Abstract
Calcium (Ca2+) signaling modules are essential for adjusting plant growth and performance to environmental constraints. Differential interactions between sensors of Ca2+ dynamics and their molecular targets are at the center of the transduction process. Calmodulin (CaM) and CaM-like (CML) proteins are principal Ca2+-sensors in plants that govern the activities of numerous downstream proteins with regulatory properties. The families of IQ67-Domain (IQD) proteins are a large class of plant-specific CaM/CML-targets (e.g., 33 members in A. thaliana) which share a unique domain of multiple varied CaM retention motifs in tandem orientation. Genetic studies in Arabidopsis and tomato revealed first roles for IQD proteins related to basal defense response and plant development. Molecular, biochemical and histochemical analysis of Arabidopsis IQD1 demonstrated association with microtubules as well as targeting to the cell nucleus and nucleolus. In vivo binding to CaM and kinesin light chain-related protein-1 (KLCR1) suggests a Ca2+-regulated scaffolding function of IQD1 in kinesin motor-dependent transport of multiprotein complexes. Furthermore, because IQD1 interacts in vitro with single-stranded nucleic acids, the prospect arises that IQD1 and other IQD family members facilitate cellular RNA localization as one mechanism to control and fine-tune gene expression and protein sorting.
Keywords: calcium, calmodulin-binding, IQ motif, cytoskeleton, microtubules, kinesin, scaffold proteins, cellular signaling
Introduction
Calmodulin (CaM) and closely related CaM-like (CML) proteins are principal sensors of dynamic intracellular calcium (Ca2+) fluctuations, also known as Ca2+-signatures, which are generated by plants in specific responses to numerous abiotic cues and biotic challenges.1 A multitude of plant CaM/CML Ca2+-sensors (e.g., encoded by 7 CaM and 50 CML genes in the reference plant Arabidopsis thaliana) control diverse biochemical activities of a broad range of regulatory downstream targets via complex Ca2+-dependent and Ca2+-independent interactions.2-4 CaM-interacting entities include, among other functional categories, proteins implicated in Ca2+-signature formation, enzymes with functions in metabolic pathways and signaling cascades, components associated with the cytoskeleton, or nuclear factors regulating gene expression.5,6 The CaM-interacting regions of target proteins are not necessarily related in primary structure and often exhibit high sequence variability, which may reflect the versatility of the Ca2+-CaM/CML sensor module. CaM binding domains typically comprise a short basic amphiphilic helix (15–35 residues), which interacts with a flexible hydrophobic pocket that is formed upon Ca2+-binding to apo-CaM/CMLs.7 Three consensus CaM recruitment motifs are currently known, although not all functionally characterized proteins contain these features. Two related motifs, termed 1-(5)-10 and 1-(8)-14, facilitate Ca2+-dependent interactions and are distinguished by their characteristic spacing of bulky amino acid residues, whereas the IQ motif (IQxxxRGxxxR) and its relaxed versions (I/L/VQxxxRxxxxR/K) are thought to mediate CaM retention in a Ca2+-independent manner.7-9
In A. thaliana, the canonical IQ motif has been identified in at least five protein families with confirmed or putative roles in CaM-regulated processes. Whereas the CNGC family of cyclic nucleotide gated channels (20 members) and the IQ-Motif (IQM) family (6 members) contain only a single IQ motif, the CAMTA family of CaM-binding transcriptional activators (6 members), the myosin family (17 members) and the IQ67-Domain (IQD) family (33 members) harbor multiple copies of this motif in an arrangement that is unique to each family.10-16 Here, we highlight and discuss emerging biochemical roles for IQD proteins, which possibly constitute the largest family of putative CaM/CML targets in plants.
Hallmarks of IQD Gene Families
Plant-specific IQD gene families have been comprehensively annotated for three genomes (Arabidopsis, rice, tomato) and encode about 30 predicted IQD proteins in each species.16,17 The presence of IQD genes in Physcomitrella but absence in algae suggests that IQD proteins are an ancient family of CaM/CML-binding proteins that originated during the early evolution of land plants, possibly before the divergence of bryophyte and vascular plant lineages.16 The common feature of IQD proteins is the presence of a central region of 67 conserved amino acid residues, referred to as the IQ67 domain. Its primary structure is characterized by the invariant spacing of up to three IQ motifs, which are separated by 11 and 15 intervening residues and partially overlap with 1–4 copies of the 1-(5)-10 as well as 1-(8)-14 CaM recruitment motif. Another distinctive hallmark of the IQ67 domain is a highly conserved exon-intron boundary that exactly interrupts codons 16 and 17 via a phase-0 intron.16 An intriguing feature of IQD gene family organization in Arabidopsis and rice is the almost exclusive presence of symmetrical exons flanked by phase-0 introns. The strong bias for symmetrical exons of variable number (2–6 exons) and length, and consequently size of the encoded IQD proteins (~100–900 amino acid residues), is consistent with the hypothesis that IQD proteins evolved by exon shuffling to generate domain diversity at both flanks of their central IQ67 domain.16 Furthermore, the extant IQD loci in Arabidopsis and rice primarily resulted from large-scale segmental genome duplication events and consist of a high fraction of homeologous gene pairs (8 retained sister pairs corresponding to 45% of the paralogous gene set). One explanation for preferential retention of sister genes, which is not observed for the average gene family in Arabidopsis (< 27%), is to counteract disturbances in gene dosage and to maintain the stoichiometry of regulatory multiprotein complexes IQD proteins are subunits of.16 Although IQDs are structurally diverse with respect to their computed molecular mass (12–90 kDa), they are, with only a few exceptions, quite uniform at the physicochemical level and share some properties with RNA-binding proteins, such as basic isoelectric points (pI~10.3) and high fractions of Arg/Lys (~17%) and Ser (~12%) residues.18,19 Interestingly, the very few atypical IQD genes and their encoded proteins of unique properties (deviating domain composition or isoelectric point) are maintained in the genomes of Arabidopsis and rice, which further suggests conserved functions of IQD family members in plants.16
Emerging Roles for IQD Proteins
Only a few biological processes are currently known to be affected by altered IQD protein expression. The first functionally characterized IQD gene, Arabidopsis IQD1, was identified in a genetic screen of T-DNA activation-tagged lines for mutants with altered glucosinolate content and composition.20,21 Glucosinolates are a class of phytoanticipins in crucifers whose derived products, most notably isothiocyanates, possess profound biological activities not only in plant defense against pathogens and herbivores, but also in the prevention of certain types of mammalian cancers.22,23 IQD1 overexpression under control of the cauliflower mosaic virus (CaMV) 35S promoter, which is also causative for the original mutant chemotype, stimulates glucosinolate production in A. thaliana and resistance to herbivory.21 Histochemical analysis of IQD1Pro:GUS lines reveals GUS expression in the vascular bundles of hypocotyls, leaves, stems, flowers and roots, which overlap with the expression patterns reported for several genes encoding enzymes of the glucosinolate pathway. Furthermore, analysis of steady-state mRNA levels indicates that IQD1 effects the expression of multiple genes related to glucosinolate metabolism. Because IQD1 was shown to encode a CaM-binding nuclear protein and its expression responds to mechanical stimuli, IQD1 was hypothesized to integrate Ca2+ signaling in defense responses to biotic challenge.21
A second member of the Arabidopsis IQD gene family, IQD22, was identified as a putative direct downstream target gene of the RGA nuclear transcription factor.24 RGA is one of five DELLA proteins in Arabidopsis that repress gibberellic acid (GA) signaling and restrict plant growth. Interestingly, IQD22 expression is repressed by GA but induced by DELLA, which suggests involvement of IQD22 in negative feedback regulation of GA responses downstream of DELLA function.24
Besides Arabidopsis IQD1, a recent genetic study in tomato (Solanum lycopersicum) uncovered a role for IQD12/SUN in controlling fruit shape.25 The sun quantitative trait locus (QTL), which is one major QTL determining tomato fruit shape, causes long and atypically formed fruits when compared with the wild ancestor. Comparative molecular analysis of the sun locus revealed a retrotransposon-mediated duplication of a 24.7-kb segment carrying the tomato IQD12 gene, which results in a much higher level of IQD12 transcripts.25 CaMV 35S promoter-mediated overexpression of tomato IQD12 recapitulates the phenotype, causing extremely elongated tomato fruits as well as dramatic changes in overall plant architecture, affecting the shape of cotyledons, leaves, floral organs, vein patterning and leads to other morphological alterations, such as twisted growth patterns.25,26 Thus, IQD12/SUN is likely involved in multiple plant developmental processes. The authors proposed that tomato IQD12 participates in the control of cell division planes, possibly by affecting auxin distribution.26 In summary, the first published reports indicate that IQD proteins are involved in the regulation of plant defense response and plant development. However, the molecular functions of IQD proteins and underlying pathways remain to be elucidated.
Functional Insights from Molecular Interactions
A recent study allowed first insight into putative cellular and biochemical functions of Arabidopsis IQD1, with implications for additional IQD family members.27 CaM pulldown and overlay assays demonstrated in vitro binding of IQD1 to various recombinant Arabidopsis CaM and CML proteins, which together suggest complex Ca2+-dependent and Ca2+-independent interactions of IQD proteins with CaM/CML sensors. Deletion mapping of IQD1 confirmed the importance of the IQ67 domain for CaM recruitment in vitro. This was corroborated by experiments with recombinant IQD20, the smallest member of the Arabidopsis IQD family, which features only a short N-terminal extension of 35 amino acid residues adjacent to the IQ67 domain at its very C-terminus.27 Thus, recruitment of apo- and Ca2+-CaM/CML sensors is likely a general property of IQD proteins. The capacity to recruit CaM/CMLs was further demonstrated by yeast two-hybrid assays, which revealed weak but differential in vivo interactions of IQD1 or IQD20 with only a small subset of the 13 CaM/CML sensors tested, but no evidence for IQD protein dimerization.27 Interestingly, a search of the recently published Arabidopsis interactome map retrieved evidence for additional interactions between IQD and CaM/CML proteins in yeast (e.g., IQD1, IQD21, IQD23, IQD31 with CaM1 and IQD31 with CML13).28
The ability of IQD1 to interact with CaM was further confirmed by taking advantage of the observation that IQD1~GFP protein fusions associate with the cytoskeleton. Nuclear targeting was previously reported for IQD1~GFP;21 however, confocal laser scanning microscopy at high resolution revealed localization of IQD1 to both the cell nucleus (including nucleoli) and the microtubular network in transiently transfected tobacco leaves as well as transgenic Arabidopsis seedlings (Fig. 1).27 Interestingly, co-transfection studies in tobacco demonstrated IQD1-dependent recruitment of RFP-tagged Arabidopsis CaM2 from the cytosol to microtubules and thus IQD1:CaM2 interaction in planta. Because many microtubule-associated proteins bind via a positively charged basic domain directly to the acidic tails of tubulins,29 a similar mode of electrostatic interaction may apply to the largely basic IQD proteins.
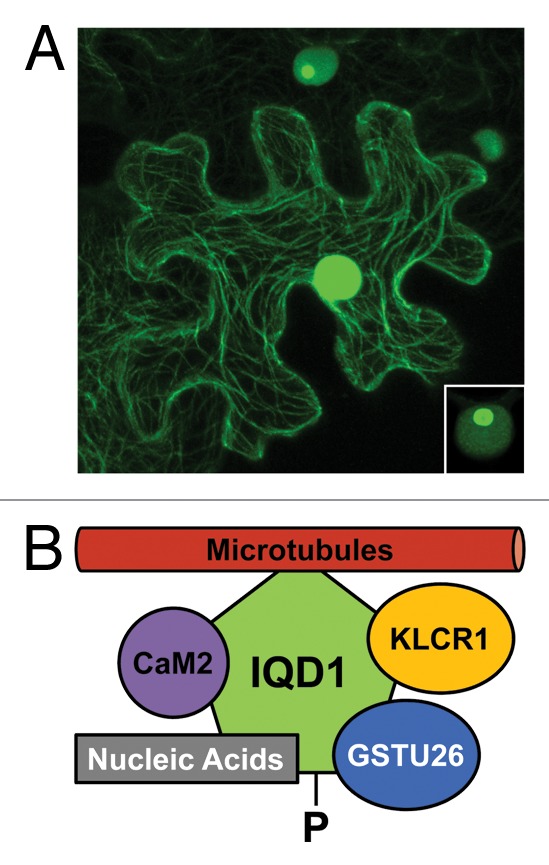
Figure 1. Subcellular localization and molecular interactors of Arabidopsis IQD1. (A) Transient expression of CaMV 35SPro:IQD1~GFP in tobacco leaves (N. benthamiana) reveals association of GFP fluorescence with the microtubular network and cell nucleus (epidermal cell in the center). Lower IQD1~GFP expression level (upper cell) or lower photomultiplier gain (inset, lower right corner) indicates targeting of IQD1~GFP to the nucleolus (see ref. 26 for original report and controls). (B) In addition to its recruitment to microtubules, IQD1 interacts in planta with Arabidopsis CaM2 and Arabidopsis KLCR1.27 A possible interaction of IQD1 in planta with GSTU26 (reproducibly identified in a yeast two-hybrid screen27) and with single nucleic acid substrates (demonstrated in vitro),27 such as cellular RNAs, remains to be tested. Querying the PhosPhAt4.0 database45 retrieved evidence for in vivo phosphorylation (-P) of IQD1on a site near to its N-terminus. To date, there is no evidence for binding of KLCR1 to kinesin motor proteins, which facilitate cellular transport of specific cargo along microtubular tracks.
A genetic yeast two-hybrid screen of an Arabidopsis flower cDNA library with IQD1 as the bait repeatedly isolated kinesin light chain-related protein-1 (KLCR1) as a novel IQD1 interactor. In vivo binding of KLCR1 to IQD1 was confirmed in yeast and in plants. Again, co-transfection studies in tobacco leaves revealed IQD1-dependent sequestration of cytosolic RFP-tagged KLCR1 to microtubules, and similar experiments suggested simultaneous binding of KLCR1 and CaM2 to microtubule-associated IQD1.27 KLCR1 and two closely related tetratricopeptide repeat (TPR) domain-containing proteins, KLCR2 and KLCR3, are similar to mammalian kinesin light chain (KLC) subunits of kinesin motor proteins.27 Kinesins are a class of microtubule-based molecular motors that are usually heterotetrameric, consisting of two heavy chain (KHC) and two light chain (KLC) subunits, and facilitate directional transport of organelles, vesicles, multiprotein or ribonucleoprotein complexes to specific cellular destinations, generally toward the cell periphery.30-32 In addition, specific kinesins play multifaceted roles during cell division and are potentially required for correct orientation of the division plane.33,34 The broader significance of the observed IQD1:KLCR1 interaction is supported by querying the Arabidopsis interactome database, which points to additional interactions between IQD and KLCR proteins, such as IQD2:KLCR1, IQD2:KLCR2, or IQD23:KLCR2.28 Given that most members of the Arabidopsis IQD family are also associated with the cytoskeleton (Bürstenbinder and Abel, unpublished), the prospect arises that microtubule-based IQD proteins are subunits of macromolecular complexes with roles in various kinesin-dependent processes and their regulation.
Are IQD Proteins Novel Scaffolds?
Kinesins typically bind to their cargo via adaptor or scaffold proteins though direct cargo attachment is possible to the globular C-terminal tail of the KHC homodimer or to its two associated KLCs. A KLC subunit contains N-terminal heptad repeats for oligomerization with the coiled-coil stalk of the KHC dimer, and several conserved TPR motifs next to its variable C-terminus may recruit adaptor proteins to KHC-KLC tetramers.30-32 Mounting evidence on kinesin-cargo interactions in animals suggests that KLC-bound scaffold proteins regulate cargo recruitment and kinesin motor activity. Even entire pre-assembled signal transduction modules (sometimes referred to as transducisomes),35 such as mitogen-activated protein kinase (MAPK) cascades associated with vesicle-bound transmembrane receptors, can be tethered to KHC-KLC tetramers via scaffold proteins, which subsequently trigger and inform transport along microtubules to their final membrane destination.30-32 The assembly, loading and unloading of scaffold-associated kinesin cargo complexes is tightly controlled by phosphorylation, GTPase activity, Ca2+-sensors, proteolysis, or other mechanisms.31,32,35 Thus, scaffold proteins composed of variable docking sites provide versatile interaction platforms and serve as critical nodes for the integration of multiple signal transduction pathways to coordinate diverse cellular activities.36,37
Although IQD genes are restricted to land plants, the small family of IQGAP proteins (IQ motif-containing GTPase-activating proteins) in yeast and animals may be instructive for IQD protein function.38-40 Two defining features of IQGAP proteins are the central conserved domain of four tandem IQ motifs, mediating highly complex Ca2+-dependent as well as Ca2+-independent interactions with CaM, and the region containing sequence similarity to Ras GTPase-activating proteins. IQGAPs harbor additional interaction domains that facilitate binding to a large number of diverse target proteins, often in a CaM-dependent manner or via regulation by protein phosphorylation.38 The ability of IQGAPs to simultaneously recruit different proteins suggests a scaffolding function in the assembly of higher order complexes with specific roles for fundamental cellular processes. For example, a large body of work indicates that IQGAP1 regulates cell growth and morphogenesis by coupling cellular signaling pathways to cytoskeleton dynamics via direct interaction with F-actin and microtubules.37 Recent studies provided strong evidence that human IQGAP1 is required as a scaffold protein for efficient signal propagation via MAPK cascades, which in turn may be regulated by Ca2+-sensors and integrate numerous other inputs from multiple signaling pathways.37,39,40 In short, IQGAPs are scaffolds that facilitate and modulate cross-talk among diverse cellular pathways in complex regulatory circuits.
The collective evidence suggests that possibly most IQD genes code for an assortment of KLCR-interacting protein scaffolds. However, it remains to be tested whether plant KLCRs are indeed subunits of plant kinesins and interact with KHC dimers. If so, IQD proteins are likely to participate in various kinesin-dependent processes, such as spatial control of cytokinesis,34 as may be the case for tomato IQD12/SUN,26 or directional transport of metabolons and perhaps ribonucleoprotein complexes, as recently discussed for Arabidopsis IQD1.27 In addition to KLCR1, the yeast two-hybrid screen for IQD1 interactors identified a second confirmed target, glutathione S-transferase GSTU26, which may participate in glucosinolate biosynthesis. Interestingly, IQD1 was also shown to bind in vitro to single-stranded but artificial nucleic acid substrates.27 Thus, microtubule-associated IQD1 seems to be capable of interacting simultaneously with multiple proteins and possibly binds to cellular but as yet unknown RNA molecules (Fig. 1). Most IQD family members share certain properties with RNA-binding proteins (basic isoelectric point, high content of Lys/Arg/Ser residues)16 and also localize to the cell nucleus, thereby often entering the nucleolus (Büstenbinder and Abel, unpublished). Therefore, we hypothesize that at least some IQD proteins participate in ribonucleoprotein complex assembly, nucleocytoplasmic transport and delivery to specific cytoplasmic sites prior to local derepression of mRNA translation, which is an increasingly recognized strategy for coupling gene expression with spatial restriction of protein synthesis and thus for efficient intracellular protein sorting.41-43 Unlike in animals, plant nucleoli are thought to exert novel functions in mRNA surveillance and nuclear export, in addition to their conventional roles for assembling and exporting ribosomal subunits.42 Not surprisingly, most Arabidopsis IQD proteins (26), including IQD1, contain predicted nucleolar localization sequences (NoLS);44 however, their functionality remains to be tested. As expected for cellular scaffold proteins with roles in regulatory processes, experimental evidence for in vivo phosphorylation of IQD1 and additional IQD proteins (19) can be found in “The Arabidopsis Protein Phosphorylation Site Database” (PhosPhAt4.0).45 The phosphorylation sites identified to date on IQD proteins are located outside of the central IQ67 domain and may thus control the interaction of IQD proteins with KLCRs and various cargo molecules.
Conclusions and Perspectives
Genetic and molecular studies initiated with Arabidopsis IQD1 have uncovered a plant-specific class of novel CaM/CML-regulated proteins with putative roles as cellular scaffolds.16,21,27 IQD1 and additional family members may connect various signaling pathways to microtubule-dependent trafficking of macromolecular complexes.21,24-26 For example, the local interactome of KLCR2 comprises more than 70 proteins with predicted functions in Ca2+-signaling, including IQD2 and IQD23, hormone action (jasmonic acid), redox homeostasis, or in the control of gene expression (transcription, RNA binding and translation).28 Some of the IQD1 complexes may originate in the cell nucleus or nucleolus and mediate RNA transport to specific intracellular sites, or even to adjoining cells via plasmodesmata (Fig. 2). Elevated IQD1 expression stimulates glucosinolate production and plant defense against herbivores.21 The highly polar architecture of cell types related to glucosinolate synthesis and accumulation (e.g., vascular bundles)46 likely necessitates cellular translocation of metabolons or ribonucleoprotein complexes for efficient targeting or localized synthesis of glucosinolate pathway enzymes. Similarly, directional transport of vesicles for rapid deposition of callose at sites of pathogen encounter is crucial for basal defense responses, which, upon pathogen perception, are in part mediated by the controlled turnover of specific glucosinolates and the signaling activities of their breakdown products.47
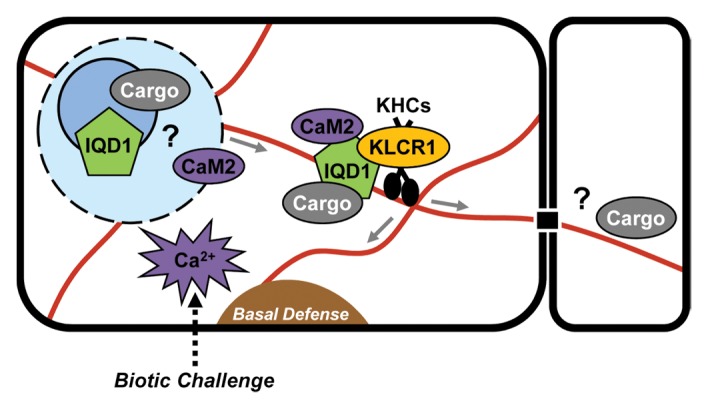
Figure 2. Current model of IQD1-regulated cellular processes. IQD1 is hypothesized to function as a scaffold protein that recruits in a Ca2+-CaM-dependent manner various cargos to kinesin (KHCs) motor complexes via KLCR1. Proteins such as GSTU26 or as yet unidentified RNAs are putative cargo molecules. Ribonucleoprotein complexes containing IQD1 may be assembled in the cell nucleolus/nucleus, exported and directionally transported along microtubule tracks to specific cellular sites related to plant defense response for local mRNA translation, or via plasmodesmata to adjoining cells. A generalized model may apply to other IQD family members.
With the thorough annotation of IQD gene families in three plant species16,17 and the emerging picture of IQD proteins as scaffolds in cellular signaling and microtubule-dependent processes,27 the stage has been set for major advances in our understanding of their biological roles and biochemical activities. A functional analysis of the entire Arabidopsis IQD gene family by various reverse genetic approaches and tools, coupled to a comprehensive phenotypic characterization, will uncover additional IQD protein-dependent processes during plant development under different growth conditions. Given that the only known biological processes regulated by IQD proteins have been recognized in lines with elevated IQD gene expression,21,25 emphasis should be placed on the generation of overexpression and higher-order knockout lines. A second focus of future research will be the study of specific IQD-regulated macromolecular complexes and of their activities in vivo. For example, work on IQD1 will aim at the isolation of IQD1 scaffold complexes formed in planta, followed by the identification and characterization of their protein and putative RNA constituents. Detailed analyses of structure-function relationships will reveal underlying structural determinants of molecular interactions and their regulation via Ca2+-sensors and protein phosphorylation.
Acknowledgments
This work was supported by a DFG Collaborative Research Center grant (SFB648), and by core funding to the Leibniz-Institute of Plant Biochemistry from the state of Saxony-Anhalt and the Federal Republic of Germany.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24369
References
- 1.Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 2.Reddy AS, Ali GS, Celesnik H, Day IS. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell. 2011;23:2010–32. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack E, Tsai YC, Braam J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10:383–9. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Reddy AS, Ben-Hur A, Day IS. Experimental and computational approaches for the study of calmodulin interactions. Phytochemistry. 2011;72:1007–19. doi: 10.1016/j.phytochem.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Reddy VS, Reddy AS. Proteomics of calcium-signaling components in plants. Phytochemistry. 2004;65:1745–76. doi: 10.1016/j.phytochem.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Bouché N, Yellin A, Snedden WA, Fromm H. Plant-specific calmodulin-binding proteins. Annu Rev Plant Biol. 2005;56:435–66. doi: 10.1146/annurev.arplant.56.032604.144224. [DOI] [PubMed] [Google Scholar]
- 7.Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–40. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 8.Bähler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–13. doi: 10.1016/S0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- 9.Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–42. doi: 10.1016/S0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 10.Fischer C, Kugler A, Hoth S, Dietrich P. An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol. 2013 doi: 10.1093/pcp/pct021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talke IN, Blaudez D, Maathuis FJ, Sanders D. CNGCs: prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 2003;8:286–93. doi: 10.1016/S1360-1385(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhou YP, Duan J, Fujibe T, Yamamoto KT, Tian CE. AtIQM1, a novel calmodulin-binding protein, is involved in stomatal movement in Arabidopsis. Plant Mol Biol. 2012;79:333–46. doi: 10.1007/s11103-012-9915-0. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Poovaiah BW. A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem. 2002;277:45049–58. doi: 10.1074/jbc.M207941200. [DOI] [PubMed] [Google Scholar]
- 14.Bouché N, Scharlat A, Snedden W, Bouchez D, Fromm H. A novel family of calmodulin-binding transcription activators in multicellular organisms. J Biol Chem. 2002;277:21851–61. doi: 10.1074/jbc.M200268200. [DOI] [PubMed] [Google Scholar]
- 15.Reddy AS, Day IS. Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2001;2:H0024. doi: 10.1186/gb-2001-2-7-research0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abel S, Savchenko T, Levy M. Genome-wide comparative analysis of the IQD gene families in Arabidopsis thaliana and Oryza sativa. BMC Evol Biol. 2005;5:72. doi: 10.1186/1471-2148-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Z, Van Houten J, Gonzalez G, Xiao H, van der Knaap E. Genome-wide identification, phylogeny and expression analysis of SUN, OFP and YABBY gene family in tomato. Mol Genet Genomics. 2013 doi: 10.1007/s00438-013-0733-0. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary N, McMahon C, Blobel G. Primary structure of a human arginine-rich nuclear protein that colocalizes with spliceosome components. Proc Natl Acad Sci USA. 1991;88:8189–93. doi: 10.1073/pnas.88.18.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Califice S, Baurain D, Hanikenne M, Motte P. A single ancient origin for prototypical serine/arginine-rich splicing factors. Plant Physiol. 2012;158:546–60. doi: 10.1104/pp.111.189019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Grubb CD, Abel S. Direct analysis of single leaf disks for chemopreventive glucosinolates. Phytochem Anal. 2002;13:152–7. doi: 10.1002/pca.636. [DOI] [PubMed] [Google Scholar]
- 21.Levy M, Wang Q, Kaspi R, Parrella MP, Abel S. Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J. 2005;43:79–96. doi: 10.1111/j.1365-313X.2005.02435.x. [DOI] [PubMed] [Google Scholar]
- 22.Grubb CD, Abel S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006;11:89–100. doi: 10.1016/j.tplants.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol. 2006;57:303–33. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 24.Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell. 2007;19:3037–57. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science. 2008;319:1527–30. doi: 10.1126/science.1153040. [DOI] [PubMed] [Google Scholar]
- 26.Wu S, Xiao H, Cabrera A, Meulia T, van der Knaap E. SUN regulates vegetative and reproductive organ shape by changing cell division patterns. Plant Physiol. 2011;157:1175–86. doi: 10.1104/pp.111.181065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bürstenbinder K, Savchenko T, Müller J, Adamson AW, Stamm G, Kwong R, et al. Arabidopsis calmodulin-binding protein IQ67-domain 1 localizes to microtubules and interacts with kinesin light chain-related protein-1. J Biol Chem. 2013;288:1871–82. doi: 10.1074/jbc.M112.396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arabidopsis Interactome Mapping Consortium Evidence for network evolution in an Arabidopsis interactome map. Science. 2011;333:601–7. doi: 10.1126/science.1203877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drevensek S, Goussot M, Duroc Y, Christodoulidou A, Steyaert S, Schaefer E, et al. The Arabidopsis TRM1-TON1 interaction reveals a recruitment network common to plant cortical microtubule arrays and eukaryotic centrosomes. Plant Cell. 2012;24:178–91. doi: 10.1105/tpc.111.089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhey KJ, Kaul N, Soppina V. Kinesin assembly and movement in cells. Annu Rev Biophys. 2011;40:267–88. doi: 10.1146/annurev-biophys-042910-155310. [DOI] [PubMed] [Google Scholar]
- 31.Akhmanova A, Hammer JA., 3rd Linking molecular motors to membrane cargo. Curr Opin Cell Biol. 2010;22:479–87. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 33.Lee YR, Liu B. Cytoskeletal motors in Arabidopsis. Sixty-one kinesins and seventeen myosins. Plant Physiol. 2004;136:3877–83. doi: 10.1104/pp.104.052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipka E, Müller S. Potential roles for Kinesins at the cortical division site. Front Plant Sci. 2012;3:158. doi: 10.3389/fpls.2012.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnapp BJ. Trafficking of signaling modules by kinesin motors. J Cell Sci. 2003;116:2125–35. doi: 10.1242/jcs.00488. [DOI] [PubMed] [Google Scholar]
- 36.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–6. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–9. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shannon KB. IQGAP family members in yeast, dictyostelium, and mammalian cells. Int J Cell Biol. 2012;2012:894817. doi: 10.1155/2012/894817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–9. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 40.White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–24. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kindler S, Wang H, Richter D, Tiedge H. RNA transport and local control of translation. Annu Rev Cell Dev Biol. 2005;21:223–45. doi: 10.1146/annurev.cellbio.21.122303.120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pendle AF, Clark GP, Boon R, Lewandowska D, Lam YW, Andersen J, et al. Proteomic analysis of the Arabidopsis nucleolus suggests novel nucleolar functions. Mol Biol Cell. 2005;16:260–9. doi: 10.1091/mbc.E04-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchand V, Gaspar I, Ephrussi A. An intracellular transmission control protocol: assembly and transport of ribonucleoprotein complexes. Curr Opin Cell Biol. 2012;24:202–10. doi: 10.1016/j.ceb.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Scott MS, Boisvert FM, McDowall MD, Lamond AI, Barton GJ. Characterization and prediction of protein nucleolar localization sequences. Nucleic Acids Res. 2010;38:7388–99. doi: 10.1093/nar/gkq653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arsova B, Schulze WX. Current status of the plant phosphorylation site database PhosPhAt and its use as a resource for molecular plant physiology. Front Plant Sci. 2012;3:132. doi: 10.3389/fpls.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koroleva OA, Gibson TM, Cramer R, Stain C. Glucosinolate-accumulating S-cells in Arabidopsis leaves and flower stalks undergo programmed cell death at early stages of differentiation. Plant J. 2010;64:456–69. doi: 10.1111/j.1365-313X.2010.04339.x. [DOI] [PubMed] [Google Scholar]
- 47.Bednarek P. Chemical warfare or modulators of defence responses - the function of secondary metabolites in plant immunity. Curr Opin Plant Biol. 2012;15:407–14. doi: 10.1016/j.pbi.2012.03.002. [DOI] [PubMed] [Google Scholar]


