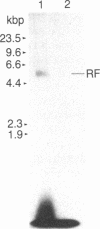
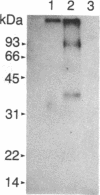
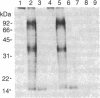
Abstract
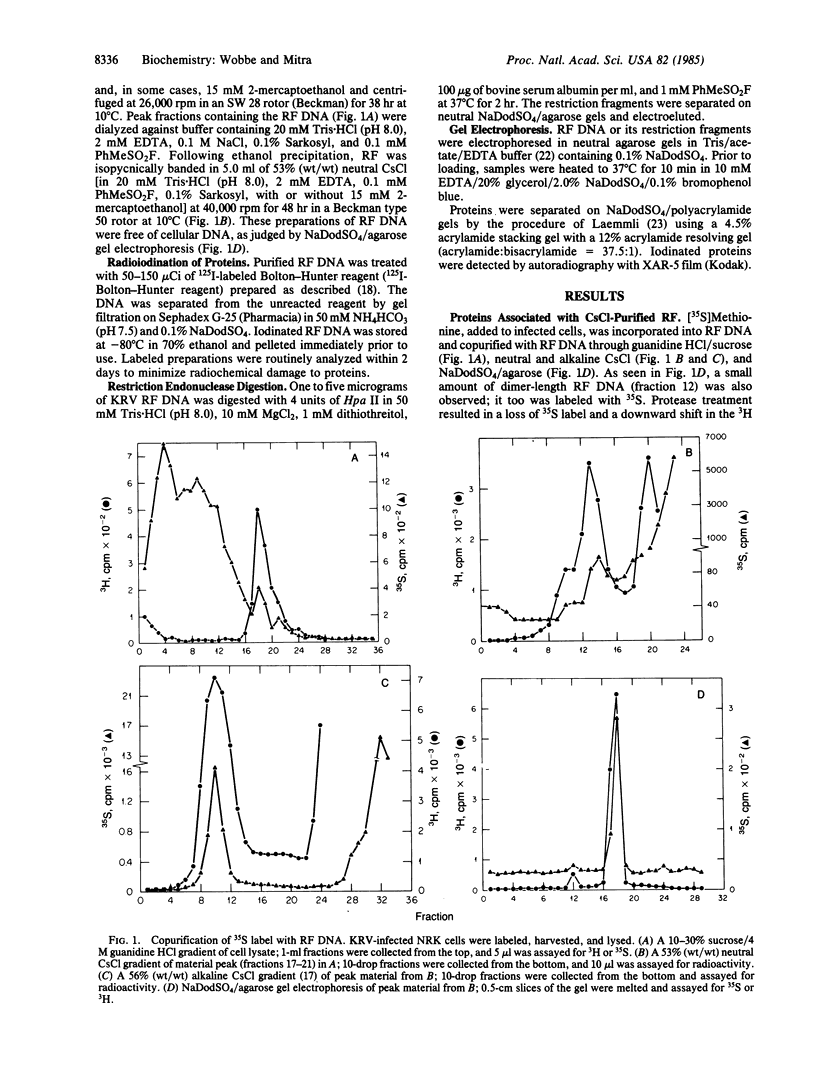
Revie et al. [Revie, D., Tseng, B. Y., Grafstrom, R. H. & Goulian, M. (1979) Proc. Natl. Acad. Sci. USA 76, 5539-5543] have proposed that the double-stranded replicative form (RF) DNA of the autonomous rodent parvovirus H-1 has protein of 60 kDa covalently bound at its 5' termini. We present evidence that the RF DNA of a similar rodent parvovirus, Kilham rat virus (KRV), also has covalently bound protein. NaDodSO4/polyacrylamide gel electrophoresis of purified, 125I-labeled RF DNA shows that proteins of 68-72, 66, 64, and 55 kDa copurify with the DNA during velocity and equilibrium sedimentation in the presence of detergents and 4 M guanidine HCl. Phenol extraction in the presence of 2-mercaptoethanol removes the 68- to 72-kDa proteins, but the 66-, 64-, and 55-kDa proteins remain tightly, but noncovalently, bound. The latter polypeptides also appear to associate with protease-treated RF DNA when mixed with uninfected cell extract. Following removal of these proteins by electrophoresis in NaDodSO4/agarose gels, two proteins (called RF TP-90 and RF TP-40), of about 90 and 40 kDa, become evident. These remain bound to the DNA and are released only after nuclease digestion of the DNA. These two proteins, apparently not of viral origin, are associated with terminal restriction fragments of the RF DNA and appear to be covalently bound to the 5' termini of both strands.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Thomson M., Chow M. B., Ward D. C. Structure and replication of minute virus of mice DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):751–762. doi: 10.1101/sqb.1983.047.01.086. [DOI] [PubMed] [Google Scholar]
- Banerjee P. T., Rothrock R., Mitra S. Restriction map of the single-stranded DNA genome of Kilham rat virus strains 171, a nondefective parvovirus. J Virol. 1981 Oct;40(1):118–125. doi: 10.1128/jvi.40.1.118-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Hauswirth W. W. Adeno-associated viruses. Adv Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Reinberg D., Schmidt-Glenewinkel T., Roth M., Zipursky S. L., Hurwitz J. DNA structures required for phi X174 A-protein-directed initiation and termination of DNA replication. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):701–715. doi: 10.1101/sqb.1983.047.01.081. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Humphrey G. W., Pigiet V. Association of polyoma T antigen and DNA with the nuclear matrix from lytically infected 3T6 cells. Cell. 1980 Nov;22(1 Pt 1):37–46. doi: 10.1016/0092-8674(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Processing of the adenovirus terminal protein. J Virol. 1981 Apr;38(1):272–277. doi: 10.1128/jvi.38.1.272-277.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Sturzenbecker L. J., Tattersall P. The autonomous parvovirus MVM encodes two nonstructural proteins in addition to its capsid polypeptides. Virology. 1983 Sep;129(2):333–343. doi: 10.1016/0042-6822(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Crawford L. V. A minute virus of mice. Virology. 1966 Aug;29(4):605–612. doi: 10.1016/0042-6822(66)90284-4. [DOI] [PubMed] [Google Scholar]
- Dobson P. R., Helleiner C. W. A replicative form of the DNA of minute virus of mice. Can J Microbiol. 1973 Jan;19(1):35–41. doi: 10.1139/m73-005. [DOI] [PubMed] [Google Scholar]
- Feitelson M. A., Wettstein F. O., Stevens J. G. Tryptic peptide mapping of picomolar quantities of protein labeled with the Bolton--Hunter reagent. Anal Biochem. 1981 Sep 15;116(2):473–479. doi: 10.1016/0003-2697(81)90391-2. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi M., Siegl G., Kronauer G. Multiplication of parvovirus LuIII in a synchronized culture system. IV. Association of viral structural polypeptides with the host cell chromatin. J Virol. 1976 Oct;20(1):29–38. doi: 10.1128/jvi.20.1.29-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavelle G., Li A. T. Isolation of intracellular replicative forms and progeny single strands of DNA from parvovirus KRV in sucrose gradients containing guanidine hydrochloride. Virology. 1977 Jan;76(1):464–467. doi: 10.1016/0042-6822(77)90324-5. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Evidence for two levels of DNA folding in histone-depleted HeLa interphase nuclei. J Mol Biol. 1982 Apr 5;156(2):309–324. doi: 10.1016/0022-2836(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Non-histone proteins and long-range organization of HeLa interphase DNA. J Mol Biol. 1982 Apr 5;156(2):325–344. doi: 10.1016/0022-2836(82)90332-1. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Godwin J., Mason D. W., Brazell I. A., Cook P. R. DNA is replicated at the nuclear cage. J Cell Sci. 1980 Dec;46:365–386. doi: 10.1242/jcs.46.1.365. [DOI] [PubMed] [Google Scholar]
- Mitra S. DNA replication in viruses. Annu Rev Genet. 1980;14:347–397. doi: 10.1146/annurev.ge.14.120180.002023. [DOI] [PubMed] [Google Scholar]
- Mitra S., Snyder C. E., Bates R. C., Banerjee P. T. Comparative physicochemical and biological properties of two strains of Kilham rat virus, a non-defective parvovirus. J Gen Virol. 1982 Jul;61(Pt 50):43–54. doi: 10.1099/0022-1317-61-1-43. [DOI] [PubMed] [Google Scholar]
- Muller D. E., Siegl G. Maturation of parvovirus LuIII in a subcellular system. I. Optimal conditions for in vitro synthesis and encapsidation of viral DNA. J Gen Virol. 1983 May;64(Pt 5):1043–1054. doi: 10.1099/0022-1317-64-5-1043. [DOI] [PubMed] [Google Scholar]
- Muller D. E., Siegl G. Maturation of parvovirus LuIII in a subcellular system. II. Isolation and characterization of nucleoprotein intermediates. J Gen Virol. 1983 May;64(Pt 5):1055–1067. doi: 10.1099/0022-1317-64-5-1055. [DOI] [PubMed] [Google Scholar]
- Nelkin B. D., Pardoll D. M., Vogelstein B. Localization of SV40 genes within supercoiled loop domains. Nucleic Acids Res. 1980 Dec 11;8(23):5623–5633. doi: 10.1093/nar/8.23.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. V., Chernokhvostov V. V., Roodyn A. V., Zbarsky I. B., Georgiev G. P. Proteins tightly bound to DNA in the regions of DNA attachment to the skeletal structures of interphase nuclei and metaphase chromosomes. Cell. 1981 Nov;27(1 Pt 2):65–73. doi: 10.1016/0092-8674(81)90361-5. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Revie D., Tseng B. Y., Grafstrom R. H., Goulian M. Covalent association of protein with replicative form DNA of parvovirus H-1. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5539–5543. doi: 10.1073/pnas.76.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Klaassen B. DNA sequence of the 5' terminus containing the replication origin of parvovirus replicative form DNA. J Virol. 1982 Mar;41(3):990–999. doi: 10.1128/jvi.41.3.990-999.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. II. Isolation and characterization of H-1 replicative form DNA. J Virol. 1974 Feb;13(2):400–410. doi: 10.1128/jvi.13.2.400-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. In vivo conversion of the single-stranded DNA of the kilham rat virus to a double-stranded form. J Virol. 1973 Feb;11(2):299–305. doi: 10.1128/jvi.11.2.299-305.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VII. Electron microscopy of replicative-form DNA synthesis. J Virol. 1977 Feb;21(2):713–723. doi: 10.1128/jvi.21.2.713-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VIII. Partial denaturation mapping and localization of the replication origin of H-1 replicative-form DNA with electron microscopy. J Virol. 1977 Feb;21(2):724–731. doi: 10.1128/jvi.21.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Hand R. E., Jr Requirement of cellular synthesis for Kilham rat virus replication. Virology. 1970 Dec;42(4):1054–1063. doi: 10.1016/0042-6822(70)90353-3. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASQUEZ C., BRAILOVSKY C. PURIFICATION AND FINE STRUCTURE OF KILHAM'S RAT VIRUS. Exp Mol Pathol. 1965 Feb;76:130–140. doi: 10.1016/0014-4800(65)90029-8. [DOI] [PubMed] [Google Scholar]
- Werner D., Petzelt C. Alkali-stably bound proteins in eukaryotic and prokaryotic DNAs show common characteristics. J Mol Biol. 1981 Aug 5;150(2):297–302. doi: 10.1016/0022-2836(81)90453-8. [DOI] [PubMed] [Google Scholar]
- Werner D., Zimmermann H. P., Rauterberg E., Spalinger J. Antibodies to the most tightly bound proteins in eukaryotic DNA. Exp Cell Res. 1981 May;133(1):149–157. doi: 10.1016/0014-4827(81)90365-7. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Mitra S., Ramakrishnan V. Structure of the capsid of Kilham rat virus from small-angle neutron scattering. Biochemistry. 1984 Dec 18;23(26):6565–6569. doi: 10.1021/bi00321a044. [DOI] [PubMed] [Google Scholar]
- Younghusband H. B., Maundrell K. Adenovirus DNA is associated with the nuclear matrix of infected cells. J Virol. 1982 Aug;43(2):705–713. doi: 10.1128/jvi.43.2.705-713.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]