Abstract
Background
Manganese superoxide dismutase (MnSOD) inhibits oxidative damage and cancer therapy effectiveness. A polymorphism in its encoding gene (SOD2: Val16Ala rs4880) may confer poorer breast cancer survival, but data are inconsistent. We examined the association of SOD2 genotype and breast cancer recurrence (BCR) among patients treated with cyclophosphamide-based chemotherapy (Cyclo). We compared our findings with published studies using meta-analyses.
Methods
We conducted a population-based case-control study of BCR among women in Jutland, Denmark. Subjects were diagnosed with non-metastatic breast cancer from 1990–2001, received adjuvant Cyclo, and were registered in the Danish Breast Cancer Cooperative Group. We identified 118 patients with BCR and 213 matched breast cancer controls. We genotyped SOD2 and used conditional logistic regression to compute the odds ratio (OR) and associated 95% confidence intervals (95% CI) of BCR. We used random-effects meta-analytic models to evaluate the association of SOD2 polymorphisms and BCR.
Results
The frequency of the SOD2-Ala allele was 70% in cases versus 71% in controls; 40% versus 44% were heterozygotes, and 30% versus 25% were homozygotes, respectively. Heterozygote and homozygote carriers of the Ala allele had no increased rate of BCR (OR = 1.1, 95%CI = 0.65, 2.0, and OR = 0.87, 95%CI = 0.47, 1.6, respectively). Five studies informed the meta-analytic models; summary estimates associating BCR for homozygote, or any inheritance of the variant Ala allele were 1.18 (95%CI = 0.74, 1.88), and 1.18, (95%CI = 0.91, 1.54), respectively.
Conclusion
Our findings do not suggest that MnSOD enzymatic activity, as measured by SOD2 genotype, affects rates of BCR among patients treated with Cyclo.
Introduction
Manganese superoxide dismutase (MnSOD) plays a crucial role in endogenous defense mechanisms against reactive oxygen species (ROS) by catalyzing their conversion into H2O2 and O2 in the mitochondria. MnSOD is encoded by the superoxide dismutase (SOD2) gene (locus 6q25.3). A single nucleotide polymorphism (SNP) in the SOD2 gene (a T to C substitution) results in a valine (Val) to alanine (Ala) amino acid change, and higher MnSOD enzymatic activity [1].
Chemotherapy exerts its anti-cancer effects in part, by inducing the production of ROS. Some studies suggest that the SOD2 polymorphism acts as a predictor of response to chemotherapy in cancer patients [2]–[5]. Research has also suggested that the Ala/Ala genotype may be associated with an approximately two-fold increase in risk of breast cancer [6]–[8], but that the Val/Val genotype may correlate with tumors with higher metastatic potential (lymph node positive disease) [9]. Its association with breast cancer recurrence is not clear because of conflicting findings from the seven former studies [10]–[15]. Two large studies (>500 patients) reported that breast cancer patients with the Ala allele had poorer survival compared with carriers of the Val allele [10], [11]. In contrast, smaller studies (n∼300 patients) reported better survival among carriers of the Ala allele, especially in patients treated with radiation in addition to chemotherapy [13], [14], [16]. Studies finding no association between MnSOD activity and breast cancer recurrence have also been reported [15], [17]. Researchers have suggested that findings from the individual studies should be assembled in a meta-analysis in order to better define the association of SOD2 polymorphisms and outcomes in patients with breast cancer [14].
In addition to their conflicting results, the previous studies have had limited generalizability due to the inclusion of selected patient groups such as those with metastatic disease [18], or hormone receptor-positive tumors [11]. Studies have lacked information on other cancer-directed therapies [10], such as radiation therapy, whose effectiveness has been shown to be modified by SOD2 polymorphisms [13], [19]. Cyclophosphamide remains a standard chemotherapy for the treatment of breast cancer patients and since 2010 has been used in combination with epirubicin and docetaxol [20]. Therefore, we conducted a population-based case-control study among women treated with cyclophosphamide-based chemotherapy (cyclophosphamide-epirubicin-5-fluorouracil – CEF) in Denmark to evaluate the effect of the SOD2 polymorphism on breast cancer recurrence. We used meta-analytic techniques to compare our findings with published studies on the association of SOD2 and breast cancer survival.
Methods
Patient consent was not required. The study was approved by the Regional Committee on Biomedical Ethics of the Central Denmark Region (20070085/1-10-72-614-12), the Danish Data Protection Agency (2012-41-1399), and the Danish Breast Cancer Cooperative Group. These permissions waived the need for written informed consent from the participants.
Study Population and Data Collection
The source population was female residents of Denmark’s Jutland peninsula, aged 35–69 years, diagnosed between 1990 and 2001 with stage I-III breast cancer and registered with the Danish Breast Cancer Cooperative Group (DBCG). Each patient had received adjuvant CEF primary chemotherapy, with or without other adjuvant treatments. Follow-up time began one year after the breast cancer diagnosis date and continued until the date of the first breast cancer recurrence, death from any cause, loss to follow-up (e.g., emigration), ten years of follow-up, or September 1, 2008.
Breast cancer patients registered in the DBCG are routinely followed-up twice yearly for the first five years after initial diagnosis, and then annually thereafter for a further five years. Cases were women in the source population diagnosed with a local or distant breast cancer recurrence during follow-up. The DBCG defines breast cancer recurrence as a recurrence in the same breast, a new primary tumor in the ipsi- or contra-lateral breast, or a recurrence at a site other than the original primary cancer site [21].
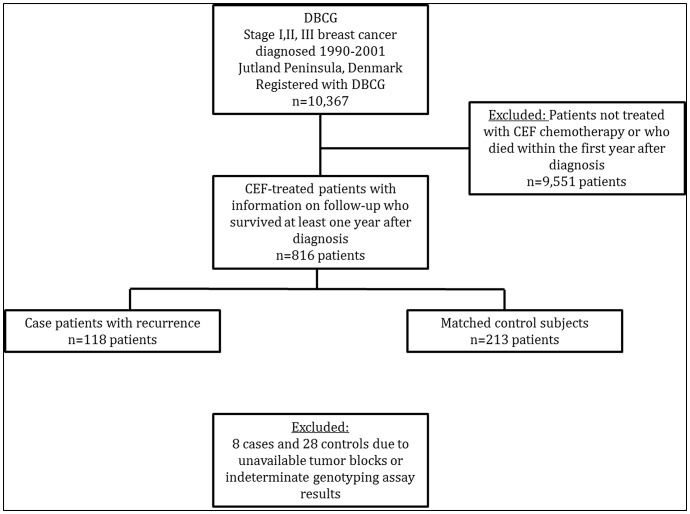
For each case, we selected controls using risk-set sampling without replacement from members of the source population. Controls were women who received CEF as their primary treatment, who survived at least one year, and who had not had a breast cancer recurrence at the time their corresponding case experienced a recurrence – risk-set sampling [22]. We individually matched up to two breast cancer controls to each case on birth year (+/−1 year), county of residence, cancer stage, estrogen receptor status, and date of diagnosis (calliper matched +/−1 year). Figure 1 shows an overview of the breast cancer patients included in this study.
Figure 1. Design used to identify the study sample.
The source population consisted of 10,367 female residents of the Jutland Peninsula in Denmark aged 35–69 years who were diagnosed with stage I, II, or III breast cancer between 1990 and 2001. Most of the women were excluded because of unknown protocol or death within the first year after diagnosis (n = 9,551). Genotyping results were missing for a small proportion of patients due to unavailable tumor blocks or indeterminate assay results.
Tissue Processing, DNA Extraction, Amplification, and Genotyping
We retrieved the formalin-fixed, paraffin-embedded (FFPE) primary tumors of cases and controls from the pathology archives of treating hospitals [23]. We reviewed hematoxylin and eosin stained sections and pathology reports to identify appropriate tumor blocks for processing. All tissue blocks were processed and handled using standard operating procedures to minimize risks of cross-contamination and nuclease digestion. Three to six 10 µm sections were cut from each tumor block and placed in sterile 1.5 mL centrifuge tube for DNA extraction.
After xylene deparaffinization, DNA was extracted by two incubations in 99% ethanol, proteinase K digestion, and purification with the QIAamp DNA FFPE Tissue Kit (Qiagen AB, Dusseldorf, Germany) following the manufacturer’s protocol.
Fifty nanograms of purified DNA from each tumor sample were amplified in 25 uL PCR reactions: 95°C for 10 minutes, followed by 40 cycles of 92°C for 15 seconds, and 60°C for 60 seconds, using primers and reagents supplied with the TaqMan genotyping kit (Applied Biosystems, Foster City, California, USA).
A commercially available TaqMan kit was used to genotype SOD2 (rs4880) (Applied Biosystems assay ID C_8709053). All samples were assayed in duplicate using the MX3000P Real-Time PCR system (Stratagene, Cedar Creek, Texas, USA). Positive genotyping controls for the variant alleles were identified by sequencing lymphocyte DNA from discarded and anonymized blood samples of 30 healthy donors, and included with each batch of assays. Negative controls, with sterile water substituted for DNA, were also included in each batch. Laboratory personnel were blinded to all clinical data, including case/control status.
Definitions of Analytic Variables
We classified SOD2 genotype as homozygous wildtype (Val/Val alleles), heterozygous (Val/Ala allele), and homozygous variants (Ala/Ala alleles), according to the auto-call feature of the analytic software (MXPro QPCR version 4.1, Stratagene). We additionally classified the SOD2 genotype as inheritance of any Ala allele (Val/Ala and Ala/Ala) versus homozygous wildtype (Val/Val).
Covariates included the year of breast cancer diagnosis, age at diagnosis, menopausal status at diagnosis (premenopausal versus postmenopausal), type of breast cancer primary surgery (mastectomy or breast conserving surgery), receipt of adjuvant tamoxifen, and receipt of adjuvant radiation therapy.
Statistical Analyses
We computed the frequency of cases and controls within each category of all analytic variables. We used conditional logistic regression to compute the odds ratio (OR) and associated 95% confidence interval (CI) (as a measure of the recurrence rate ratio) to analyse the association of the SOD2 genotype with the rate of breast cancer recurrence. Because of the matched design, the conditional OR was adjusted for confounding by the matching factors (birth year, stage, county, ER status and date of diagnosis), menopausal status, primary surgery type, and receipt of radiation therapy. We tested whether genotypes at the SOD2 locus observed among control subjects were in Hardy-Weinberg equilibrium by computing the χ2 test statistic, with expected genotype frequencies based on the observed prevalence of major and minor alleles [24].
Meta-analysis
Search strategy
We searched for the terms “manganese superoxide dismutase,” “MnSOD,” “SOD2,” “breast cancer” and “survival” in PubMed. No language restrictions were imposed. All papers published through November 2013 regarding the association between SOD2 gene variants and risks of breast cancer recurrence or mortality were reviewed to determine whether their results should be included.
Data sources and searches
We searched for the terms “manganese superoxide dismutase,” “breast cancer” and “survival” in PubMed, and imposed no language restrictions. We also searched references of the included articles.
Study selection
Our search targeted articles that met the following criteria: evaluated a prognostic outcome (breast cancer recurrence, mortality, or breast cancer survival) in breast cancer patients by MnSOD expression, evaluated original data, and reported a risk estimate with an associated estimate of precision (standard error or 95%CI). We included studies that genotyped SOD2 in breast cancer patients, and correlated it with breast cancer prognosis (breast cancer recurrence, mortality or breast cancer survival) in patients treated with cyclophosphamide-based chemotherapy. We excluded studies where SOD2 had been correlated with cancer therapies other than chemotherapy.
Data extraction
DCF performed the PubMed searches, reviewed each of the retrieved abstracts, selected those that appeared to meet the study’s inclusion criteria, and reviewed each of the selected manuscripts in full. Where a study met the inclusion criteria, DCF extracted the data from each study (effect estimates and associated 95% confidence intervals), of the included manuscripts, and performed the meta-analysis. All authors reviewed and agreed on the study inclusion criteria, and on the selected manuscripts. Where there was a disagreement, the paper was re-reviewed and an agreement was reached.
Extracted data variables
We retrieved information on several breast cancer prognostic outcomes namely, breast cancer mortality, breast cancer recurrence, disease-free survival, and breast cancer-specific mortality.
Given the differences in study design, we expected substantial heterogeneity between the studies. We formally assessed this by using Cochran Q and I2 [25] and observed substantial heterogeneity (Q, 12.74 on 4 df, P = 0.013; I2, 68.6%, P = 0.02). We calculated a pooled effect estimate using the Der Simonian-Laird methods for random-effects models. [26].
Bias assessment
We evaluated publication bias by constructing a funnel plot (Figures A & B in File S1). We also performed Duval and Tweedie nonparametric “trim and fill” procedure to further investigate any potential effects of publication bias. This method creates hypothetical missing studies, imputes their effect estimates, and recalculates a pooled effect estimate [27].
Meta-analytic statistical models
We created two separate meta-analytic models to investigate the gene-dose effect of SOD2 alleles and breast cancer outcomes. The first, a recessive model, evaluated recurrence risks associated with inheritance of two variant alleles (Ala/Ala) compared with homozygous or heterozygous carriers of the wild-type allele (Val/Val and Val/Ala, respectively). The second, a dominant model, evaluated recurrence risks associated with inheritance of any Ala allele (Ala/Ala or Val/Ala), compared with homozygous carriers of the wild-type allele. For the second model, where studies presented associations for heterozygote and homozygote variant alleles separately, we estimated an inverse-variance-weighted average of these two associations and used this estimate in the model.
All analyses were performed using STATA software, version 11.0 (StataCorp LP, College Station, Texas). All statistical tests were two-sided.
Results
We identified 118 patients with recurrent breast cancer and 213 matched controls. Table 1 provides descriptive characteristics of the study population. Because of the matching, the proportions of cases and controls by age group, stage and ER status were similar. More cases than controls had a mastectomy as their primary surgical treatment (84% versus 76%). A higher proportion of cases than controls received CEF alone rather than in combination with pamidronate (PAM), tamoxifen (TAM), or herceptin (HER).
Table 1. Frequency of patients with breast cancer recurrence (n = 118) and matched controls (n = 213), by clinical characteristics, among women aged 35 to 69 years at the time of breast cancer diagnosis who resided in Jutland, Denmark, 1991–2001.
| Category | Subcategory | Cases (n,%) | Controls (n,%) |
| Age at diagnosis | |||
| 35–44 | 49 (42) | 84 (39) | |
| 45–54 | 55 (47) | 106 (50) | |
| 55–64 | 8 (6.8) | 18 (8.5) | |
| 65–69 | 6 (5.1) | 5 (2.3) | |
| Menopausal status | |||
| Premenopausal | 95 (81) | 187 (88) | |
| Postmenopausal | 23 (19) | 26 (12) | |
| Stage | |||
| I | 11 (9.3) | 25 (12) | |
| II | 49 (42) | 86 (40) | |
| III | 58 (49) | 102 (48) | |
| Estrogen receptor status | |||
| Poorˆ | 72 (61) | 119 (56) | |
| Positive | 46 (39) | 94 (44) | |
| Surgery type | |||
| Mastectomy | 99 (84) | 162 (76) | |
| Breast conserving surgery | 19 (16) | 51 (24) | |
| Radiation therapy | |||
| No | 35 (30) | 61 (29) | |
| Yes | 83 (70) | 152 (71) | |
| Adjuvant therapy | |||
| CEF | 74 (63) | 113 (53) | |
| CEF+PAM* | 12 (10) | 22 (10) | |
| CEF+TAM§ | 31 (26) | 78 (37) | |
| CEF+TAM+HER¤ | 1 (0.9) | 0 | |
| DBCG Protocol | |||
| DBCG89 | 55 (47) | 86 (40) | |
| DBCG99 | 63 (53) | 127 (60) |
PAM = pamidronate; §TAM = tamoxifen; ¤HER = Herceptin; ?ER ”poor” refers to <10% ER positivity as per the diagnostic period of patients included in the current study.
MnSOD genotypes were in Hardy-Weinberg equilibrium among the controls (P = 0.07) (Table 2). Table 3 presents the association between SOD2 genotype and breast cancer recurrence. Forty percent of cases versus 44% of controls were heterozygotes (Val/Ala), while 30% of cases and 25% of controls were homozygote variants (Ala/Ala). In both conditional and adjusted analyses, we found no evidence of an increased rate of breast cancer recurrence associated with the inheritance of at least one Ala allele [adjusted OR = 1.1 (95%CI = 0.65, 2.0) among heterozygotes, and adjusted OR = 0.87 (95%CI = 0.47, 1.6) among homozygotes]. The inheritance of two Ala alleles was also not associated with an increased rate of breast cancer recurrence when compared with wild-type homozygotes and heterozygotes (adjusted OR = 0.81, 95%CI = 0.47, 1.4).
Table 2. Observed and expected allelic frequency and Hardy-Weinberg Equilibrium (HWE).
| Wildtype | Heterozygote | Variant | |
| Observed | 32 | 44 | 25 |
| Expected | 29 | 50 | 22 |
| HWE | P = 0.07 | ||
Table 3. Association between MnSOD (SOD2) genotype and breast cancer recurrence among women who received cyclophosphamide-epirubicin-5-fluorouracil (CEF) adjuvant chemotherapy; women aged 35 to 69 years at breast cancer diagnosis who resided in Jutland, Denmark, 1991–2001.
| Cases | Controls | Matched* OR (95% CI) | Adjusted§ OR (95% CI) | |
| SOD2 | ||||
| Wildtype | 35 (30) | 66 (32) | 1.0 | 1.0 |
| Heterozygote | 47 (40) | 91 (44) | 1.1 (0.67, 2.0) | 1.1 (0.65, 2.0) |
| Homozygote variant | 35 (30) | 52 (25) | 0.82 (0.44, 1.5) | 0.87 (0.47, 1.6) |
| Unknown | 1 (1) | 4 (2) | – | – |
| SOD2 | ||||
| Homozygous wildtype & heterozygote | 82 (70) | 157 (75) | 1.0 | 1.0 |
| Homozygous variant | 35 (30) | 52 (25) | 0.76 (0.45, 1.3) | 0.81 (0.47, 1.4) |
| Unknown | 1 (1) | 4 (2) | – | – |
Conditioned on the matched factors.
Adjusted for surgery type, type of adjuvant treatment (i.e., CEF +/− pamidronate, tamoxifen, herceptin) and radiation therapy.
Meta-analyses
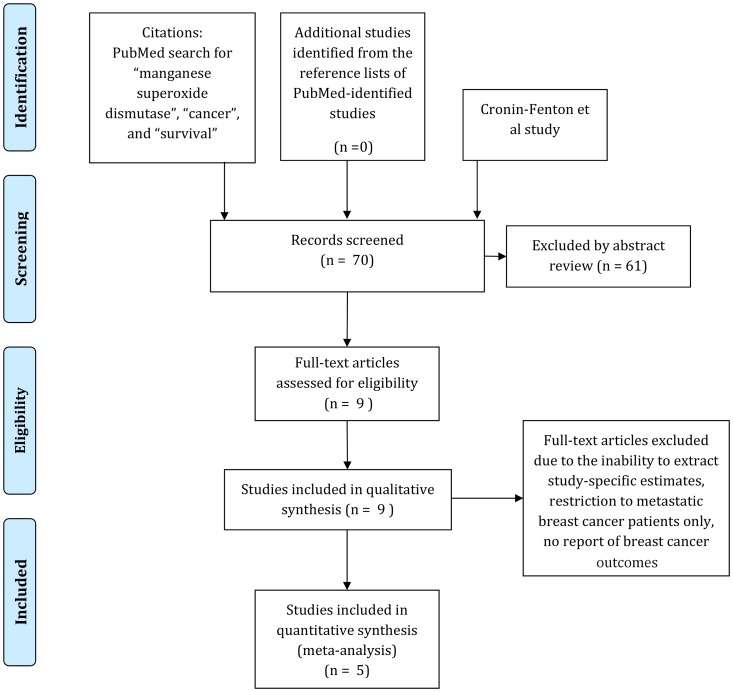
Figure 2 presents a flowchart outlining the process of evaluating articles for inclusion in our meta-analysis. We identified 69 unique records in PubMed based on our primary search terms. Sixty-one of these papers were excluded based on abstract review – most of these studies were based in in vitro systems. Nine studies, including our own unpublished work, investigated the association between MnSOD activity and breast cancer outcomes. Two studies were excluded from our meta-analytic models due to the inability to extract study-specific estimates [14], and the investigation of the association of MnSOD protein, rather than genotype, with breast cancer outcome [17]. We also excluded a study by Martin et al that investigated SOD2_102 variant (Genbank AY397775), [16] as the same patients were investigated in the earlier study by Ambrosone et al. Therefore all of the studies included in our meta-analysis investigated the SOD2_47 variant (rs4880). Two studies included between 80–90% Caucasians [11], [13]; the Ji study included 100% Chinese Han patients [15]; the Glynn study included a Norwegian population (100% European descent), and a US population (57% African American, 43% European descent) [10]; and our study is likely to have been 100% patients of European descent (Table S1).
Figure 2. Selection of studies for inclusion in qualitative and quantitative review.
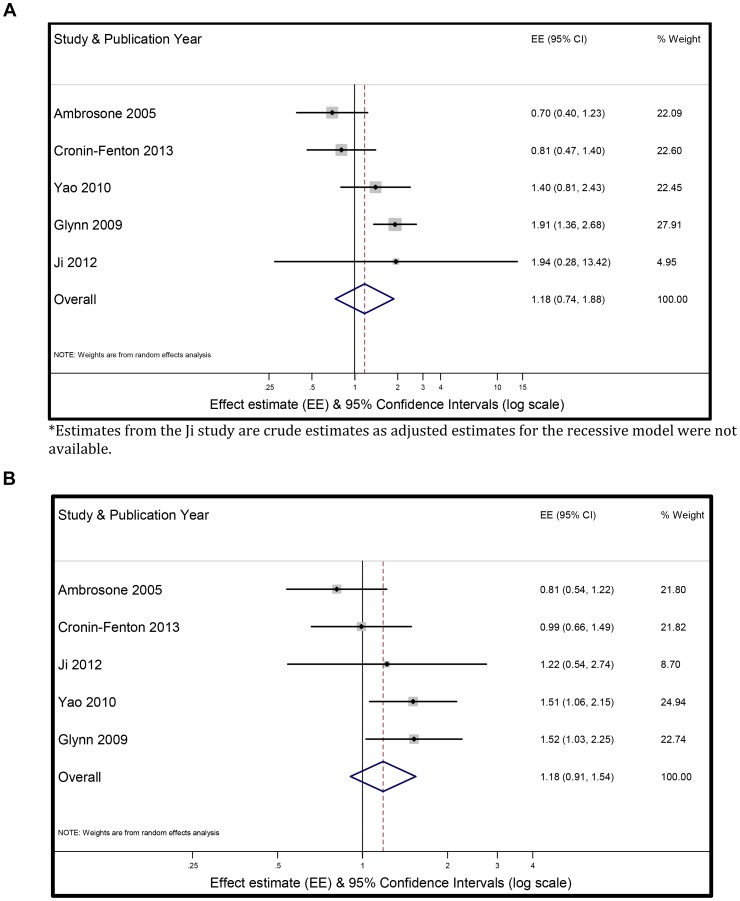
Figure 3a shows a forest plot of the random-effects meta-analytic model of carriers of two Ala alleles (homozygote variant) of SOD2 and breast cancer outcomes, compared with carriers of at least one wild-type allele. The summary random-effects estimate was 1.18 (95%CI = 0.74, 1.88). Figure 3b shows the random-effects meta-analytic model of carriers of any Ala allele (heterozygote or homozygote Ala allele) of SOD2 and breast cancer outcomes, compared with wild-type homozygotes. The summary random-effects estimate was 1.18 (95%CI = 0.91, 1.54). We used the trim and fill method to calculate an adjusted pooled random-effects effect estimate [27]. This method did not add any additional estimates to the funnel plot and the adjusted risk estimate remained unchanged.
Figure 3. Summary relative effect size (ES) and 95% confidence intervals (95%CI) for the association between inheritance of (3a) two SOD2 Ala alleles (recessive model) versus heterozygote or homozygote wild-type allele and (3b) inheritance of any SOD2 Ala allele (dominant model) (homozygote or heterozygote Ala allele) versus homozygote wild-type (Val/Val) and breast cancer outcome.
Summary effect size and 95% CI were estimated using a random-effects meta-analytic model. The size of each square is proportional to the corresponding study’s weight. The horizontal lines represent the confidence intervals. The diamond represents the summary effect size and 95%CI. *.
Discussion
In this population-based study, we found no evidence of an association between genetic modification of MnSOD activity and rates of breast cancer recurrence in breast cancer patients treated with CEF adjuvant chemotherapy. This null association persisted even after adjusting for other cancer-directed treatments, including radiation therapy. Our meta-analyses enabled us to contextualize our findings with those in the published literature. They suggest little evidence of an association between a polymorphism in SOD2 and breast cancer outcomes among women treated with adjuvant chemotherapy.
Several issues should be considered when interpreting our findings. We used the DBCG as a source of comprehensive data on breast cancer patients, tumors, and treatment. The clinical breast cancer database, managed by the DBCG, is considered to be one of the most comprehensive in the world, with exceptionally high data quality [21]. Validation studies of DBCG data have shown that breast cancer recurrence has a positive predictive value of 99.4% [28]. We linked the DBCG records to pathology archives. Thus except for genotyping results, all data and tumor tissues were prospectively collected, minimizing selection bias. As we used risk-set sampling for control selection, the conditional ORs are unbiased estimates of the recurrence rate ratio in the underlying source population [22]. There was little difference between crude (matched) and adjusted analyses. Furthermore, any factor strongly related to breast cancer recurrence is unlikely also to be related to genotype.
There are several plausible explanations for our null results. First, the effect of the SOD2 variant likely depends on the balance between non-toxic neutralization and toxic ROS generation. We focused specifically on the effect of a polymorphism in an enzyme that acts upstream of other detoxifying enzymes. These other antioxidant enzymes (e.g., myeloperoxidase, catalase) may compensate for a shift in the balance of MnSOD expression in order to maintain cellular homeostasis. A study by Ambrosone et al. found that the combined effect of polymorphisms in SOD2 and MPO (the gene encoding myeloperoxidase) was associated with better survival compared with heterozygous or homozygous wild-type carriers of these genes [13]. However, estimates were imprecise given only six exposed cases. Ambrosone et al. were also unable to assess the combined effect of polymorphisms in SOD2 and CAT, the gene encoding catalase. Nonetheless, as in our study, they found a near-null association between the SOD2 polymorphism and the effectiveness of chemotherapy, as measured by breast cancer recurrence (hazard ratio = 0.66, 95% CI = 0.34, 1.29). A null association was also reported in a Chinese study of breast cancer patients [15], in an Italian study focused on MnSOD protein expression and breast cancer survival [17], and in studies evaluating the effect of SOD2 on survival in patients with other cancers [5].
We included breast cancer patients from a relatively homogeneous population (the Danish population), all of whom had received CEF chemotherapy either alone or combined with endocrine therapy or radiation therapy. This contrasts with some of the other studies, which included patients who received different cancer-directed treatments, across different populations [10], [13], [16]. We note also that our meta-analyses were based on studies that included primarily populations of European descent, and so may not be representative of populations of other racial/ethnic distributions. We were unable to extract an effect estimate from a study in Czech breast cancer patients, which reported an increased risk of recurrence among patients homozygote for the SOD2 polymorphism [14]. However, they only evaluated the effect of the SOD2 polymorphism in 30 patients treated with cyclophosphamide. In addition, some of the published studies had very imprecise estimates [10].
We used DNA extracted from tumor tissue rather than non-tumor tissue to assay SOD2 genotype – similar to the Glynn study [10]. Ideally, we would have used blood samples as a source of DNA, however these were unavailable. An earlier validation study in an overlapping patient population showed excellent genotyping concordance between tumor and non-tumor tissue [29]. Furthermore, a recent paper by Rae et al genotyped several CYP2D6 genes in paired tumor and blood and also found good concordance [30]. We note that the paper by Hubackova et al [14] reported an increase in SOD2 transcript levels in tumor compared with paired normal tissue. However, they did not compare SOD2 genotype in the paired tumor and normal samples. The SOD2 locus is adjacent to chromosomal regions that are frequently deleted in breast cancer [31], [32]. Although our genotyping results conformed to Hardy-Weinberg equilibrium, we observed a slightly lower proportion of heterozygotes than expected, which may account for the low P-value (0.07). This potential loss of heterozygosity may be an effect of such chromosome six aberrations, and could also have contributed to our null findings.
Conclusions
In conclusion, we found no association between a functional polymorphism in SOD2 and breast cancer recurrence. Taken together with the published research, our findings suggest that MnSOD activity, as measured by SOD2 genotype, is unlikely to be an important factor in predicting response to cyclophosphamide-based chemotherapy.
Supporting Information
Figure A, Funnel plot showing little evidence of publication bias in studies investigating the association of two SOD2 polymorphisms with outcomes in breast cancer patients. Figure B, Funnel plot showing little evidence of publication bias in studies investigating the association of any SOD2 polymorphisms with outcomes in breast cancer patients.
(PDF)
Studies included in the qualitative and quantitative review of manganese superoxide dismutase and outcomes in patients with breast cancer.
(DOCX)
Acknowledgments
The authors thank the Danish Breast Cancer Cooperative Group for access to its registry data and for preparing the initial dataset. The authors thank Kristina Lystlund Lauridsen for technical assistance.
Funding Statement
This project was supported primarily by a research grant from the Danish Medical Research Council (to SHD), and secondarily by research grants from the US NCI (to TLL), the Danish Medical Research Council (to TLL), and the Karen Elise Jensen Foundation (to HTS). TPA was supported by a US NIH postdoctoral training award (NIH 5 T32 CA 009001). In addition, the Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sutton A, Imbert A, Igoudjil A, Descatoire V, Cazanave S, et al. (2005) The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet Genomics 15: 311–319. [DOI] [PubMed] [Google Scholar]
- 2.Chen PM, Wu YH, Li MC, Cheng YW, Chen CY, et al.. (2012) MnSOD promotes tumor invasion via upregulation of FoxM1-MMP2 axis and related with poor survival and relapse in lung adenocarcinomas. Mol Cancer Res. [DOI] [PubMed]
- 3. Fu TY, Hou YY, Chu ST, Liu CF, Huang CH, et al. (2011) Manganese superoxide dismutase and glutathione peroxidase as prognostic markers in patients with buccal mucosal squamous cell carcinomas. Head Neck 33: 1606–1615. [DOI] [PubMed] [Google Scholar]
- 4. Funke S, Risch A, Nieters A, Hoffmeister M, Stegmaier C, et al. (2009) Genetic polymorphisms in genes related to oxidative stress (GSTP1, GSTM1, GSTT1, CAT, MnSOD, MPO, eNOS) and survival of rectal cancer patients after radiotherapy. J Cancer Epidemiol 2009: 302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnatty SE, Nagle CM, Spurdle AB, Chen X, Australian Breast Cancer Family Study, et al. (2007) The MnSOD Val9Ala polymorphism, dietary antioxidant intake, risk and survival in ovarian cancer (australia). Gynecol Oncol 107: 388–391. [DOI] [PubMed] [Google Scholar]
- 6. Cai Q, Shu XO, Wen W, Cheng JR, Dai Q, et al. (2004) Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: Results from the shanghai breast cancer study. Breast Cancer Res 6: R647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slanger TE, Chang-Claude J, Wang-Gohrke S (2006) Manganese superoxide dismutase ala-9Val polymorphism, environmental modifiers, and risk of breast cancer in a german population. Cancer Causes Control 17: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 8. Mitrunen K, Sillanpaa P, Kataja V, Eskelinen M, Kosma VM, et al. (2001) Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis 22: 827–829. [DOI] [PubMed] [Google Scholar]
- 9. Bica CG, da Silva LL, Toscani NV, Zettler CG, Gottlieb MG, et al. (2010) Polymorphism (ALA16VAL) correlates with regional lymph node status in breast cancer. Cancer Genet Cytogenet 196: 153–158. [DOI] [PubMed] [Google Scholar]
- 10. Glynn SA, Boersma BJ, Howe TM, Edvardsen H, Geisler SB, et al. (2009) A mitochondrial target sequence polymorphism in manganese superoxide dismutase predicts inferior survival in breast cancer patients treated with cyclophosphamide. Clin Cancer Res 15: 4165–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao S, Barlow WE, Albain KS, Choi JY, Zhao H, et al. (2010) Manganese superoxide dismutase polymorphism, treatment-related toxicity and disease-free survival in SWOG 8897 clinical trial for breast cancer. Breast Cancer Res Treat 124: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin RC, Li Y, Liu Q, Jensen NS, Barker DF, et al. (2009) Manganese superoxide dismutase V16A single-nucleotide polymorphism in the mitochondrial targeting sequence is associated with reduced enzymatic activity in cryopreserved human hepatocytes. DNA Cell Biol 28: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ambrosone CB, Ahn J, Singh KK, Rezaishiraz H, Furberg H, et al. (2005) Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res 65: 1105–1111. [PubMed] [Google Scholar]
- 14. Hubackova M, Vaclavikova R, Ehrlichova M, Mrhalova M, Kodet R, et al. (2012) Association of superoxide dismutases and NAD(P)H quinone oxidoreductases with prognosis of patients with breast carcinomas. Int J Cancer 130: 338–348. [DOI] [PubMed] [Google Scholar]
- 15. Ji M, Tang J, Zhao J, Xu B, Qin J, et al. (2012) Polymorphisms in genes involved in drug detoxification and clinical outcomes of anthracycline-based neoadjuvant chemotherapy in chinese han breast cancer patients. Cancer Biol Ther 13: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin RC, Ahn J, Nowell SA, Hein DW, Doll MA, et al. (2006) Association between manganese superoxide dismutase promoter gene polymorphism and breast cancer survival. Breast Cancer Res 8: R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sgambato A, Camerini A, Collecchi P, Graziani C, Bevilacqua G, et al. (2009) Cyclin E correlates with manganese superoxide dismutase expression and predicts survival in early breast cancer patients receiving adjuvant epirubicin-based chemotherapy. Cancer Sci 100: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bewick MA, Conlon MS, Lafrenie RM (2008) Polymorphisms in manganese superoxide dismutase, myeloperoxidase and glutathione-S-transferase and survival after treatment for metastatic breast cancer. Breast Cancer Res Treat 111: 93–101. [DOI] [PubMed] [Google Scholar]
- 19. Ahn J, Ambrosone CB, Kanetsky PA, Tian C, Lehman TA, et al. (2006) Polymorphisms in genes related to oxidative stress (CAT, MnSOD, MPO, and eNOS) and acute toxicities from radiation therapy following lumpectomy for breast cancer. Clin Cancer Res 12: 7063–7070. [DOI] [PubMed] [Google Scholar]
- 20. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, et al. (2008) The clinical database and the treatment guidelines of the danish breast cancer cooperative group (DBCG); its 30-years experience and future promise. Acta Oncol 47: 506–524. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S, Lash TL (2008) Case-control studies. In: Rothman K, Greenland S, Lash TL, editors. Modern Epidemiology, 3rd Edition. Philadelphia: Lippincott, Williams & Wilkins. 111–127.
- 23. Erichsen R, Lash TL, Hamilton-Dutoit S, Bjerregaard B, Vyberg M, et al. (2010) Existing data sources for clinical epidemiology: The danish national pathology registry and data bank. Clin Epidemiol 2: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weir B (1996) Genetic data analysis II: Methods for discrete population genetic data. Sunderland, MA: Sinauer Associates. [DOI] [PubMed]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 27. Duval S, Tweedie R (2000) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 28. Hansen PS, Andersen E, Andersen KW, Mouridsen HT (1997) Quality control of end results in a danish adjuvant breast cancer multi-center study. Acta Oncol 36: 711–714. [DOI] [PubMed] [Google Scholar]
- 29. Ahern TP, Christensen M, Cronin-Fenton D, Lunetta KL, Rosenberg CL, et al. (2010) Concordance of metabolic enzyme genotypes assayed from paraffin-embedded, formalin-fixed breast tumors and normal lymphatic tissue. Clin Epidemiol 2: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rae JM, Regan MM, Thibert JN, Gersch C, Thomas D, et al. (2013) Concordance between CYP2D6 genotypes obtained from tumor-derived and germline DNA. J Natl Cancer Inst 105: 1332–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noviello C, Courjal F, Theillet C (1996) Loss of heterozygosity on the long arm of chromosome 6 in breast cancer: Possibly four regions of deletion. Clin Cancer Res 2: 1601–1606. [PubMed] [Google Scholar]
- 32. Sheng ZM, Marchetti A, Buttitta F, Champeme MH, Campani D, et al. (1996) Multiple regions of chromosome 6q affected by loss of heterozygosity in primary human breast carcinomas. Br J Cancer 73: 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A, Funnel plot showing little evidence of publication bias in studies investigating the association of two SOD2 polymorphisms with outcomes in breast cancer patients. Figure B, Funnel plot showing little evidence of publication bias in studies investigating the association of any SOD2 polymorphisms with outcomes in breast cancer patients.
(PDF)
Studies included in the qualitative and quantitative review of manganese superoxide dismutase and outcomes in patients with breast cancer.
(DOCX)