin D reduced the risk of a fall by 34% (OR = 0.66 [0.53, 0.82] P = 0.0002), while no reduction of falls was observed at the lower dosage level (OR = 1.14 [0.69, 1.87]).21
In addition to the crucial role of vitamin D for bone health several studies have shown an association between alveolar bone density, osteoporosis and tooth loss. Low bone mass may be a risk factor for periodontal disease. A recent systematic review and meta-analysis of controlled clinical trials indicate that vitamin D is important for dental health and a promising factor in the prevention of caries. This could be due to the fact that vitamin D has a direct effect on bone metabolism and can also act as an anti-inflammatory agent and stimulate the production of anti-microbial peptides.24,25
Comment: Improvement in 25(OH)D status by supplementation with vitamin D2 or vitamin D3 is an important health strategy to promote bone health at all age levels and to reduce the risk of fractures and falling in the elderly. To maximize the osseous effect and intestinal calcium absorption, supplementation should achieve a 25(OH)D status of ≥75 nmol/l or ≥30 ng/ml, this particularly applies in the pharmacotherapy of osteoporosis with bisphosphonates as well as other medications. Apart from bone health vitamin D seems to be an important factor for dental health.
Ecological Association Studies
Over the past 100 years there have been a variety of ecological studies that have associated living at higher latitudes with many acute and chronic illnesses. One of the first association studies was reported by Palm in 1889 when he realized that children living in the inner cities of Great Britain were at extremely high risk for developing the devastating bone deforming disease rickets while children living in India had little evidence for the disease.26 He was one of the first to recommend sunbathing for promoting skeletal health and reducing risk for rickets. In 1921 Hess and Unger reported that sun exposure was effective in treating and preventing rickets.27 The relationship between sun exposure, rickets and vitamin D3 was finally appreciated in the early 1930s when Windaus determined that vitamin D3 was produced in mammalian skin and was identified as the antirachtic factor by many investigators.28
One of the first ecological studies relating latitude and cancer was reported by Hoffman in 1916.29 He observed that cancer mortality between 1908 and 1912 was increased in people living at higher latitudes. Peller and Stephensen reported on the incidence of cancer in navy personnel in the United States and noted that although navy personnel who were outside all the time were 8 times more likely to develop skin cancer compared with age-matched controls who work indoors they had a 60% reduced risk of dying of cancer than the civilian population.30 This was followed by Apperly who in 1941 reported total cancer mortality in Americans and Canadians in the same population who were engaged in agriculture. He concluded that cancer mortality was highest in farmers living in the northeast compared with those living in the south.31 In the 1980s through the early 2000s there were several reports of associations with increased risk for colon, ovarian, prostate cancer and many other cancers for people living at higher latitudes in the United States as well as in Europe.32-37 Grant reported a dramatic inverse relationship between premature mortality due to cancer with UV exposure in both men and women.38 A meta-analysis of cancer incidence rates for more than 100 countries including Australia, and China among others confirm previous studies where it was concluded that there was an inverse relationship with solar UVB exposure for 15 types of cancers including endometrial, gastric, cervical, bladder, pancreatic and colorectal cancer among others.39,39a Not only the relative risk for developing deadly cancers was associated with less solar UVB exposure but also mortality from these numerous malignancies was also related to less solar UVB exposure.40 A study conducted in Canada reported that women who had the most sun exposure as teenagers and young adults had a more than 60% reduced risk of developing breast cancer compared with women living in the same locale who had minimum sun exposure during the same period of time.41 Luscombe et al. made a similar observation in men who worked outdoors; these men had a 3 y hiatus before developing prostate cancer compared with indoor workers.42
The connection between increased sun exposure and living at lower latitudes with reduced risk for cancers with vitamin D was first described by Garland and Garland.32 They first related a strong significant negative correlation with colon cancer and mortality with mean daily radiation in the United States. This was followed by an 8 y prospective case-controlled study of adults living in Washington County where the risk of developing colon cancer was reduced 3-fold in people who had a baseline 25(OH)D >20 ng/mL.33 Giovannucci et al. conducted a prospective study in men and found an inverse association with cancer incidence of GI related cancers and predictors of vitamin D status.43
These studies have been followed by a large number of publications that have related increased sun exposure, living at lower latitudes and increased vitamin D intake with reduced risk not only for cancers but also autoimmune diseases including type 1 diabetes, rheumatoid arthritis, and multiple sclerosis, neurological disorders including depression and schizophrenia, infectious diseases including tuberculosis and lower blood pressure. Evidence suggests that living for the first 10 years of your life below 35° north and above 35° south latitude reduces risk for developing multiple sclerosis by 50%.44,45 Being born and living near the equator reduces risk of type 1 diabetes by more than 10-fold compared with living in the far northern and southern regions of the globe.90 Women living at higher latitudes in the US were at higher risk for developing rheumatoid arthritis.46 People in Scandinavia are more likely to develop schizophrenia compared with people living near the equator and babies born at the end of the winter are more likely to develop schizophrenia even those born in Australia.47,48 Patients with TB were found to do better when exposed to sunlight and curiously it was known that living in the Alps above 5000 feet the incidence of TB was very low.49 Finally, it was demonstrated that the higher the latitude that you live in the northern hemisphere and the lower the latitude that you live in the southern hemisphere, was associated with a higher overall blood pressure.50
Many of these ecologic observations which suggest a direct role of increased sun exposure and improved vitamin D status has been supported by association and prospective and retrospective studies relating vitamin D intake or vitamin D status with these chronic illnesses.112
Gene Expression: Link Between Vitamin D and Prevention
In a recent randomized, placebo-controlled, double-blind study, the influence of a daily supplementation of 400 IU or 2000 IU of vitamin D3 over a period of two months in winter on the gene expression of white blood cells (leukocytes) in healthy adults was investigated for the first time. The improvement of 25(OH)D status observed thereby was associated with a change in gene expression of at least 1.5-fold in 291 genes. There was a significant difference in the expression of 66 genes between subjects at baseline with vitamin D deficiency [25(OH)D <20 ng/mL] and subjects with a 25(OH)D >20 ng/mL. After vitamin D3 supplementation gene expression of these 66 genes was similar for both groups. Seventeen vitamin D-regulated genes with new candidate vitamin D response elements including TRIM27, CD83, COPB2, YRNA, and CETN3 which have been shown to be important for transcriptional regulation, immune function, response to stress and DNA repair were identified. The results of this study suggest that any improvement in vitamin D status will significantly affect the expression of genes, which have a wide variety of biological functions and are involved in more than 80 metabolic pathways associated with the pathogenesis of autoimmune diseases, cancers and cardiovascular diseases. This study reveals, for the first time, genetic fingerprints contributing significantly to an understanding of the non-skeletal effects of the sunshine vitamin on health at a molecular biochemical level.4
Cardiovascular System: Hypertension and Cardiac Insufficiency
A deficiency of vitamin D [25(OH)D <20 ng/mL or 50 nmol/L] significantly increases overall and cardiovascular mortality.25 The vitamin D receptor is present in endothelium, vascular smooth muscle, and cardiomyocytes and may protect against atherosclerosis through the inhibition of macrophage cholesterol uptake and foam cell formation, reduced vascular smooth muscle cell proliferation, and reduced expression of adhesion molecules in endothelial cells and through inhibition of cytokine release from lymphocytes.51,52
In the Intermountain Heart Collaborative Study, a prospective study with 41 504 participants, an inadequate vitamin D supply was determined in 63.6% [25(OH)D: < 30 ng/mL). A 25(OH)D level <15 ng/mL compared with a 25(OH)D levels >30 ng/mL was associated with a highly significant increase in the prevalence of type 2 diabetes, high blood pressure, dyslipoproteinaemia, peripheral vascular diseases, coronary heart disease, myocardial infarction, cardiac insufficiency and stroke (P < 0.0001) as well as in the incidence of overall mortality, cardiac insufficiency, coronary heart disease / myocardial infarction (P < 0.0001), stroke (P = 0.003), and their combination (P < 0.0001).53,54 The results of a meta-analysis covering the vitamin D status with the risk for cerbrovascular events, including >1200 cases of stroke, revealed that 25(OH)D levels ≤12.4 ng/ml compared with 25(OH)D levels of >18.8 ng/ml was associated with an increase in the risk level for strokes of 53%.55
A systematic review and a meta-analysis conclude that vitamin D lowers systolic blood pressure by −6.18 mmHg and reduces diastolic blood pressure by -3.1 mmHg in hypertensive patients. No change in blood pressure was observed in normotensive persons.56 Black US. Americans suffer significantly more frequently from high blood pressure than whites. Reduced blood levels of 25(OH)D could be responsible for the higher risk of hypertension, since people with darker skin color generally produce less vitamin D3 in the skin due to the higher content of melanin and thus have lower levels of 25(OH)D. In a recent 4-arm, double-blind, placebo-controlled and randomized study of 283 blacks (age: ± 51), the influence of 1000 IU, 2000 IU and 4000 IU vitamin D3 per day or placebo on blood pressure was investigated for a period of 3 months. Blood pressure and 25(OH)D levels were determined at onset, after 3 months and after 6 months. The difference between the systolic blood pressure at baseline and after 3 months in the placebo group was +1.7 mmHg in the placebo group, −0.66 mg Hg in the group with 1000 IU vitamin D3 per day, −3.44 mmHg in the group with 2000 IU vitamin D3 per day and −4.0 mmHg in the group with 4000 IU vitamin D3 per day (−1.4 mmHg for every 1000 I.U. of additional vitamin D3 intake, P = 0.04). For each increase of 25(OH)D levels by 1 ng/ml, a significant reduction in systolic blood pressure of 0.2 mmHg was detected (P = 0.02). However, no significant reduction in diastolic blood pressure was detected (P = 0.37).57 In another 16-week randomized clinical trial with normotensive black boys and girls the relation between 25(OH)D concentrations and total body fat mass by dual-energy X-ray absorptiometry, and the arterial stiffness measured by pulse wave velocity (PWV) in response to 2000 IU vitamin D supplementation was analyzed. The results demonstrate that vitamin D supplementation may be effective in optimizing vitamin D status and counteracting the progression of aortic stiffness in black youth. Plasma 25(OH)D concentrations in response to the 2000 IU/d supplementation were negatively influenced by adiposity.58
The suppression of parathyroid hormone (PTH) by vitamin D, which has been known for some time, must now be seen in a new light, since PTH has been increasingly recognized in recent years as a major risk factor for cardiovascular diseases such as high blood pressure or cardiac insufficiency. PTH can cause damage to the cardiovascular system at different levels, either directly or indirectly. Elevated PTH levels, as well as a hypercalcaemia, can promote the development of hypertension. In addition, hyperparathyroidism is associated with a high incidence of hypercontractility of the heart muscle with consecutive left-ventricular hypertrophy and calcification of the myocardium. Vitamin D counteracts these processes, in that it, among other things, promotes the synthesis of anti-inflammatory cytokines, such as interleukin 10, and of other substances that reduce vascular calcification (e.g., matrix Gla protein). In addition, vitamin D counteracts the adverse effects of the so-called “advanced glycation endproducts” (AGEs) on the endothelium.59 In a recent placebo-controlled, double-blind study of 80 infants with cardiac insufficiency, daily supplementation of 1200 IU vitamin D3 for a period of 12 weeks in the 42 children from the vitamin D group compared with the 38 children in the placebo group resulted in a significant improvement in the performance of the heart muscle (e.g., LVEF ↑) and the reduction of various cardiovascular risk parameters (e.g., PTH, IL-6, TNFα levels ↓) (P < 0.001) in addition to a significant rise in 25(OH)D status (13.4 → 32,9 ng/ml).60 Another recent study evaluated the effect of vitamin D insufficiency (< 30 ng/mL) on epicardial coronary flow rate, subclinical atherosclerosis, and endothelial function in 222 consecutive patients who had undergone coronary angiography for suspected ischemic heart disease and were found to have normal or near-normal coronary arteries. The mean level of 25(OH)D was 31.8 ng/ml, and 47% (n = 106) of the patients had insufficient 25(OH)D levels (<30 ng/ml). Baseline characteristics were similar between vitamin D insufficient and vitamin D sufficient groups. The incidence of slow coronary flow (SCF) was significantly higher in the vitamin D insufficient group than in patients with sufficient vitamin D (RR = 3.5, 95% CI = 1.1–10.5, P = 0.01). After adjusting for cardiovascular disease risk factors, VD insufficiency was independently associated with SCF. The linear regression analysis showed that VD insufficiency was correlated independently with % flow-mediated dilatation (P < 0.001) and carotid intima-media thickness (P < 0.001).61
Comment: According to the data available to date from epidemiological and prospective cohort studies as well as from smaller interventional studies of vitamin D status in cardiovascular diseases such as hypertension and cardiac insufficiency, 25(OH)D levels should always be checked and be appropriately compensated by vitamin D2 or vitamin D3 supplementation. Normalization of 25(OH)D status could also help reduce therapeutic requirements for antihypertensives and cardiac drugs (e.g., diuretics, ACE inhibitors, calcium antagonists).
Diabetology
Type 1 diabetes
The worldwide incidence rate of type 1 diabetes is increasing and accumulating data show that it is associated with vitamin D deficiency. The chronic autoimmune disease type 1 diabetes usually results from a T cell mediated destruction of insulin producing, pancreatic β-cells with a typical onset in childhood or adolescence. There is evidence that vitamin D supplementation early in life is a protective factor against the development of type 1 diabetes. Furthermore, in animal models, such as the NOD mice, the administration of 1α,25(OH)2D or vitamin D analogs prevented or at least delayed the onset of diabetes.62-65
In a Finnish cohort study involving 12 058 children, the influence of supplementation of vitamin D in the first year of life on incidence of diabetes was followed up over a period of 30 y. It was found that newborn babies who receive 2000 IU of vitamin D3 daily as rickets prophylaxis showed a 88% lower risk for type 1 diabetes mellitus compared with those with lower-dosed supplementation. Children who suffered from rickets in the first year of life had a 3-fold higher risk for type 1 diabetes compared with healthy children.66 In a meta-analysis of 4 case-control studies, the risk for type 1 diabetes in infants who received a vitamin D supplement compared with those who received no vitamin D was reduced by 29% (OR 0.71, 95% CI 0.60 to 0.84).67 The importance of maternal vitamin D status on subsequent development of type 1 diabetes in newborns is described by a Norwegian cohort study of 20 072 women. A low maternal 25(OH)D status (≤54 nmol/L or 21.6 ng/mL) during pregnancy was associated with a more than 2-fold risk increase for the development of type 1 diabetes later in life compared with a good maternal 25(OH)D status (>89 nmol/L or 35.6 ng/mL).68
Type 2 diabetes and metabolic syndrome
Vitamin D deficiency has been implicated in decreased insulin secretion and increased insulin resistance, hallmarks of type 2 diabetes mellitus. The results of a recent published prospective cohort study and meta-analysis with 9841 participants of whom 810 developed type 2 diabetes during a 29 years follow-up confirm once more an association of low 25(OH)D serum levels with an increased risk of type 2 diabetes. Lower 25(OH)D concentrations were associated with higher cumulative incidence of type 2 diabetes (trend, P = 2 × 10(−7) and p = 4 × 10(−10)). Multivariable adjusted hazard ratios of type 2 diabetes were 1.22 (95% CI 0.85–1.74) for 25(OH)D <5 vs ≥20 μg/L and 1.35 (1.09–1.66) for lowest vs highest quartile. Also, the multivariable adjusted hazard ratio of type 2 diabetes for a 50% lower concentration of 25(OH)D was 1.12 (1.03–1.21); the corresponding hazard ratio for those ≤58 years old was 1.26 (1.15–1.41). Finally, in a meta-analysis of 16 studies, the odds ratio for type 2 diabetes was 1.50 (1.33–1.70) for the bottom vs top quartile of 25(OH)D.69 In a randomized, placebo-controlled study involving insulin-resistant South Asian women (age: 23–68 years) with median 25(OH)D baseline levels of <10 ng/ml, daily supplementation of 4,000 IU vitamin D3 led to a significant improvement in insulin sensitivity and reduction of insulin resistance (P = 0.003 and P = 0.02) compared with placebo. Insulin resistance was particularly reduced when the 25(OH)D levels rose to above 32 ng/mL (= 80 nmol/L). Optimum concentrations of 25(OH)D for the improvement of insulin resistance ranged from 32 to 47.6 ng/mL ( = 80–119 nmol/L).70
Several studies showed an inverse correlation of 25(OH)D concentration with metabolic syndrome risk or with the incidence or severity of its components. Subjects with hypovitaminosis D are at higher risk of insulin resistance and the metabolic syndrome.71,72 The association of 25(OH)D level with the incidence of metabolic syndrome was analyzed in 4,164 Australian adults (age ±50 years) in a recent prospective study. Waist measurement and the classic risk factors for metabolic syndrome were recorded for all study participants. After 5 years of follow-up, the scientists observed a significantly increased likelihood (OR 1.41 and 1.74; CI 95%) of developing metabolic syndrome in study participants with 25(OH)D levels <18 ng/mL and 18–23 ng/mL compared with those with a good vitamin D status of >34 ng/mL. They concluded that in Australian adults vitamin D deficiency [25(OH)D <20 ng/mL] and vitamin D insufficiency [25-OH-D: 21–29 ng/mL] were associated with a significantly increased risk for metabolic syndrome (P < 0.01), insulin resistance (P < 0.01), high waist circumference (P < 0.001) and raised glucose and triglyceride levels (P < 0.01).73
The results of a further prospective study provide additional meaningful results in support of the thesis that a vitamin D deficiency accelerates the progression of pre-diabetes to manifest type 2 diabetes. In this connection, the scientists studied glucose tolerance and 25(OH)D levels in 980 women and 1398 men (age: 35–56 y old) with no type 2 diabetes prior to study onset. After 8–10 y of follow-up, the study participants with pre-diabetes or type 2 diabetes were compared with age-correlated and sex-correlated controls with normal glucose tolerance. After elimination of potential confounding variables, the male study participants from the highest quartile showed a 48% reduced risk for the progression of pre-diabetes to type 2 diabetes compared with those in the lowest quartile of the 25-OH-D level (OR 0.52, 95% CI 0.30, 0.90). Men and women with pre-diabetes at baseline showed a remarkable 25% reduction in type 2 diabetes incidence per 4 ng/mL (=10 nmol/L) increase in 25(OH)D level.74 According to the current data, a vitamin D deficiency [25(OH)D <20 ng/ml] seems to increase not only the progression of pre-diabetes to manifest type 2 diabetes, but also influences mortality in metabolic syndrome: In the LURIC study of 1801 patients with metabolic syndrome, a good vitamin D status [25(OH)D ≥30 ng/mL] was associated with a 66% reduction in cardiovascular mortality and a 75% reduction in total mortality (Fig. 2) compared with a severe vitamin D deficiency [25(OH)D <10 ng/ml]. Patients with a good vitamin D status [25(OH)D ≥30 ng/mL] showed a mortality risk reduced by 85% and 76% due to sudden cardiac death or cardiac insufficiency respectively compared with those with a severe vitamin D deficiency. Even when patients with type 2 diabetes were eliminated from the analysis, those with an optimum vitiman D status showed a 64% reduction in overall mortality compared with the subjects with severe vitamin D deficiency.75
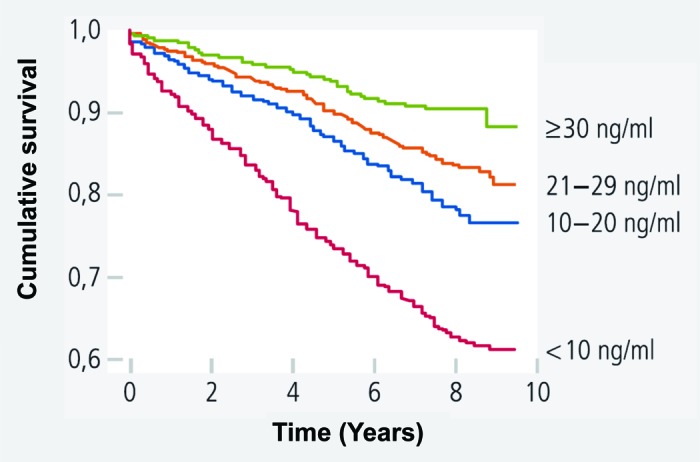
Figure 2. 25(OH)D levels are dose-dependently associated with a robust reduction in all-cause mortality in subjects with the metabolic syndrome. Metabolic Syndrome and Kaplan-Meier plot for all-cause mortality according to 25(OH)D groups in those with the metabolic syndrome. Log-rank analysis indicated a significant difference between all 25(OH)D groups (p < 0.001).35
In a just-published interventional study of 100 patients (age: 54.11 ± 11) with type 2 diabetes, oral supplementation of 50 000 IU of vitamin D3 per week over a period of 8 weeks resulted in a significant improvement of the HOMA index (HOMA-IR: 3.57 ± 3.18 → 2.89 ± 3.28; P = 0.008), insulin resistance (insulin: 10.76 ± 8.9 → 8.6 ± 8.25 μU/ml; P = 0.02) and the fasting glucose levels (FPG (mg/dl)): 138.48 ± 36.74 → 131.02 ± 39 0.05; P = 0.05) in addition to an increase of 25(OH)D levels (43.03 ± 19.28 → 60.12 ± 17.2; P = 0.02).76
Comment: According to current data concerning metabolic control, comorbidities and increased mortality, patients with diabetes mellitus, insulin resistance and metabolic syndrome apparently benefit from vitamin D supplementation.
Immune system
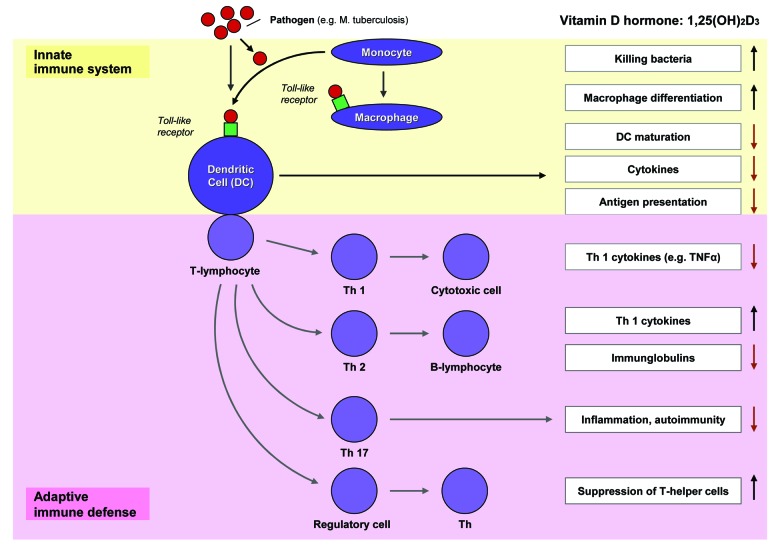
In addition to the endocrine effects, vitamin D hormone [1,25(OH)2D] has also autocrine and paracrine effects. Many body cells, including immunocompetent cells, such as dendritic cells, macrophages and B and T lymphocytes, have VDR and the enzymatic capability to synthesize 1,25(OH)2D from its precursor 25(OH)D. 1,25(OH)2D is a potent modulator of acquired immunity and the immune balance between Th1 and Th2 cells (Fig. 3). Local or systemically produced vitamin D hormone inhibits the maturation of dendritic cells, among others, reduces Th1-mediated secretion of proinflammatory cytokines such as TNFα, increases the differentiation of monocytes to macrophages and their rate of phagocytosis as well as the activity of lysosomal enzymes in macrophages.1-3
Figure 3. Vitamin D and immune system. 1α,25(OH)2D appears to influence susceptibility to and severity of infection via multiple mechanisms via the innate and adaptive immune system.145
Respiratory tract diseases (RTI)
A series of observational and epidemiological studies, supported by interventional studies and the ubiquitous evidence of vitamin D receptors in all major organ systems, show an association between 25(OH)D levels and a reduced incidence of infections of the upper respiratory system. In a recent systematic review and meta-analysis of 11 randomized controlled trials with 5660 patients vitamin D showed a protective effect against RTI (OR, 0.64; 95% CI, 0.49 to 0.84). The protective effect was larger in studies using once-daily dosing compared with bolus doses (OR = 0.51 vs OR = 0.86, P = 0.01).77
In a US study of 18,883 persons (age > 12 y) – a representative cross-section of the US population (3rd National Health and Nutrition Examination Survey) – the relationship between serum 25-OH-D levels and the susceptibility to infections of the upper respiratory tract in relation to the season was examined. Vitamin D status correlated inversely with the rate of infection of the upper respiratory tract: Compared with subjects with normal 25(OH)D status (≥30 ng/mL), the subjects with insufficient status (10–30 ng/mL) showed a 1.24-fold increase in infection rate, while the subjects with a pronounced vitamin D deficiency (<10 ng/mL) showed a 1.36-fold increase in infection rate (odds ratio [OR], 1.36; 95% CI, 1.01–1.84 at < 10 ng/mL and 1.24; 1.07–1.43 at 10–29 ng/ml). In patients with bronchial asthma or chronic obstructive pulmonary disease (COPD), the infection rate was even higher showing a 2.26-fold/5.67-fold increase (P = 0.007) (OR, 5.67 and 2.26). The average 25(OH)D level of all participants was 29 ng/mL.78
In a recent randomized double-blinded trial with 247 Mongolian schoolchildren the effect of daily ingestion of vitamin D unfortified or fortified milk (fortified with 300 IU vitamin D) was evaluated (control: n = 104; D-group: n = 143). At baseline, the median serum 25(OH)D level was 7 ng/mL (interquartile range: 5–10 ng/mL). At the end of the trial, follow-up was 99% (n = 244), and the median 25(OH)D levels of children in the control vs. vitamin D groups was significantly different (7 vs. 19 ng/mL; P < 0.001). Compared with controls, children receiving vitamin D reported significantly fewer acute respiratory infections (ARI) during the study period (mean: 0.80 vs. 0.45; P = 0.047), with a rate ratio of 0.52 (95% CI: 0.31–0.89). Adjusting for age, gender, and history of wheezing, vitamin D continued to halve the risk of ARI (rate ratio: 0.50 [95% CI: 0.28–0.88]). Similar results were found among children either below or above the median 25(OH)D level at baseline (rate ratio: 0.41 vs. 0.57; p(interaction) = 0.27). Vitamin D supplementation significantly reduced the risk of ARIs in winter among Mongolian children with vitamin D deficiency.79
In a randomized, placebo-controlled, double-blind study of 334 Japanese schoolchildren, the influence of vitamin D3 supplementation on respiratory diseases such as influenza A and asthma was investigated. The children received a placebo or 1200 IU vitamin D3 daily during the intervention period from December 2008 to March 2009 (Fig. 4). The risk of influenza A was reduced by the supplementation of vitamin D3 by 42% compared with placebo (RR: 0.58; 95% CI: 0.34, 0.99; P = 0.04). The protective effect was particularly pronounced in the children who took no other vitamin D-containing supplements (RR: 0.36; 95% CI: 0.17, 0.79; P = 0.006). The result in relation to the frequency of asthma attacks is even more impressive: In the vitamin D group, the frequency of asthma attacks decreased by 83% (RR: 0.17; 95% CI: 0.04, 0.73; P = 0.006).80 Also in interventional studies with adults, the supplementation of vitamin D3 led to a significant reduction in seasonal flu-like infections.81
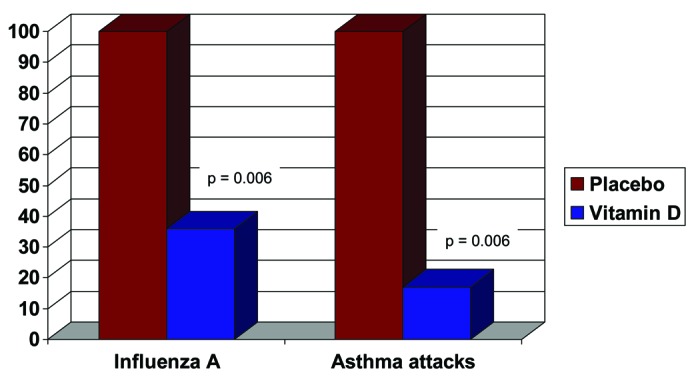
Figure 4. Respiratory tract infections and vitamin D: influence of vitamin D on respiratory diseases such as influenza A and asthma in schoolchildren.39
Comment: With regard to the prevention of respiratory tract infections, children and adults can benefit from a normalization of vitamin D status based on the data from interventional studies. Further interventional studies in the coming years must show whether patients with asthma and COPD can benefit from a supplementation with vitamin D in conjunction with enhancing the effectiveness of some medications (e.g., corticoids).
Atopic dermatitis, psoriasis other dermatides
The ability of vitamin D to regulate local immune and inflammatory responses offers exciting potential for understanding and treating chronic inflammatory dermatitides. That is why vitamin D and its analogs are playing an increasing role in the management of atopic dermatitis, psoriasis, vitiligo, acne and rosacea.82
Vitamin D hormone [1,25(OH)2D] has a pronounced modulating effect on the balance between the Th1 and Th-2 cells. Th1:Th2 imbalances play a pathogenic role in atopic disease in addition to autoimmune diseases such as multiple sclerosis.2 In two randomized, placebo-controlled, double-blind studies of vitamin D supplementation only (1,600 IU / day, PO) and in combination with vitamin E (600 IU/ day, PO) over a period of 60 d led to significant improvement of skin findings in patients (age: 13–45 years of age) with mild, moderate, and severe atopic dermatitis. To assess the extent and intensity of atopic eczema, the SCORAD score (Scoring Atopic Dermatitis) was used. In atopic dermatitis, the inflammatory processes in the skin are associated with an intensive infiltration of lymphocytes and eosinophils, which release proinflammatory cytokines, superoxide radicals, hydrogen peroxides and peroxynitrite. Remarkably, these studies demonstrated that not only vitamin E, but also vitamin D reduces the oxidative load and inflammatory processes in the skin and significantly increases the activity level of the erythrocytic superoxide dismutase (SOD) (P = 0.002) and catalase (CAT) (P = 0.004).83-851,25(OH)2D and its analogs have been shown to be effective when applied topically for the treatment of psoriasis.3
Autoimmune disease: rheumatoid arthritis, IBD and multiple sclerosis
In addition to infectious diseases Vitamin D plays a contributory role in the pathophysiology of autoimmune diseases. This is further supported by various experimental findings showing vitamin D’s capability to regulate chemokine production, counteracting autoimmune inflammation and to induce differentiation of immune cells in a way that promotes self-tolerance. This involves the enhancement of the innate and the inhibition of the adaptive immune system by regulating the interactions between lymphocytes and antigen presenting cells. By increasing the quantity of Th2 lymphocytes and by inducing proliferation of dendritic cells with tolerance properties, vitamin D exerts anti-inflammatory and immunoregulatory effects.86
The increased prevalence of auto-immune diseases at higher latitudes has been shown for multiple sclerosis (MS),86,87 inflammatory bowel disease,88 rheumatoid arthritis89 and type 1 diabetes.86,87,90 The positive effect of supplementation and an adequate 25(OH)D blood level to prevent diabetes type 1 has already been mentioned in the section about diabetology. Meanwhile there exist almost as many studies about vitamin D and rheumatoid arthritis (RA) as with Diabetes type 1. Recent evidence indicates that vitamin D’s effects on the innate immune system are predominantly through the toll-like receptors (TLR) and on the adaptive immune system through T cell differentiation, particularly the Th17 response. As Th17 cells are critical in the pathogenesis of RA, this has led to an interest in the effects of vitamin D deficiency in RA.91 Several studies have looked at the association of vitamin D deficiency with markers of disease activity in RA with somewhat mixed results. In the Iowa womens health study of 29 368 women of ages 55–69 years without a history of rheumatoid arthritis at study baseline showed that greater intake (highest vs. lowest tertile) of vitamin D was inversely associated with the risk of rheumatoid arthritis (RR 0.67; 95% CI: 0.44–1.00; p for trend = 0.05).92 In contrast in a larger cohort of 186389 women followed in the Nurses Health Studies there was no correlation between serum levels of Vitamin D and later development of RA.93
On the other hand recently an evaluation of in vitro effects of 1α,25(OH)2D3 in primary cultures of peripheral blood monocyte-derived macrophages of RA patients and healthy subjects was reported. A significant dose-dependent decrease in TNFα and RANKL production by cultured RA macrophages after 1α,25(OH)2D3 treatment was observed, whereas a significant reduction in normal cells was observed only at higher concentrations. IL-1α, IL-1β and IL-6 levels were reduced by 1α,25(OH)2D3 at higher concentrations in all cell populations. TNFα immunostaining was less intense in treated cells compared with untreated. 1α,25(OH)2D3 significantly reduced NO levels regardless of the concentration used. Vitamin D downregulated pro-inflammatory mediators in monocyte-derived macrophages, and RA cells appeared more sensitive than normal cells. These effects further provide a rationale for the therapeutic value of vitamin D supplementation in the treatment for RA.94 Another just published clinical trial of 4,793 Japanese patients with RA shows a high prevalence of vitamin D deficiency (<20ng/mL) and severe deficiency (<10 ng/mL) (71.8 and 11.5%, respectively). In multivariate analysis, female gender, younger age, high Japanese version of health assessment questionnaire (HAQ) disability score, low serum total protein levels, low serum total cholesterol levels, high serum alkaline phosphate (ALP) levels, and non-steroidal anti-inflammatory drug (NSAID) use were significantly associated with vitamin D deficiency (P < 0.01).95
Inflammatory bowel disease (IBD) is associated with industrialization, and its incidence has increased markedly over time. A review in 2013 described converging data sets that suggest that local activation of vitamin D coordinates the activity of the innate and adaptive arms of immunity, and of the intestinal epithelium, in a manner that promotes barrier integrity, facilitates the clearance of translocated flora, and diverts CD4 T cell development away from inflammatory phenotypes.96 In a small randomized double-blind placebo-controlled trial the benefits of oral vitamin D3 treatment in 108 patients with Crohn’s disease in remission was assessed. Supplementation of vitamin D3 (1200 IU/day) increased significantly the 25(OH)D serum level from mean 69 nmol/L to mean 96 nmol/L after 3 months (P < 0.001). The relapse rate was lower among patients treated with vitamin D3 (6/46 or 13%) than among patients treated with placebo (14/48 or 29%), (P = 0.06).97 In a recently published pilot study the influence of vitamin D3 supplementation on Crohn’s disease activity index (CDAI) was tested in patients with mild-to-moderate Crohn’s disease. Vitamin D3 oral therapy was initiated at 1000 IU/d and after 2 weeks, the dose was escalated incrementally until patients' serum concentrations reached 40 ng/ml 25(OH)D3 or they were taking 5000 IU/d. Vitamin D supplementation significantly increased serum 25(OH)D3 levels from 16 ± 10 ng/ml to 45 ± 19 ng/ml (P < 0.0001) and reduced the unadjusted mean CDAI scores by 112 ± 81 points from 230 ± 74 to 118 ± 66 (P < 0.0001). Quality-of-life scores also improved following vitamin D supplementation (P = 0.0004) (Fig. 5).144 It is particularly notable that vitamin D receptor knockout and vitamin D deficient mice have a surplus of effector T cells that have been implicated in the pathology of inflammatory bowel disease and multiple sclerosis (MS). 1α,25(OH)2D directly and indirectly suppresses the function of these pathogenic T cells while inducing several regulatory T cells that suppress the development of IBD and MS. Nonetheless, current evidence suggests that improving 25(OH)D status and/or using vitamin D receptor agonists may be useful in the treatment of inflammatory bowel disease and multiple sclerosis.98
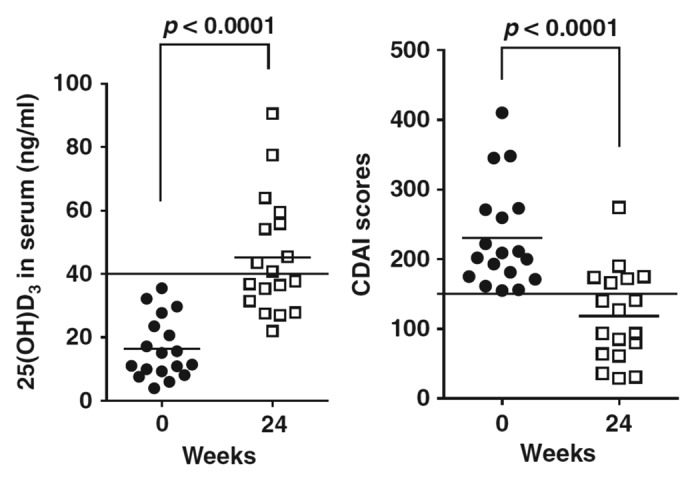
Figure 5. Vitamin D and Crohn’s disease. Twenty-four weeks supplementation with up to 5,000 IU/d vitamin D3 effectively raised serum 25(OH)D3 and reduced CDAI scores in a small cohort of Crohn’s patients suggesting that restoration of normal vitamin D serum levels may be useful in the management of patients with mild–moderate Crohn’s disease.144
Even though the autoimmune origin of multiple sclerosis (MS) is increasingly discussed there is no doubt about the important role vitamin D plays in the development and progression of MS.99,100 Epidemiologic evidence supports an association between vitamin D and susceptibility to and severity of autoimmune disorders. In the specific case of MS, correlations of lower MS prevalence, activity and mortality with high levels of vitamin D nutrition have led to the hypothesis that high levels of vitamin D could be beneficial for MS. Most convincingly, the risk of relapse decreased by up to 12% for every 10-nmol/l (4 ng/mL) increase in serum 25(OH)D in a prospective population-based cohort study.101 A study addressing vitamin D’s effect on multiple sclerosis showed the safety of high-dose vitamin D (~14 000 IU/day). It appeared to have immunomodulatory effects including a persistent reduction in T-cell proliferation and resulted in a trend for fewer relapse events.102 When examining the association between 25(OH)D-serum levels and the relapse rate in MS patients before and after supplementation with ~3000 IU vitamin D per day, a significant strong inverse relationship between the relapse incidence rate and the 25(OH)D level (P < 0.0001) was found.103 However, there are unresolved clinical questions related to the effect of vitamin D supplementation and MS. In general the literature is often limited by small study sizes (ranging from 23 to 68 patients), heterogeneity of dosing, form of vitamin D tested (vitamin D3 in 4 trials and vitamin D2 in 1) and clinical outcome measures. The evidence for vitamin D as a treatment for MS is inconclusive. Larger studies are warranted to assess the effect of vitamin D on clinical outcomes in patients with MS.104,105
Comment: There is reason to believe that vitamin D could be an environmental factor that plays a crucial role in the development of different autoimmune diseases. It seems to be reasonable to maintain a healthy 25(OH)D-status especially in patients with RA, IBD and MS, especially during winter [25(OH)D target value: 40–60 ng/mL or 100–150 nmol/L].
Degenerative Brain Disease and Traumatic Injury
The links between vitamin D and brain function have strengthened considerably in the past decade. The combination of in vitro, ex vivo, and animal model data provide compelling evidence that vitamin D has a crucial role in proliferation, differentiation, neurotrophism, neuroprotection, neurotransmission, and neuroplasticity. Vitamin D exerts its biological function not only by influencing cellular processes directly, but also by influencing gene expression through vitamin D response elements.106 Occasionally, the evidence from these different research domains converges.107 The strength of evidence varies for schizophrenia, autism, Parkinson disease, amyotrophic lateral sclerosis and Alzheimer disease.
In 2010 Knekt et al. looked at the Mini-Finland Health Survey, which was conducted from 1978 to 1980, with Parkinson disease occurrence follow-up through the end of 2007 with three thousand one hundred 73 men and women, aged 50 to 79 years and free of Parkinson disease at baseline. Main Outcome Measure was Parkinson disease incidence. Individuals with higher serum 25(OH)D concentrations showed a reduced risk of Parkinson disease. The relative risk between the highest and lowest quartiles was 0.33 (95% confidence interval, 0.14–0.80) after adjustment for sex, age, marital status, education, alcohol consumption, leisure-time physical activity, smoking, body mass index, and month of blood draw.108 114 patients with Parkinson disease were randomly assigned to receive vitamin D3 supplements (n = 56; 1200 IU/d) or a placebo (n = 58) for 12 mo in a double-blind setting. Outcomes were clinical changes from baseline and the percentage of patients who showed no worsening of the modified Hoehn and Yahr (HY) stage and Unified Parkinson Disease Rating Scale (UPDRS). Compared with the placebo, vitamin D3 significantly prevented the deterioration of the HY stage in patients (P = 0.005). Compared with the placebo, vitamin D3 significantly prevented deterioration of the HY stage in patients with FokI TT (P = 0.009) and and FokI CT (P = 0.020) but not FokI CC.109
In addition to chronic brain diseases injuries arising from brain trauma (TBI) are among the causes of death and severe disabilities. Finding drugs which are effectively neuroprotective is of special importance for prevention of secondary brain injury after trauma. Similar to progesterone, 1α,25(OH)2D is a neurosteroid acting like progesterone in neuroprotective process, e.g., 1α,25(OH)2D reduces the pro-inflammatory level of TH1 cytokines such as IL6, IL12, IL1B, and TNFα.110,111 Recent studies have shown that vitamin D deficiency may intensify traumatic brain injury and reduce the effects of other therapies for TBI.112 Meanwhile, Vitamin D therapy in combination with progesterone was evaluated in a clinical study with 60 patients and showed improved results: The recovery rate in patients with severe brain trauma in the group receiving progesterone and vitamin D together was significantly higher than that of progesterone group, which was in turn higher than that of placebo group.113
Comment: 25(OH)D status should be monitored in all patients with degenerative brain disease (e.g., Alzheimer, Parkinson disease) and be treated by adequate vitamin D supplementation [25(OH)D target value: 40–60 ng/mL or 100–150 nmol/L).
Cancers
A large body of evidence indicates that solar UV-B (UVB) irradiance and vitamin D reduce the risk of incidence and death for many types of cancer. For men, the UVB index was significantly inversely correlated with 14 types of internal cancer-bladder, breast, colon, gallbladder, kidney, laryngeal, liver, lung, oral, pancreatic, pharyngeal, prostate, rectal and small intestine cancer. For women, the same UVB index was inversely correlated with bladder, breast and colon cancer. The results of many studies provide support for the UVB-vitamin D-cancer hypothesis and suggest that the widespread fear of chronic solar UV (UV) irradiance may be misplaced.114,115
Vitamin D deficiency is common in cancer patients and correlates with disease progression. In observational studies, vitamin D deficiency is associated with increased incidence of breast and colon cancer as well as with an unfavorable course of non-Hodgkin lymphoma.116-120 In a placebo-controlled, double-blind study of 1179 postmenopausal women aged over 55 years, the influence of 1400 mg of calcium daily, the combination of 1,400 mg of calcium and 1100 I.U. of vitamin D3 or placebo on the cancer risk was studied over a period of 4 years. In the woman who received the combination of calcium and vitamin D, the 25(OH)D level rose from 28.7 ng/mL to 38.4 ng/mL. Vitamin D status remained unchanged in the two other groups. At the end of the four-year period, the relative risk (RR) of developing cancer was reduced by 60% in the calcium + vitamin D3 group as compared with the placebo group relative risk (RR) cancer (RR: 0.402, CI: 0.20, 0.82; p = 0.013), while in the group with calcium alone it was reduced by 47% (RR: 0.532, CI: 0.27, 1.03; p = 0.063). A reevaluation using logistic regression to cancer-free survival at 12 mo showed that the relative risk in the calcium + vitamin D3 group had been significantly reduced by 77% (RR 0.232, CI: 0.09, 0.60, P < 0.005). The data in the calcium group alone remained virtually unchanged (RR: 0.587, CI: 0.29, 1.21; p = 0.147).121 Some studies indicate that the intake of vitamin D in the range of 1100 to 4000 IU/d and a 25(OH)D serum levels between 60 and 80 ng/ml may be needed to reduce the cancer risk.122
In a prospective cohort study, Canadian researchers from the Mount Sinai Hospital in Toronto observed the course of disease in 512 women with breast cancer for about 12 years from 1997 to 2008. The average age of the women was 50.4 years at diagnosis. 37.5 percent of the breast cancer patients had a vitamin D deficiency [25(OH)D <20 ng/mL or <50 nmol/L] when diagnosed. Only 24 percent of the affected women had an almost normal vitamin D status [25(OH)D > 29 ng/mL or 72 nmol/L]. Vitamin D deficiency was associated with the occurrence of more aggressive forms of breast cancer. After 12 y, the risk of a metastasis in women with a vitamin D deficiency was increased by 94 percent compared with those with normal vitamin D status (hazard ratio [HR] = 1.94; 95% CI, 1.16 to 3.25). The probability of premature death due to the disease rose in the presence of a vitamin D deficiency by 73 percent (HR = 1.73; 95% CI, 1.05 to 2.86).123
In breast cancer patients under polychemotherapy with anthracycline and taxane, a significant drop in 25(OH)D levels was observed.124 Some cytostatics (e.g., cyclophosphamide, paclitaxel) are ligands of the pregnane X receptor and can therefore increase the enzymatic degradation of 25(OH)D and 1α,25(OH)2D via the induction of 24-hydroxylase in the course of chemotherapy.112,132 Docetaxel is a known trigger for cutaneous adverse reactions and taste disorders. A vitamin D deficiency can lead to the occurrence of chemotherapy-induced mucositis and dysgeusia. There have been case reports of mucocutaneous side effects (e.g., stomatitis) and taste disorders occurring in cancer patients under polychemotherapy with TCH (T: docetaxel, C: carboplatin, H: trastuzumab) or FOLFOX6, which could be treated successfully with supplementation of vitamin D3.126 Also, arthralgias and fatigue during treatment with aromatase inhibitors such as letrozole were significantly reduced by supplementation of vitamin D3 (e.g., 50 000 IU vitamin D3/week for 12 weeks, PO) in breast cancer patients with vitamin D deficiency.127,128 Similar results are on record for use of bisphosphonates. The osseous effectiveness of bisphosphonates is improved by an adequate vitamin D status [25(OH)D ≥33 ng/ml]. This could be related to the fact that a cessation of the parathyroid hormone increase is not achieved until a 25(OH)D level ≥40 ng/ml is reached.2,5,129 Necrotic bone exposure in the oral cavity has recently been reported in patients treated with nitrogen-containing bisphosphonates as part of their therapeutic regimen for multiple myeloma or metastatic cancers to bone. It is suggested that the pathophysiologic mechanism(s) underpinning osteonecrosis of the jaw may involve the interaction between bisphosphonates and compromised vitamin D functions in the realm of skeletal homeostasis and innate immunity.138 In a recently published case-control study with 43 patients, 77% of patients with BRONJ were osteomalacic compared with 5% of patients without BRONJ, according to histomorphometry (P < 0.001). Osteomalacia represents a new and previously unreported risk factor for the development of bisphosphonate-related osteonecrosis of the jaw (BRONJ).146 In vitamin D deficiency (until it is corrected) oral bisphosphonates should not be used.139,140
Comment: Vitamin D status should be monitored in all cancer patients and treated by adequate vitamin D supplementation [25(OH)D target value: 40–60 ng/mL or 100–150 nmol/L). This applies in particular to cancer patients with poor nutritional status, treatment with aromatase inhibitors, bisphosphonates, and CTX containing anthracycline taxane as well as in cases of muscular or mucocutaneous disorders, fatigue and tumor cachexia.
Medical Drugs and Vitamin D
Drug-induced vitamin D imbalances must be reconsidered in the light of the high prophylactic potential of the sunshine vitamin. It is known that many drugs can interfere with vitamin D metabolism. Already in 1967 the association between osteomalacia and antiepileptic drug therapy was reported. Since then there have been many reports of abnormalities in calcium, vitamin D, and bone metabolism in subjects chronically treated not only with antiepileptic drugs but also with corticoids, rifampicin, and antiretroviral drugs.130,131 A drug-induced vitamin D deficiency [25(OH)D <20 ng/mL] may manifest as secondary hyperparathyroidism, bone mineralization disorders including the development of osteoporosis, and osteomalacia.
One possible mechanism discussed by various authors that explains the abnormalities in bone metabolism under medication is the activation of pregnane X rececptor (PXR) by some drugs (Table 1), that may be responsible for the acceleration of vitamin D catabolism through the upregulation of CYP3A4 and CYP24A1, leading to vitamin D deficiency and, eventually, to osteopenia or osteomalacia. Human PXR (hPXR) is also named steroid and xenobiotic receptor (SXR). CYP24 is a multifunctional 24-hydroxylase and the major vitamin D catabolic enzyme that directs the side-chain oxidation and cleavage of 1α,25(OH)2D and 25(OH)D to catabolic carboxylic acid end products. This could mean: drugs that can stimulate the pregnane X receptor can potentially trigger all of the negative consequences of a vitamin D deficiency. But the effects of PXR on bone homeostasis are tissue specific and signal specific, and drug-induced osteomalacia may be far more complicated than just a phenomenon of enhanced CYP3A4 (or CYP24A1) expression and induced 25(OH)D and 1α,25(OH)2D catabolism. A drug-induced vitamin D deficiency manifests mainly at the level of bone and muscle metabolism.132-134
Table 1. Prescription and over the counter drugs that can activate the pregnane X receptor (PXR) (selection).
| PXR ligands | Examples |
|---|---|
| Androgen receptor antagonists | Cyproterone acetate |
| Antiepileptics | Phenytoin, carbamazepine |
| Antiestrogens | Tamoxifen |
| Antihypertensives | Nifedipine, spironolactone |
| Antimycotics | Clotrimazole |
| Antiretroviral drugs (NNRTI/protease inhibitors) | Efavirenz, nevirapine / ritononavir, saquinavir |
| Antituberculosis drugs | Rifampicin |
| Glucocorticoids | Dexamethasone |
| Phytopharmaceuticals | Kava-kava, St. John’s wort (hyperforin) |
| Cytostatics | Cyclophosphamide, paclitaxel |
Comment: As a general rule, the vitamin D status of all patients undergoing long-term medication regimens should be monitored in view of the fact that not all of the agonists of the pregnane X receptor in pharmaceutical substances that can degrade vitamin D have been described to date, often this patients need more vitamin d to achieve an optimum 25(OH)D status of 40–60 ng/ml or 100–150 nmol/l!
Treatment Strategies
Sensible sun exposure is the least expensive and most efficient way of obtaining an adequate amount of vitamin D. It has been estimated that a healthy adult in a bathing suit exposed to one minimal erythemal dose (MED) of sunlight is equivalent to ingesting about 20 000 IUs of vitamin D.3,52 Thus the skin has a large capacity to produce vitamin D. Because time of day, season of the year and latitude along with degree of skin pigmentation has a dramatic effect on the cutaneous production of vitamin D there is no simple recommendation as to how much time to be exposed to obtain an adequate amount of vitamin D. However for example if a person knows that they are going to get a light pinkness to their skin 24 h later, i.e., a MED, by being exposed to 30 min of sun in their locale, the recommendation is to expose arms, legs and abdomen and back when possible for about 10–15 min followed by good sun protection. One way to determine how much vitamin D a child or adult is producing is to use a free app dminder.info which not only provides the user with how much vitamin D they are producing but also tells them when to stop being exposed to sunlight without sun protection to prevent sunburn.
Oral vitamin D (vitamin D2 or D3) can be taken on an empty stomach or with a meal. Both vitamin D2 and vitamin D3 at physiologic doses are effective in raising the blood level of 25(OH)D.141 The meal does not need to contain fat in order for the fat-soluble vitamin D to be absorbed. Furthermore vitamin D can be taken daily or the total amount can be taken once a week or even once a month as long as the total is the same i.e., 3000 IUs daily or 21 000 IUs weekly or 90 000 IUs monthly are equally effective in maintaining serum 25(OH)D levels in the desired range of 40–60 ng/mL.
To guarantee vitamin D sufficiency there are a variety of strategies to both treat and prevent vitamin D deficiency. One simple strategy that is effective is to fill the empty vitamin D tank with 50 000 IUs of vitamin D once a week for 8–12 weeks.3,142 This is equivalent to ingesting approximately 6600 IUs daily. To prevent recurrence of vitamin D deficiency 50 000 IUs of vitamin D once every 2 weeks (equivalent to 3300 IUs daily) forever is effective in maintaining a healthy vitamin D status without causing toxicity.142 Even young children have been effectively treated for vitamin D deficiency with 50 000 IUs of vitamin D weekly for 6 weeks or 2000 IUs of vitamin D daily.143
Concluding Remarks
Closer attention should be paid to vitamin D deficiency in medical and pharmaceutical practice than has been the case hitherto. The data available to date on vitamin D from experimental, ecological, case-control, retrospective and prospective observational studies, as well as smaller intervention studies, are significant and confirm the sunshine vitamin’s essential role in a variety of physiological and preventative functions, including neuropsychiatric disorders. The results of these studies justify the recommendation to improve the general vitamin D status in children and adults by means of a healthy approach to sunlight exposure, consumption of foods containing vitamin D and supplementation with vitamin D preparations. Nevertheless, in a number of fields, we must await the results of controlled and randomized interventional studies involving the use of vitamin D in sufficiently high doses, which are still pending and can be expected to be published in the coming years.1,2,135-137
Citation: Gröber U, Spitz J, Reichrath J, Kisters K, Holick MF.Vitamin D―Update 2013: From rickets prophylaxis to general preventive healthcare. Dermato-Endocrinology 2013; 5: - ;
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/26738
References
- 1.Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111–48. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gröber U, Holick MF, Vitamin D. Die Heilkraft des Sonnenvitamins. 2.Auflage, 304 S., Wissenschaftliche Verlagsgesellschaft, Stuttgart, 2013. [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS One. 2013;8:e58725. doi: 10.1371/journal.pone.0058725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gröber U, Mücke R, Adamietz IA, et al. Komplementärer Einsatz von Antioxidanzien und Mikronährstoffen in der Onkologie - Update 2013. Onkologie. 2013;19:136–43. doi: 10.1007/s00761-012-2385-9. [DOI] [Google Scholar]
- 6.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 7.Grant WB, Tangpricha V, Vitamin D. Vitamin D: Its role in disease prevention. Dermatoendocrinol. 2012;4:81–3. doi: 10.4161/derm.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohr SB, Gorham ED, Alcaraz JE, Kane CI, Macera CA, Parsons JK, Wingard DL, Garland CF. Does the evidence for an inverse relationship between serum vitamin D status and breast cancer risk satisfy the Hill criteria? Dermatoendocrinol. 2012;4:152–7. doi: 10.4161/derm.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 10.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ, Kienreich K, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12:976–89. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Schöttker B, Haug U, Schomburg L, Köhrle J, Perna L, Müller H, Holleczek B, Brenner H. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97:782–93. doi: 10.3945/ajcn.112.047712. [DOI] [PubMed] [Google Scholar]
- 12.Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab. 2012;97:3989–95. doi: 10.1210/jc.2012-2276. [DOI] [PubMed] [Google Scholar]
- 13.Peiris AN, Youssef D, Grant WB. Secondary hyperparathyroidism: benign bystander or culpable contributor to adverse health outcomes? South Med J. 2012;105:36–42. doi: 10.1097/SMJ.0b013e31823c4155. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153–8. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 15.Sohl E, van Schoor NM, de Jongh RT, Visser M, Deeg DJ, Lips P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J Clin Endocrinol Metab. 2013;98:E1483–90. doi: 10.1210/jc.2013-1698. [DOI] [PubMed] [Google Scholar]
- 16.Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, Vettorazzi E, Zustin J, Hahn M, Ager JW, 3rd, et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci Transl Med. 2013;5:93ra88. doi: 10.1126/scitranslmed.3006286. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–9. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 18.Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–9. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 19.Ceglia L, da Silva Morais M, Park LK, Morris E, Harris SS, Bischoff-Ferrari HA, Fielding RA, Dawson-Hughes B. Multi-step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. J Mol Histol. 2010;41:137–42. doi: 10.1007/s10735-010-9270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. Re: Fall prevention with Vitamin D. Clarifications needed. http://wwwbmjcom/content/339/bmjb3692?tab=responses (access: Feb132012), 2011.
- 22.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–12. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 23.Stein SH, Tipton DA. Vitamin D and its impact on oral health--an update. J Tenn Dent Assoc. 2011;91:30–3, quiz 34-5. [PubMed] [Google Scholar]
- 24.Hujoel PP. Vitamin D and dental caries in controlled clinical trials: systematic review and meta-analysis. Nutr Rev. 2013;71:88–97. doi: 10.1111/j.1753-4887.2012.00544.x. [DOI] [PubMed] [Google Scholar]
- 25.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 26.Palm TA. The geographical distribution and aetiology of rickets. Practitioner. 1890;XLV:270–342. [Google Scholar]
- 27.Hess AF, Unger LJ. The cure of infantile rickets by sunlight. J Am Med Assoc. 1921;77:39–41. doi: 10.1001/jama.1921.02630270037013. [DOI] [Google Scholar]
- 28.Havinga E. Vitamin D, example and challenge. Experientia. 1973;29:1181–93. doi: 10.1007/BF01935064. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman FL. The mortality from cancer throughout the world: Prudential Press; 1916. [Google Scholar]
- 30.Peller S, Stephenson CS. Skin Irritation and Cancer in the US Navy. Am J Med Sci. 1937;194:326–33. doi: 10.1097/00000441-193709000-00004. [DOI] [Google Scholar]
- 31.Apperly FL. The Relation of Solar Radiation to Cancer Mortality in North America. Cancer Res. 1941;1:191–5. doi: 10.1158/0008-5472.CAN-15-3169. [DOI] [PubMed] [Google Scholar]
- 32.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–31. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 33.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–8. doi: 10.1016/S0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 34.Lefkowitz ES, Garland CF. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23:1133–6. doi: 10.1093/ije/23.6.1133. [DOI] [PubMed] [Google Scholar]
- 35.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–22. doi: 10.1016/0091-7435(90)90058-R. [DOI] [PubMed] [Google Scholar]
- 36.Mizoue T. Ecological study of solar radiation and cancer mortality in Japan. Health Phys. 2004;87:532–8. doi: 10.1097/01.HP.0000137179.03423.0b. [DOI] [PubMed] [Google Scholar]
- 37.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::AID-CNCR2820701224>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 38.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 39.Grant WB. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012;32:223–36. [PubMed] [Google Scholar]
- 39a.Grant WB. Update on evidence that support a role of solar ultraviolet-B irradiance in reducing cancer risk. Anticancer Agents Med Chem. 2013;13:140–6. doi: 10.2174/187152013804487425. [DOI] [PubMed] [Google Scholar]
- 40.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:422–9. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- 42.Luscombe CJ, Fryer AA, French ME, Liu S, Saxby MF, Jones PW, Strange RC. Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet. 2001;358:641–2. doi: 10.1016/S0140-6736(01)05788-9. [DOI] [PubMed] [Google Scholar]
- 43.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 44.Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;181-182:71–8. doi: 10.1016/S0300-483X(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 45.Grant WB. The prevalence of multiple sclerosis in 3 US communities: the role of vitamin D. Prev Chronic Dis. 2010;7:A89–, author reply A90. [PMC free article] [PubMed] [Google Scholar]
- 46.Vieira VM, Hart JE, Webster TF, Weinberg J, Puett R, Laden F, Costenbader KH, Karlson EW. Association between residences in U.S. northern latitudes and rheumatoid arthritis: A spatial analysis of the Nurses’ Health Study. Environ Health Perspect. 2010;118:957–61. doi: 10.1289/ehp.0901861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinney DK, Teixeira P, Hsu D, Napoleon SC, Crowley DJ, Miller A, Hyman W, Huang E. Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: a role for prenatal vitamin d deficiency and infections? Schizophr Bull. 2009;35:582–95. doi: 10.1093/schbul/sbp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Callaghan E, Gibson T, Colohan HA, Walshe D, Buckley P, Larkin C, Waddington JL. Season of birth in schizophrenia. Evidence for confinement of an excess of winter births to patients without a family history of mental disorder. Br J Psychiatry. 1991;158:764–9. doi: 10.1192/bjp.158.6.764. [DOI] [PubMed] [Google Scholar]
- 49.The Nobel Prize in Physiology or Medicine 1903. Niels Ryberg Finsen. 2012-09-08. URL:http://www.nobelprize.org/nobel_prizes/medicine/laureates/1903/ Accessed: 2012-09 08 (Archived by WebCiteR at http://www.webcitation.org/6AWpWACU0). 1903.
- 50.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.HYP.30.2.150. [DOI] [PubMed] [Google Scholar]
- 51.Reid IR, Bolland MJ. Role of vitamin D deficiency in cardiovascular disease. Heart. 2012;98:609–14. doi: 10.1136/heartjnl-2011-301356. [DOI] [PubMed] [Google Scholar]
- 52.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB, Intermountain Heart Collaborative (IHC) Study Group Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 54.Mullie P, Autier P. Relation of vitamin d deficiency to cardiovascular disease. Am J Cardiol. 2011;107:956–, author reply 956-7. doi: 10.1016/j.amjcard.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 55.Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43:1470–7. doi: 10.1161/STROKEAHA.111.636910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–54. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 57.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, Bennett GG, Chandler PD, Hollis BW, Emmons KM, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–85. doi: 10.1161/HYPERTENSIONAHA.111.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, Li K, Bassali R, Guo DH, Thomas J, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–91. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 59.Salum E, Kals J, Kampus P, Salum T, Zilmer K, Aunapuu M, Arend A, Eha J, Zilmer M. Vitamin D reduces deposition of advanced glycation end-products in the aortic wall and systemic oxidative stress in diabetic rats. Diabetes Res Clin Pract. 2013;100:243–9. doi: 10.1016/j.diabres.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Shedeed SA. Vitamin D supplementation in infants with chronic congestive heart failure. Pediatr Cardiol. 2012;33:713–9. doi: 10.1007/s00246-012-0199-6. [DOI] [PubMed] [Google Scholar]
- 61.Oz F, Cizgici AY, Oflaz H, Elitok A, Karaayvaz EB, Mercanoglu F, Bugra Z, Omer B, Adalet K, Oncul A. Impact of vitamin D insufficiency on the epicardial coronary flow velocity and endothelial function. Coron Artery Dis. 2013;24:392–7. doi: 10.1097/MCA.0b013e328362b2c8. [DOI] [PubMed] [Google Scholar]
- 62.Hyppönen E. Vitamin D and increasing incidence of type 1 diabetes-evidence for an association? Diabetes Obes Metab. 2010;12:737–43. doi: 10.1111/j.1463-1326.2010.01211.x. [DOI] [PubMed] [Google Scholar]
- 63.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39:419–46. doi: 10.1016/j.ecl.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Driver JP, Foreman O, Mathieu C, van Etten E, Serreze DV. Comparative therapeutic effects of orally administered 1,25-dihydroxyvitamin D(3) and 1α-hydroxyvitamin D(3) on type-1 diabetes in non-obese diabetic mice fed a normal-calcaemic diet. Clin Exp Immunol. 2008;151:76–85. doi: 10.1111/j.1365-2249.2007.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–21. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 67.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–7. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 68.Sørensen IM, Joner G, Jenum PA, Eskild A, Torjesen PA, Stene LC. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61:175–8. doi: 10.2337/db11-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59:381–91. doi: 10.1373/clinchem.2012.193003. [DOI] [PubMed] [Google Scholar]
- 70.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–55. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 71.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 72.Boucher BJ. Is vitamin D status relevant to metabolic syndrome? Dermatoendocrinol. 2012;4:212–24. doi: 10.4161/derm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab) J Clin Endocrinol Metab. 2012;97:1953–61. doi: 10.1210/jc.2011-3187. [DOI] [PubMed] [Google Scholar]
- 74.Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Östenson CG. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55:1668–78. doi: 10.1007/s00125-012-2529-x. [DOI] [PubMed] [Google Scholar]
- 75.Thomas GN, ó Hartaigh B, Bosch JA, Pilz S, Loerbroks A, Kleber ME, Fischer JE, Grammer TB, Böhm BO, März W. Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Diabetes Care. 2012;35:1158–64. doi: 10.2337/dc11-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Talaei A, Mohamadi M, Adgi Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol Metab Syndr. 2013;5:8. doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergman P, Lindh AU, Björkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ginde AA, Mansbach JM, Camargo CA., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Camargo CA, Jr., Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, Sumberzul N, Rich-Edwards JW. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130:e561–7. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 80.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 81.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6, author reply 1097-8. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Youssef DA, Miller CW, El-Abbassi AM, Cutchins DC, Cutchins C, Grant WB, Peiris AN. Antimicrobial implications of vitamin D. Dermatoendocrinol. 2011;3:220–9. doi: 10.4161/derm.3.4.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amestejani M, Salehi BS, Vasigh M, Sobhkhiz A, Karami M, Alinia H, Kamrava SK, Shamspour N, Ghalehbaghi B, Behzadi AH. Vitamin D supplementation in the treatment of atopic dermatitis: a clinical trial study. J Drugs Dermatol. 2012;11:327–30. [PubMed] [Google Scholar]
- 84.Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, Seirafi H, Ehsani AH, Chamari M, Mirshafiey A. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat. 2011;22:144–50. doi: 10.3109/09546630903578566. [DOI] [PubMed] [Google Scholar]
- 85.Javanbakht M, Keshavarz S, Mirshafiey A, Djalali M, Siassi F, Eshraghian M, Firooz A, Seirafi H, Ehsani A, Chamari M. The effects of vitamins e and d supplementation on erythrocyte superoxide dismutase and catalase in atopic dermatitis. Iran J Public Health. 2010;39:57–63. [PMC free article] [PubMed] [Google Scholar]
- 86.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–36. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;181-182:71–8. doi: 10.1016/S0300-483X(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 88.Peyrin-Biroulet L, Oussalah A, Bigard MA. Crohn’s disease: the hot hypothesis. Med Hypotheses. 2009;73:94–6. doi: 10.1016/j.mehy.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 89.Vieira VM, Hart JE, Webster TF, Weinberg J, Puett R, Laden F, Costenbader KH, Karlson EW. Association between residences in U.S. northern latitudes and rheumatoid arthritis: A spatial analysis of the Nurses’ Health Study. Environ Health Perspect. 2010;118:957–61. doi: 10.1289/ehp.0901861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51:1391–8. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 91.Sen D, Ranganathan P. Vitamin D in rheumatoid arthritis: panacea or placebo? Discov Med. 2012;14:311–9. [PubMed] [Google Scholar]
- 92.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG, Iowa Women’s Health Study Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 93.Nielen MM, van Schaardenburg D, Lems WF, van de Stadt RJ, de Koning MH, Reesink HW, Habibuw MR, van der Horst-Bruinsma IE, Twisk JW, Dijkmans BA. Vitamin D deficiency does not increase the risk of rheumatoid arthritis: comment on the article by Merlino et al. Arthritis Rheum. 2006;54:3719–20. doi: 10.1002/art.22191. [DOI] [PubMed] [Google Scholar]
- 94.Neve A, Corrado A, Cantatore FP. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med. 2013 doi: 10.1007/s10238-013-0249-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 95.Furuya T, Hosoi T, Tanaka E, Nakajima A, Taniguchi A, Momohara S, Yamanaka H. Prevalence of and factors associated with vitamin D deficiency in 4,793 Japanese patients with rheumatoid arthritis. Clin Rheumatol. 2013;32:1081–7. doi: 10.1007/s10067-013-2216-4. [DOI] [PubMed] [Google Scholar]
- 96.Palmer MT, Weaver CT. Linking vitamin d deficiency to inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2245–56. doi: 10.1097/MIB.0b013e31828a3b6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 98.Cantorna MT, Vitamin D. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys. 2012;523:103–6. doi: 10.1016/j.abb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ho SL, Alappat L, Awad AB. Vitamin D and multiple sclerosis. Crit Rev Food Sci Nutr. 2012;52:980–7. doi: 10.1080/10408398.2010.516034. [DOI] [PubMed] [Google Scholar]
- 100.Karmon Y, Ramanathan M, Minagar A, Zivadinov R, Weinstock-Guttman B. Arterial, venous and other vascular risk factors in multiple sclerosis. Neurol Res. 2012;34:754–60. doi: 10.1179/1743132812Y.0000000077. [DOI] [PubMed] [Google Scholar]
- 101.Simpson S, Jr., Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, Dwyer T, Gies P, van der Mei I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 102.Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch HM, Cheung R, Gagne D, D’Souza C, Ursell M, O’Connor P. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74:1852–9. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pierrot-Deseilligny C, Rivaud-Péchoux S, Clerson P, de Paz R, Souberbielle JC. Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord. 2012;5:187–98. doi: 10.1177/1756285612447090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pozuelo-Moyano B, Benito-León J, Mitchell AJ, Hernández-Gallego J. A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in multiple sclerosis. Neuroepidemiology. 2013;40:147–53. doi: 10.1159/000345122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dörr J, Döring A, Paul F. Can we prevent or treat multiple sclerosis by individualised vitamin D supply? EPMA J. 2013;4:4. doi: 10.1186/1878-5085-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39:458–84. doi: 10.1111/nan.12020. [DOI] [PubMed] [Google Scholar]
- 107.Cui X, Groves NJ, Burne TH, Eyles DW, McGrath JJ. Low vitamin D concentration exacerbates adult brain dysfunction. Am J Clin Nutr. 2013;97:907–8. doi: 10.3945/ajcn.113.061176. [DOI] [PubMed] [Google Scholar]
- 108.Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808–11. doi: 10.1001/archneurol.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D, Urashima M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013;97:1004–13. doi: 10.3945/ajcn.112.051664. [DOI] [PubMed] [Google Scholar]
- 110.Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol Med. 2009;15:328–36. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–62. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 112.Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–35. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aminmansour B, Nikbakht H, Ghorbani A, Rezvani M, Rahmani P, Torkashvand M, Nourian M, Moradi M. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: A randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58. doi: 10.4103/2277-9175.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grant WB. Role of solar UVB irradiance and smoking in cancer as inferred from cancer incidence rates by occupation in Nordic countries. Dermatoendocrinol. 2012;4:203–11. doi: 10.4161/derm.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin SW, Wheeler DC, Park Y, Cahoon EK, Hollenbeck AR, Freedman DM, Abnet CC. Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. Int J Cancer. 2012;131:E1015–23. doi: 10.1002/ijc.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer--results of a large case-control study. Carcinogenesis. 2008;29:93–9. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 117.Churilla TM, Brereton HD, Klem M, Peters CA. Vitamin D deficiency is widespread in cancer patients and correlates with advanced stage disease: a community oncology experience. Nutr Cancer. 2012;64:521–5. doi: 10.1080/01635581.2012.661515. [DOI] [PubMed] [Google Scholar]
- 118.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 119.Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, Kelly JL, Macon WR, Nowakowski GS, Inwards DJ, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28:4191–8. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30:113–25. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 121.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 122.Garland CF, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. 2011;31:607–11. [PubMed] [Google Scholar]
- 123.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–63. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 124.Santini D, Galluzzo S, Vincenzi B, Zoccoli A, Ferraro E, Lippi C, Altomare V, Tonini G, Bertoldo F. Longitudinal evaluation of vitamin D plasma levels during anthracycline- and docetaxel-based adjuvant chemotherapy in early-stage breast cancer patients. Ann Oncol. 2010;21:185–6. doi: 10.1093/annonc/mdp497. [DOI] [PubMed] [Google Scholar]
- 125.Fink M. Vitamin D deficiency is a cofactor of chemotherapy-induced mucocutaneous toxicity and dysgeusia. J Clin Oncol. 2011;29:e81–2. doi: 10.1200/JCO.2010.31.5317. [DOI] [PubMed] [Google Scholar]
- 126.Prieto-Alhambra D, Javaid MK, Servitja S, Arden NK, Martinez-García M, Diez-Perez A, Albanell J, Tusquets I, Nogues X. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat. 2011;125:869–78. doi: 10.1007/s10549-010-1075-9. [DOI] [PubMed] [Google Scholar]
- 127.Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O’Dea AP, Klemp JR, Fabian CJ. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–8. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carmel AS, Shieh A, Bang H, Bockman RS. The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/ml. Osteoporos Int. 2012;23:2479–87. doi: 10.1007/s00198-011-1868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berruti A, Cook R, Saad F, Buttigliero C, Lipton A, Tampellini M, Lee KA, Coleman RE, Smith MR. Prognostic role of serum parathyroid hormone levels in advanced prostate cancer patients undergoing zoledronic acid administration. Oncologist. 2012;17:645–52. doi: 10.1634/theoncologist.2011-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schmid F. Osteopathien bei antiepileptischer Dauerbehandlung. Fortschr Med. 1967;38:381–2. [Google Scholar]
- 131.Holick MF. Stay tuned to PXR: an orphan actor that may not be D-structive only to bone. J Clin Invest. 2005;115:32–4. doi: 10.1172/JCI23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang B, Xie W, Krasowski MD. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics. 2008;9:1695–709. doi: 10.2217/14622416.9.11.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou C, Assem M, Tay JC, Watkins PB, Blumberg B, Schuetz EG, Thummel KE. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006;116:1703–12. doi: 10.1172/JCI27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gröber U, Kisters K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinol. 2012;4:158–66. doi: 10.4161/derm.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gröber U. Arzneimittel und Mikronährstoffe. 3., korrigierte Auflage, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 2014. [Google Scholar]
- 136.Hossein-Nezhad A, Holick MF. Vitamin d for health: a global perspective. Mayo Clin Proc. 2013;88:720–55. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eyles DW, Burne TH, McGrath JJ, Vitamin D. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, Garrett N, Adams JS, Nishimura I. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res. 2010;25:1337–49. doi: 10.1002/jbmr.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med. 2010;363:2027–35. doi: 10.1056/NEJMct1004903. [DOI] [PubMed] [Google Scholar]
- 140.Fink M. Pathophysiologie der Kiefernekrose: multifaktoriell und letztlich unklar. InFo Onkologie. 2013;16:23–5. doi: 10.1007/s15004-013-0557-4. [DOI] [Google Scholar]
- 141.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–81. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pietras SM, Obayan BK, Cai MH, Holick MF. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 2009;169:1806–8. doi: 10.1001/archinternmed.2009.361. [DOI] [PubMed] [Google Scholar]
- 143.Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93:2716–21. doi: 10.1210/jc.2007-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang L, Weaver V, Smith JP, Bingaman S, Hartman TJ, Cantorna MT. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin Transl Gastroenterol. 2013;4:e33. doi: 10.1038/ctg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gunville CF, Mourani PM, Ginde AA. The role of vitamin D in prevention and treatment of infection. Inflamm Allergy Drug Targets. 2013;12:239–45. doi: 10.2174/18715281113129990046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bedogni A, Saia G, Bettini G, Tronchet A, Totola A, Bedogni G, Tregnago P, Valenti MT, Bertoldo F, Ferronato G, et al. Osteomalacia: the missing link in the pathogenesis of bisphosphonate-related osteonecrosis of the jaws? Oncologist. 2012;17:1114–9. doi: 10.1634/theoncologist.2012-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]